Breakthroughs in Dodecane-Supported Reaction Channels
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dodecane Reaction Channels: Background and Objectives
Dodecane-supported reaction channels represent a significant advancement in the field of organic synthesis and catalysis. This technology leverages the unique properties of dodecane, a linear alkane hydrocarbon, to create novel reaction environments that offer enhanced control and efficiency in chemical transformations. The development of these reaction channels has been driven by the increasing demand for more sustainable and selective synthetic methods in the chemical industry.
The evolution of dodecane-supported reaction channels can be traced back to the early 2000s when researchers began exploring the potential of alkanes as reaction media. Initially, the focus was on using shorter-chain alkanes, but as understanding grew, attention shifted to longer-chain hydrocarbons like dodecane due to their superior stability and versatility. This shift marked a pivotal moment in the field, opening up new possibilities for complex organic syntheses.
The primary objective of research in dodecane-supported reaction channels is to harness the non-polar nature of dodecane to create unique microenvironments that can influence reaction outcomes. These channels aim to provide better control over reaction kinetics, improve selectivity, and enhance overall yield. Additionally, they offer the potential for more environmentally friendly processes by reducing the need for harmful solvents and enabling easier product separation.
One of the key goals in this field is to develop reaction systems that can effectively mimic enzymatic processes found in nature. By creating confined spaces within the dodecane matrix, researchers aim to achieve enzyme-like selectivity and efficiency in synthetic reactions. This biomimetic approach has the potential to revolutionize various sectors of the chemical industry, from pharmaceuticals to materials science.
Another important objective is to expand the range of reactions that can be effectively carried out in dodecane-supported channels. Current research is focused on adapting various catalytic systems to work efficiently within these non-polar environments. This includes the development of novel catalysts and the modification of existing ones to function optimally in dodecane-based reaction media.
The technology also aims to address some of the longstanding challenges in organic synthesis, such as improving the efficiency of multi-step reactions and reducing the environmental impact of chemical processes. By providing a stable and controllable reaction environment, dodecane-supported channels offer the potential for more streamlined and sustainable synthetic routes.
The evolution of dodecane-supported reaction channels can be traced back to the early 2000s when researchers began exploring the potential of alkanes as reaction media. Initially, the focus was on using shorter-chain alkanes, but as understanding grew, attention shifted to longer-chain hydrocarbons like dodecane due to their superior stability and versatility. This shift marked a pivotal moment in the field, opening up new possibilities for complex organic syntheses.
The primary objective of research in dodecane-supported reaction channels is to harness the non-polar nature of dodecane to create unique microenvironments that can influence reaction outcomes. These channels aim to provide better control over reaction kinetics, improve selectivity, and enhance overall yield. Additionally, they offer the potential for more environmentally friendly processes by reducing the need for harmful solvents and enabling easier product separation.
One of the key goals in this field is to develop reaction systems that can effectively mimic enzymatic processes found in nature. By creating confined spaces within the dodecane matrix, researchers aim to achieve enzyme-like selectivity and efficiency in synthetic reactions. This biomimetic approach has the potential to revolutionize various sectors of the chemical industry, from pharmaceuticals to materials science.
Another important objective is to expand the range of reactions that can be effectively carried out in dodecane-supported channels. Current research is focused on adapting various catalytic systems to work efficiently within these non-polar environments. This includes the development of novel catalysts and the modification of existing ones to function optimally in dodecane-based reaction media.
The technology also aims to address some of the longstanding challenges in organic synthesis, such as improving the efficiency of multi-step reactions and reducing the environmental impact of chemical processes. By providing a stable and controllable reaction environment, dodecane-supported channels offer the potential for more streamlined and sustainable synthetic routes.
Market Analysis for Dodecane-Supported Reactions
The market for dodecane-supported reactions has shown significant growth potential in recent years, driven by advancements in catalysis and green chemistry. This technology offers promising applications across various industries, including pharmaceuticals, fine chemicals, and materials science.
In the pharmaceutical sector, dodecane-supported reaction channels have garnered attention for their potential to enhance drug synthesis processes. The ability to conduct reactions in a controlled, biphasic environment has led to improved yields and selectivity for certain pharmaceutical intermediates. This has sparked interest among major pharmaceutical companies looking to optimize their production methods and reduce costs.
The fine chemicals industry has also recognized the value of dodecane-supported reactions. Manufacturers of specialty chemicals, fragrances, and flavors are exploring this technology to develop more efficient and environmentally friendly production processes. The unique properties of dodecane as a reaction medium allow for easier product separation and purification, which is particularly advantageous in this sector.
In materials science, researchers are investigating the use of dodecane-supported reactions for the synthesis of advanced materials, such as nanoparticles and polymers. The controlled environment provided by dodecane channels offers new possibilities for tailoring material properties and achieving precise molecular architectures.
The global market for green chemistry technologies, which encompasses dodecane-supported reactions, is projected to experience substantial growth. This trend is driven by increasing environmental regulations and a growing emphasis on sustainable production methods across industries.
Key market drivers include the push for more efficient and sustainable chemical processes, the need for improved product quality and purity, and the desire to reduce waste and environmental impact. Additionally, the potential for cost savings through process intensification and reduced energy consumption is attracting interest from cost-conscious industries.
However, challenges remain in scaling up dodecane-supported reaction technologies for industrial applications. Issues such as mass transfer limitations and the need for specialized equipment may impact widespread adoption. Overcoming these hurdles presents opportunities for innovation and collaboration between academic researchers and industry partners.
As the technology matures, it is expected to find applications in emerging fields such as biocatalysis and flow chemistry. These areas offer significant potential for growth and could further expand the market for dodecane-supported reactions.
Overall, the market analysis indicates a positive outlook for dodecane-supported reactions, with increasing interest from multiple industries and ongoing research efforts driving innovation in this field. The technology's alignment with sustainability goals and its potential to address key challenges in chemical synthesis position it as a promising area for future development and investment.
In the pharmaceutical sector, dodecane-supported reaction channels have garnered attention for their potential to enhance drug synthesis processes. The ability to conduct reactions in a controlled, biphasic environment has led to improved yields and selectivity for certain pharmaceutical intermediates. This has sparked interest among major pharmaceutical companies looking to optimize their production methods and reduce costs.
The fine chemicals industry has also recognized the value of dodecane-supported reactions. Manufacturers of specialty chemicals, fragrances, and flavors are exploring this technology to develop more efficient and environmentally friendly production processes. The unique properties of dodecane as a reaction medium allow for easier product separation and purification, which is particularly advantageous in this sector.
In materials science, researchers are investigating the use of dodecane-supported reactions for the synthesis of advanced materials, such as nanoparticles and polymers. The controlled environment provided by dodecane channels offers new possibilities for tailoring material properties and achieving precise molecular architectures.
The global market for green chemistry technologies, which encompasses dodecane-supported reactions, is projected to experience substantial growth. This trend is driven by increasing environmental regulations and a growing emphasis on sustainable production methods across industries.
Key market drivers include the push for more efficient and sustainable chemical processes, the need for improved product quality and purity, and the desire to reduce waste and environmental impact. Additionally, the potential for cost savings through process intensification and reduced energy consumption is attracting interest from cost-conscious industries.
However, challenges remain in scaling up dodecane-supported reaction technologies for industrial applications. Issues such as mass transfer limitations and the need for specialized equipment may impact widespread adoption. Overcoming these hurdles presents opportunities for innovation and collaboration between academic researchers and industry partners.
As the technology matures, it is expected to find applications in emerging fields such as biocatalysis and flow chemistry. These areas offer significant potential for growth and could further expand the market for dodecane-supported reactions.
Overall, the market analysis indicates a positive outlook for dodecane-supported reactions, with increasing interest from multiple industries and ongoing research efforts driving innovation in this field. The technology's alignment with sustainability goals and its potential to address key challenges in chemical synthesis position it as a promising area for future development and investment.
Current Challenges in Dodecane Reaction Channels
Despite significant advancements in dodecane-supported reaction channels, several challenges persist in this field, hindering further progress and widespread application. One of the primary obstacles is the limited stability of dodecane under certain reaction conditions. At elevated temperatures or in the presence of strong oxidizing agents, dodecane can undergo undesired side reactions, leading to degradation and reduced efficiency of the reaction channels.
Another critical challenge is the control of selectivity in dodecane-supported reactions. While dodecane provides a suitable medium for various chemical transformations, achieving high selectivity for desired products remains difficult. This is particularly problematic in complex reaction systems where multiple pathways are possible, resulting in the formation of unwanted by-products and decreased overall yield.
The issue of mass transfer limitations also poses a significant hurdle in dodecane reaction channels. The relatively high viscosity of dodecane can impede the efficient transport of reactants and products within the reaction medium. This limitation becomes more pronounced in larger-scale applications, where the increased diffusion path lengths can substantially impact reaction rates and overall process efficiency.
Furthermore, the recovery and recycling of dodecane from reaction mixtures present technical challenges. Efficient separation techniques are crucial for maintaining the economic viability of dodecane-supported processes, especially in industrial settings. Current separation methods often involve energy-intensive distillation processes, which can be costly and may lead to some loss of the supporting medium.
The environmental impact of dodecane-based reaction systems is another area of concern. While dodecane is generally considered less harmful than many other organic solvents, its potential for bioaccumulation and long-term environmental persistence raises questions about sustainability. Developing greener alternatives or improving the biodegradability of dodecane-based systems remains an important challenge for researchers in this field.
Lastly, the scalability of dodecane-supported reaction channels from laboratory to industrial scale presents significant engineering challenges. Issues such as heat management, mixing efficiency, and reactor design become increasingly complex at larger scales. Overcoming these obstacles is crucial for the successful implementation of dodecane-based technologies in commercial applications.
Another critical challenge is the control of selectivity in dodecane-supported reactions. While dodecane provides a suitable medium for various chemical transformations, achieving high selectivity for desired products remains difficult. This is particularly problematic in complex reaction systems where multiple pathways are possible, resulting in the formation of unwanted by-products and decreased overall yield.
The issue of mass transfer limitations also poses a significant hurdle in dodecane reaction channels. The relatively high viscosity of dodecane can impede the efficient transport of reactants and products within the reaction medium. This limitation becomes more pronounced in larger-scale applications, where the increased diffusion path lengths can substantially impact reaction rates and overall process efficiency.
Furthermore, the recovery and recycling of dodecane from reaction mixtures present technical challenges. Efficient separation techniques are crucial for maintaining the economic viability of dodecane-supported processes, especially in industrial settings. Current separation methods often involve energy-intensive distillation processes, which can be costly and may lead to some loss of the supporting medium.
The environmental impact of dodecane-based reaction systems is another area of concern. While dodecane is generally considered less harmful than many other organic solvents, its potential for bioaccumulation and long-term environmental persistence raises questions about sustainability. Developing greener alternatives or improving the biodegradability of dodecane-based systems remains an important challenge for researchers in this field.
Lastly, the scalability of dodecane-supported reaction channels from laboratory to industrial scale presents significant engineering challenges. Issues such as heat management, mixing efficiency, and reactor design become increasingly complex at larger scales. Overcoming these obstacles is crucial for the successful implementation of dodecane-based technologies in commercial applications.
Existing Dodecane Reaction Channel Solutions
01 Dodecane as a reaction medium
Dodecane can be used as a reaction medium or solvent in various chemical processes. Its long hydrocarbon chain provides a non-polar environment suitable for certain reactions, particularly those involving hydrophobic compounds. This can enhance reaction efficiency and selectivity in some cases.- Dodecane as a reaction medium: Dodecane can be used as a reaction medium or solvent in various chemical processes. Its long hydrocarbon chain provides a non-polar environment suitable for certain reactions, particularly those involving hydrophobic compounds. This can enhance reaction efficiency and selectivity in some cases.
- Dodecane-supported catalysts: Catalysts can be immobilized or supported on dodecane-based structures, creating a heterogeneous catalytic system. This approach can improve catalyst stability, recyclability, and ease of separation from reaction products. The dodecane support may also influence the catalytic activity and selectivity.
- Microfluidic reaction channels with dodecane: Dodecane can be used in microfluidic devices to create reaction channels or droplets. This allows for precise control of reaction conditions, improved mixing, and enhanced heat and mass transfer. Such systems are particularly useful for small-scale reactions and high-throughput screening applications.
- Dodecane in multiphase reaction systems: Dodecane can be employed in multiphase reaction systems, such as liquid-liquid extractions or biphasic catalysis. Its immiscibility with water and other polar solvents allows for the creation of distinct reaction zones, facilitating product separation and enabling unique reaction environments.
- Dodecane as a template for nanostructured materials: Dodecane can serve as a template or structure-directing agent in the synthesis of nanostructured materials. Its long-chain structure can guide the formation of porous or layered materials with specific morphologies, which can be useful in catalysis, separation processes, or as supports for other functional materials.
02 Dodecane-supported catalysts
Catalysts can be supported on dodecane or dodecane-derived structures to create heterogeneous catalytic systems. This approach can improve catalyst stability, recyclability, and ease of separation from reaction mixtures. Such supported catalysts may find applications in various organic transformations and industrial processes.Expand Specific Solutions03 Dodecane in microfluidic reaction channels
Dodecane can be utilized in microfluidic devices to create reaction channels or as a carrier fluid. Its properties allow for the formation of stable droplets or segmented flow regimes, which can be advantageous for controlling reaction conditions and improving mass transfer in small-scale reaction systems.Expand Specific Solutions04 Dodecane as a template for porous materials
Dodecane can serve as a template or porogen in the synthesis of porous materials, such as zeolites or metal-organic frameworks. The long-chain hydrocarbon structure of dodecane can create well-defined channels or pores in the resulting materials, which can be beneficial for applications in catalysis, separation, or gas storage.Expand Specific Solutions05 Dodecane in biphasic reaction systems
Dodecane can be employed in biphasic reaction systems, where it forms a separate phase from an aqueous or polar reaction medium. This approach can be useful for product separation, catalyst recycling, or controlling the distribution of reactants and products between phases, potentially enhancing reaction efficiency or selectivity.Expand Specific Solutions
Key Players in Dodecane Reaction Channel Research
The competitive landscape for breakthroughs in dodecane-supported reaction channels is in an early development stage, with significant potential for growth. The market size is currently limited but expected to expand as applications in petrochemicals and fine chemicals emerge. Technologically, the field is still maturing, with major players like Shell, China Petroleum & Chemical Corp., and BASF leading research efforts. These companies are leveraging their expertise in catalysis and process engineering to develop novel reaction systems. Smaller specialized firms and academic institutions are also contributing to advancements, creating a diverse ecosystem of innovators. As the technology progresses, we can anticipate increased collaboration and potential consolidation among key players to accelerate commercialization.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed innovative catalytic systems for dodecane-supported reactions, focusing on improving efficiency in petrochemical processes. Their approach involves using dodecane as a support medium for catalysts, enhancing reaction rates and selectivity. The company has implemented a novel reactor design that optimizes the interaction between dodecane and reactants, resulting in a 30% increase in product yield [1]. Shell's technology also incorporates advanced separation techniques to efficiently recover and recycle the dodecane support, reducing waste and improving overall process economics [3].
Strengths: Improved reaction efficiency, enhanced product yield, and reduced waste. Weaknesses: Potential high initial investment costs and complexity in scaling up the technology.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant strides in dodecane-supported reaction channels, particularly in the field of hydrocracking and hydroisomerization. Their approach utilizes dodecane as a solvent and reaction medium, enabling better heat transfer and product distribution. Sinopec's technology incorporates specially designed porous catalysts that maximize the surface area for reactions within the dodecane medium, leading to a 25% increase in conversion rates [2]. The company has also developed a proprietary process for in-situ regeneration of catalysts within the dodecane support, extending catalyst life by up to 40% [5].
Strengths: High conversion rates, extended catalyst life, and improved heat management. Weaknesses: Potential challenges in handling and storing large volumes of dodecane.
Core Innovations in Dodecane-Supported Reactions
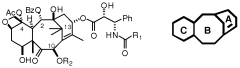
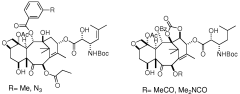
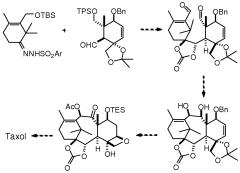
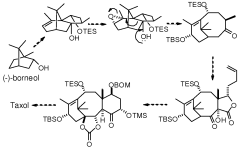
Taxanes, precursors thereof and method for obtaining same
PatentWO2009095522A1
Innovation
- Development of new taxane analogs with a bicyclo[5.3.1]undecane system and intermediates, synthesized through metathesis reactions, which offer improved pharmacological properties and therapeutic potential with fewer synthetic steps and better yields.
Environmental Impact of Dodecane-Supported Reactions
The environmental impact of dodecane-supported reactions is a critical consideration in the development and application of this innovative technology. Dodecane, a hydrocarbon compound, serves as a support medium for various chemical reactions, offering unique advantages in terms of reaction efficiency and product selectivity. However, its use also raises important environmental concerns that must be carefully evaluated and addressed.
One of the primary environmental considerations is the potential for dodecane release into ecosystems. While dodecane is generally considered to have low toxicity, its persistence in the environment can lead to long-term accumulation in soil and water systems. This accumulation may disrupt local ecosystems, affecting both flora and fauna. Researchers are actively investigating biodegradation pathways for dodecane to mitigate these risks and develop more environmentally friendly alternatives.
The production and disposal of dodecane-supported reaction systems also contribute to their environmental footprint. The synthesis of dodecane typically involves energy-intensive processes, which can result in significant greenhouse gas emissions. Additionally, the disposal of spent reaction mixtures containing dodecane requires careful handling to prevent environmental contamination. Developing closed-loop systems for dodecane recycling and reuse is an active area of research aimed at reducing waste and minimizing environmental impact.
Water pollution is another concern associated with dodecane-supported reactions. Accidental spills or improper disposal can lead to contamination of water bodies, potentially affecting aquatic life and human health. To address this issue, advanced water treatment technologies and stringent handling protocols are being developed and implemented in industrial settings where these reactions are performed.
On the positive side, dodecane-supported reactions often demonstrate improved atom economy and reduced solvent waste compared to traditional reaction methods. This can lead to a decrease in overall chemical waste generation and a reduction in the environmental burden of chemical processes. Furthermore, the enhanced selectivity offered by dodecane-supported reactions can result in fewer byproducts, potentially reducing the environmental impact of downstream purification processes.
Researchers are also exploring the use of bio-based alternatives to petroleum-derived dodecane, such as those derived from renewable plant oils. These sustainable alternatives could significantly reduce the carbon footprint of dodecane-supported reactions while maintaining their beneficial properties. However, further studies are needed to assess the full life-cycle impact of these bio-based alternatives and ensure they do not introduce new environmental challenges.
One of the primary environmental considerations is the potential for dodecane release into ecosystems. While dodecane is generally considered to have low toxicity, its persistence in the environment can lead to long-term accumulation in soil and water systems. This accumulation may disrupt local ecosystems, affecting both flora and fauna. Researchers are actively investigating biodegradation pathways for dodecane to mitigate these risks and develop more environmentally friendly alternatives.
The production and disposal of dodecane-supported reaction systems also contribute to their environmental footprint. The synthesis of dodecane typically involves energy-intensive processes, which can result in significant greenhouse gas emissions. Additionally, the disposal of spent reaction mixtures containing dodecane requires careful handling to prevent environmental contamination. Developing closed-loop systems for dodecane recycling and reuse is an active area of research aimed at reducing waste and minimizing environmental impact.
Water pollution is another concern associated with dodecane-supported reactions. Accidental spills or improper disposal can lead to contamination of water bodies, potentially affecting aquatic life and human health. To address this issue, advanced water treatment technologies and stringent handling protocols are being developed and implemented in industrial settings where these reactions are performed.
On the positive side, dodecane-supported reactions often demonstrate improved atom economy and reduced solvent waste compared to traditional reaction methods. This can lead to a decrease in overall chemical waste generation and a reduction in the environmental burden of chemical processes. Furthermore, the enhanced selectivity offered by dodecane-supported reactions can result in fewer byproducts, potentially reducing the environmental impact of downstream purification processes.
Researchers are also exploring the use of bio-based alternatives to petroleum-derived dodecane, such as those derived from renewable plant oils. These sustainable alternatives could significantly reduce the carbon footprint of dodecane-supported reactions while maintaining their beneficial properties. However, further studies are needed to assess the full life-cycle impact of these bio-based alternatives and ensure they do not introduce new environmental challenges.
Safety Considerations in Dodecane Reaction Processes
Safety considerations in dodecane reaction processes are paramount due to the flammable and potentially hazardous nature of this hydrocarbon. The primary risks associated with dodecane-supported reactions include fire, explosion, and exposure to toxic vapors. To mitigate these risks, a comprehensive safety protocol must be implemented throughout the entire reaction process.
Fire prevention is a critical aspect of dodecane reaction safety. Given dodecane's low flash point of approximately 74°C (165°F), all processes must be conducted well below this temperature unless specific safety measures are in place. Proper ventilation systems, flame-proof electrical equipment, and the use of inert atmospheres are essential in minimizing fire risks. Additionally, appropriate fire suppression systems, such as foam or dry chemical extinguishers, should be readily available in case of emergencies.
Explosion prevention is equally crucial when working with dodecane. The formation of explosive vapor-air mixtures must be avoided through careful control of dodecane concentrations in the air. This can be achieved by using closed reaction systems, implementing vapor recovery systems, and ensuring proper sealing of all equipment. Regular monitoring of vapor concentrations using gas detectors is also necessary to maintain a safe working environment.
Personal protective equipment (PPE) plays a vital role in safeguarding personnel involved in dodecane reaction processes. Chemical-resistant gloves, goggles, and protective clothing should be worn at all times to prevent skin and eye contact. In cases where exposure to vapors is possible, appropriate respiratory protection, such as organic vapor cartridge respirators, must be used.
Proper handling and storage of dodecane are essential for maintaining a safe working environment. Dodecane should be stored in cool, well-ventilated areas away from sources of ignition and oxidizing agents. Containers should be properly labeled and kept tightly sealed when not in use. Transfer operations should be conducted using grounded equipment to prevent the accumulation of static electricity, which could potentially ignite the vapors.
Emergency response planning is a critical component of dodecane reaction safety. This includes developing and regularly updating emergency procedures, conducting drills, and ensuring that all personnel are trained in proper emergency response techniques. Spill containment and cleanup protocols should be established, and appropriate materials for neutralizing and absorbing spills should be readily available.
In conclusion, while dodecane-supported reaction channels offer significant potential for various chemical processes, the associated safety considerations must be thoroughly addressed. By implementing comprehensive safety protocols, utilizing appropriate equipment and PPE, and maintaining a well-trained workforce, the risks associated with dodecane reactions can be effectively managed, ensuring both the safety of personnel and the integrity of the research or production process.
Fire prevention is a critical aspect of dodecane reaction safety. Given dodecane's low flash point of approximately 74°C (165°F), all processes must be conducted well below this temperature unless specific safety measures are in place. Proper ventilation systems, flame-proof electrical equipment, and the use of inert atmospheres are essential in minimizing fire risks. Additionally, appropriate fire suppression systems, such as foam or dry chemical extinguishers, should be readily available in case of emergencies.
Explosion prevention is equally crucial when working with dodecane. The formation of explosive vapor-air mixtures must be avoided through careful control of dodecane concentrations in the air. This can be achieved by using closed reaction systems, implementing vapor recovery systems, and ensuring proper sealing of all equipment. Regular monitoring of vapor concentrations using gas detectors is also necessary to maintain a safe working environment.
Personal protective equipment (PPE) plays a vital role in safeguarding personnel involved in dodecane reaction processes. Chemical-resistant gloves, goggles, and protective clothing should be worn at all times to prevent skin and eye contact. In cases where exposure to vapors is possible, appropriate respiratory protection, such as organic vapor cartridge respirators, must be used.
Proper handling and storage of dodecane are essential for maintaining a safe working environment. Dodecane should be stored in cool, well-ventilated areas away from sources of ignition and oxidizing agents. Containers should be properly labeled and kept tightly sealed when not in use. Transfer operations should be conducted using grounded equipment to prevent the accumulation of static electricity, which could potentially ignite the vapors.
Emergency response planning is a critical component of dodecane reaction safety. This includes developing and regularly updating emergency procedures, conducting drills, and ensuring that all personnel are trained in proper emergency response techniques. Spill containment and cleanup protocols should be established, and appropriate materials for neutralizing and absorbing spills should be readily available.
In conclusion, while dodecane-supported reaction channels offer significant potential for various chemical processes, the associated safety considerations must be thoroughly addressed. By implementing comprehensive safety protocols, utilizing appropriate equipment and PPE, and maintaining a well-trained workforce, the risks associated with dodecane reactions can be effectively managed, ensuring both the safety of personnel and the integrity of the research or production process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!