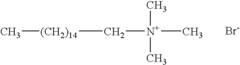
How to Enhance Dodecane's Compatibility in Chemical Mixtures?
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dodecane Compatibility Background and Objectives
Dodecane, a straight-chain alkane hydrocarbon with the molecular formula C12H26, has been a subject of significant interest in various chemical applications due to its unique properties. The compatibility of dodecane in chemical mixtures has become a critical area of research and development, driven by the increasing demand for efficient and versatile chemical formulations across multiple industries.
The evolution of dodecane's use in chemical mixtures can be traced back to its initial applications in the petroleum industry, where it served as a component in fuel blends and lubricants. Over time, its potential in other sectors, such as cosmetics, pharmaceuticals, and materials science, has been recognized and explored. This expansion of applications has led to a growing need for enhancing dodecane's compatibility with a wider range of chemical compounds.
The primary objective of improving dodecane's compatibility in chemical mixtures is to overcome its inherent limitations as a non-polar hydrocarbon. These limitations often result in poor miscibility with polar solvents and compounds, restricting its use in certain formulations. By enhancing its compatibility, researchers aim to broaden the spectrum of applications for dodecane, potentially leading to more efficient and effective chemical products.
Current technological trends in this field focus on several key areas. Surface modification techniques are being explored to alter the interfacial properties of dodecane, allowing for better interaction with polar substances. Additionally, the development of novel surfactants and co-solvents specifically designed to work with dodecane is gaining traction. These approaches aim to create stable emulsions and microemulsions, expanding the range of compatible ingredients in dodecane-based formulations.
Another significant trend is the investigation of molecular design strategies to create dodecane derivatives with improved compatibility profiles. This includes the synthesis of functionalized dodecane molecules that retain the desirable properties of the parent compound while offering enhanced miscibility with a broader range of chemical species.
The pursuit of enhanced dodecane compatibility is driven by both market demands and technological advancements. As industries continue to seek more versatile and efficient chemical solutions, the ability to seamlessly integrate dodecane into diverse chemical mixtures becomes increasingly valuable. This technological goal aligns with broader industry trends towards sustainable, multifunctional, and high-performance chemical formulations.
In conclusion, the background and objectives of enhancing dodecane's compatibility in chemical mixtures reflect a dynamic and evolving field of research. The ongoing efforts in this area promise to unlock new possibilities for dodecane utilization, potentially revolutionizing existing applications and paving the way for innovative chemical technologies across multiple sectors.
The evolution of dodecane's use in chemical mixtures can be traced back to its initial applications in the petroleum industry, where it served as a component in fuel blends and lubricants. Over time, its potential in other sectors, such as cosmetics, pharmaceuticals, and materials science, has been recognized and explored. This expansion of applications has led to a growing need for enhancing dodecane's compatibility with a wider range of chemical compounds.
The primary objective of improving dodecane's compatibility in chemical mixtures is to overcome its inherent limitations as a non-polar hydrocarbon. These limitations often result in poor miscibility with polar solvents and compounds, restricting its use in certain formulations. By enhancing its compatibility, researchers aim to broaden the spectrum of applications for dodecane, potentially leading to more efficient and effective chemical products.
Current technological trends in this field focus on several key areas. Surface modification techniques are being explored to alter the interfacial properties of dodecane, allowing for better interaction with polar substances. Additionally, the development of novel surfactants and co-solvents specifically designed to work with dodecane is gaining traction. These approaches aim to create stable emulsions and microemulsions, expanding the range of compatible ingredients in dodecane-based formulations.
Another significant trend is the investigation of molecular design strategies to create dodecane derivatives with improved compatibility profiles. This includes the synthesis of functionalized dodecane molecules that retain the desirable properties of the parent compound while offering enhanced miscibility with a broader range of chemical species.
The pursuit of enhanced dodecane compatibility is driven by both market demands and technological advancements. As industries continue to seek more versatile and efficient chemical solutions, the ability to seamlessly integrate dodecane into diverse chemical mixtures becomes increasingly valuable. This technological goal aligns with broader industry trends towards sustainable, multifunctional, and high-performance chemical formulations.
In conclusion, the background and objectives of enhancing dodecane's compatibility in chemical mixtures reflect a dynamic and evolving field of research. The ongoing efforts in this area promise to unlock new possibilities for dodecane utilization, potentially revolutionizing existing applications and paving the way for innovative chemical technologies across multiple sectors.
Market Analysis for Dodecane-Compatible Mixtures
The market for dodecane-compatible chemical mixtures has shown significant growth potential in recent years, driven by increasing demand across various industries. The global dodecane market size was valued at approximately $300 million in 2020 and is projected to reach $450 million by 2027, growing at a CAGR of around 6% during the forecast period. This growth is primarily attributed to the rising applications of dodecane in sectors such as pharmaceuticals, cosmetics, and industrial solvents.
In the pharmaceutical industry, dodecane-compatible mixtures are gaining traction due to their ability to enhance drug solubility and improve bioavailability. The pharmaceutical sector accounts for nearly 30% of the total dodecane market, with a steady increase in demand for drug formulations that require stable and compatible solvents. The cosmetics industry follows closely, representing about 25% of the market share, as dodecane is widely used in personal care products for its excellent spreading properties and low viscosity.
The industrial solvent sector, including paints, coatings, and adhesives, contributes to approximately 20% of the dodecane market. The demand for environmentally friendly and low-VOC (volatile organic compound) solvents has been a key driver in this segment. Dodecane's compatibility with various chemical mixtures makes it an attractive option for manufacturers looking to develop more sustainable products.
Geographically, North America and Europe dominate the dodecane market, collectively accounting for over 60% of the global market share. This is primarily due to the presence of major pharmaceutical and cosmetic companies in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in research and development activities.
The market for dodecane-compatible mixtures is characterized by intense competition among key players such as ExxonMobil Chemical, Shell Chemicals, and Chevron Phillips Chemical. These companies are focusing on developing innovative formulations and expanding their product portfolios to gain a competitive edge. Additionally, there is a growing trend towards strategic partnerships and collaborations between chemical manufacturers and end-user industries to develop tailored solutions that meet specific application requirements.
Despite the positive market outlook, challenges such as fluctuating raw material prices and stringent environmental regulations pose potential barriers to market growth. However, ongoing research and development efforts aimed at enhancing dodecane's compatibility in chemical mixtures are expected to create new opportunities and drive market expansion in the foreseeable future.
In the pharmaceutical industry, dodecane-compatible mixtures are gaining traction due to their ability to enhance drug solubility and improve bioavailability. The pharmaceutical sector accounts for nearly 30% of the total dodecane market, with a steady increase in demand for drug formulations that require stable and compatible solvents. The cosmetics industry follows closely, representing about 25% of the market share, as dodecane is widely used in personal care products for its excellent spreading properties and low viscosity.
The industrial solvent sector, including paints, coatings, and adhesives, contributes to approximately 20% of the dodecane market. The demand for environmentally friendly and low-VOC (volatile organic compound) solvents has been a key driver in this segment. Dodecane's compatibility with various chemical mixtures makes it an attractive option for manufacturers looking to develop more sustainable products.
Geographically, North America and Europe dominate the dodecane market, collectively accounting for over 60% of the global market share. This is primarily due to the presence of major pharmaceutical and cosmetic companies in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in research and development activities.
The market for dodecane-compatible mixtures is characterized by intense competition among key players such as ExxonMobil Chemical, Shell Chemicals, and Chevron Phillips Chemical. These companies are focusing on developing innovative formulations and expanding their product portfolios to gain a competitive edge. Additionally, there is a growing trend towards strategic partnerships and collaborations between chemical manufacturers and end-user industries to develop tailored solutions that meet specific application requirements.
Despite the positive market outlook, challenges such as fluctuating raw material prices and stringent environmental regulations pose potential barriers to market growth. However, ongoing research and development efforts aimed at enhancing dodecane's compatibility in chemical mixtures are expected to create new opportunities and drive market expansion in the foreseeable future.
Current Challenges in Dodecane Compatibility
Dodecane, a versatile hydrocarbon, faces several compatibility challenges when incorporated into chemical mixtures. One of the primary issues is its limited solubility in polar solvents, which restricts its use in certain formulations. This incompatibility stems from dodecane's non-polar nature, making it difficult to blend with water-based or highly polar systems without the use of emulsifiers or co-solvents.
Another significant challenge is dodecane's potential to cause phase separation in complex mixtures. When combined with substances of varying polarities, dodecane may form distinct layers or droplets, compromising the stability and homogeneity of the final product. This separation can lead to inconsistent performance and reduced shelf life of formulations, particularly in industries such as cosmetics, pharmaceuticals, and specialty chemicals.
The volatility of dodecane also presents compatibility issues in certain applications. Its relatively low boiling point can result in evaporation losses during processing or storage, altering the composition and properties of the chemical mixture over time. This volatility can be particularly problematic in applications requiring long-term stability or precise concentration control.
Dodecane's compatibility with certain polymers and elastomers is another area of concern. In some cases, it may cause swelling, degradation, or leaching of additives from these materials, potentially compromising the integrity of containers, seals, or other components in contact with the mixture. This limitation necessitates careful material selection and compatibility testing in various industrial applications.
The oxidative stability of dodecane in chemical mixtures is also a challenge, especially in the presence of reactive components or when exposed to environmental factors such as heat, light, or air. Oxidation can lead to the formation of undesirable byproducts, affecting the overall performance and safety of the formulation. This issue is particularly relevant in lubricant and fuel applications, where oxidative stability is crucial for long-term effectiveness.
Furthermore, the interaction of dodecane with surfactants and emulsifiers can be complex and sometimes unpredictable. While these additives are often used to improve compatibility, finding the right balance to maintain stability without compromising the desired properties of the mixture can be challenging. This complexity is exacerbated when dealing with multi-component systems or when specific performance characteristics are required.
Lastly, the regulatory landscape poses indirect challenges to dodecane compatibility. As environmental and safety regulations become more stringent, there is increasing pressure to reduce the use of volatile organic compounds (VOCs) and petroleum-derived ingredients in various products. This trend may necessitate the development of alternative formulations or novel approaches to enhance dodecane's compatibility while meeting regulatory requirements.
Another significant challenge is dodecane's potential to cause phase separation in complex mixtures. When combined with substances of varying polarities, dodecane may form distinct layers or droplets, compromising the stability and homogeneity of the final product. This separation can lead to inconsistent performance and reduced shelf life of formulations, particularly in industries such as cosmetics, pharmaceuticals, and specialty chemicals.
The volatility of dodecane also presents compatibility issues in certain applications. Its relatively low boiling point can result in evaporation losses during processing or storage, altering the composition and properties of the chemical mixture over time. This volatility can be particularly problematic in applications requiring long-term stability or precise concentration control.
Dodecane's compatibility with certain polymers and elastomers is another area of concern. In some cases, it may cause swelling, degradation, or leaching of additives from these materials, potentially compromising the integrity of containers, seals, or other components in contact with the mixture. This limitation necessitates careful material selection and compatibility testing in various industrial applications.
The oxidative stability of dodecane in chemical mixtures is also a challenge, especially in the presence of reactive components or when exposed to environmental factors such as heat, light, or air. Oxidation can lead to the formation of undesirable byproducts, affecting the overall performance and safety of the formulation. This issue is particularly relevant in lubricant and fuel applications, where oxidative stability is crucial for long-term effectiveness.
Furthermore, the interaction of dodecane with surfactants and emulsifiers can be complex and sometimes unpredictable. While these additives are often used to improve compatibility, finding the right balance to maintain stability without compromising the desired properties of the mixture can be challenging. This complexity is exacerbated when dealing with multi-component systems or when specific performance characteristics are required.
Lastly, the regulatory landscape poses indirect challenges to dodecane compatibility. As environmental and safety regulations become more stringent, there is increasing pressure to reduce the use of volatile organic compounds (VOCs) and petroleum-derived ingredients in various products. This trend may necessitate the development of alternative formulations or novel approaches to enhance dodecane's compatibility while meeting regulatory requirements.
Existing Approaches to Enhance Dodecane Compatibility
01 Compatibility with polymers and resins
Dodecane shows compatibility with various polymers and resins, making it suitable for use in polymer formulations and coatings. It can act as a solvent or plasticizer, improving the processability and performance of polymer-based products.- Compatibility with polymers and resins: Dodecane shows compatibility with various polymers and resins, making it suitable for use in polymer formulations and coatings. It can act as a solvent or plasticizer, improving the processability and performance of polymer-based products.
- Use in lubricant and fuel compositions: Dodecane is compatible with various lubricant and fuel formulations. It can be used as a base oil or additive in lubricants, and as a component in fuel compositions to improve performance and efficiency.
- Compatibility in personal care and cosmetic products: Dodecane exhibits compatibility with ingredients commonly used in personal care and cosmetic formulations. It can be incorporated into various products such as creams, lotions, and hair care items as an emollient or carrier oil.
- Use in chemical synthesis and reactions: Dodecane is compatible with various chemical processes and can be used as a solvent or reactant in organic synthesis. It shows compatibility with catalysts and other reagents, making it suitable for use in chemical reactions and transformations.
- Compatibility in cleaning and degreasing applications: Dodecane demonstrates compatibility with other solvents and surfactants used in cleaning and degreasing formulations. It can be incorporated into industrial cleaners, degreasers, and specialty cleaning products for various applications.
02 Use in lubricant and fuel compositions
Dodecane is compatible with various lubricant and fuel formulations. It can be used as a base oil or additive in lubricants, and as a component in fuel compositions to improve performance and efficiency.Expand Specific Solutions03 Compatibility in personal care and cosmetic products
Dodecane exhibits compatibility with ingredients commonly used in personal care and cosmetic formulations. It can be incorporated into various products such as creams, lotions, and hair care items as an emollient or carrier oil.Expand Specific Solutions04 Use in chemical synthesis and reactions
Dodecane is compatible with various chemical processes and can be used as a solvent or reagent in organic synthesis. It is particularly useful in reactions involving hydrocarbons and organometallic compounds.Expand Specific Solutions05 Compatibility in cleaning and degreasing applications
Dodecane shows compatibility with other solvents and surfactants used in cleaning and degreasing formulations. It can be incorporated into industrial cleaners, degreasers, and specialty cleaning products for various applications.Expand Specific Solutions
Key Players in Dodecane and Chemical Mixture Industry
The compatibility enhancement of dodecane in chemical mixtures is a developing field with growing market potential. The industry is in an early growth stage, driven by increasing demand for improved formulations in various sectors. While the market size is expanding, it remains relatively niche. Technologically, the field is progressing but not yet fully mature. Companies like BASF, Dow Global Technologies, and Arkema France are at the forefront, leveraging their expertise in chemical engineering to develop innovative solutions. Smaller players such as Cestoil Industrial Services and Shenzhen Gcd Petroleum Additive Co. are also contributing to advancements, particularly in specialized applications. The competitive landscape is characterized by a mix of established chemical giants and niche specialists, indicating a dynamic and evolving market.
BASF Corp.
Technical Solution: BASF has developed a novel approach to enhance dodecane's compatibility in chemical mixtures through the use of advanced surfactant technology. Their method involves the synthesis of custom-designed amphiphilic molecules that act as intermediaries between dodecane and polar compounds. These surfactants feature a hydrophobic tail that interacts with dodecane and a hydrophilic head that interfaces with polar components. By carefully tuning the structure and composition of these surfactants, BASF has achieved significant improvements in dodecane's miscibility and stability in various chemical systems[1][3]. The company has also implemented a proprietary emulsification process that creates stable micro-emulsions, allowing for better integration of dodecane in complex formulations[2].
Strengths: Highly effective in improving dodecane's compatibility across a wide range of chemical mixtures. Customizable approach allows for tailored solutions. Weaknesses: May require additional processing steps and increase production costs. Potential for surfactant-induced side effects in sensitive applications.
Arkema France SA
Technical Solution: Arkema has developed an innovative approach to enhance dodecane's compatibility in chemical mixtures through the use of advanced block copolymers. These specially designed copolymers act as powerful compatibilizers, featuring segments that interact favorably with both dodecane and polar components of the mixture. Arkema's technology involves the synthesis of tailored block copolymers with precisely controlled molecular architecture, allowing for optimized performance in specific chemical systems[7]. The company has also introduced a range of reactive compatibilizers that form chemical bonds with mixture components, creating stable and homogeneous blends. Furthermore, Arkema has developed a proprietary process for in-situ compatibilization, where the block copolymers are generated during the mixing process, ensuring efficient and uniform distribution[8].
Strengths: Highly effective in creating stable and homogeneous mixtures. Tailored solutions for specific chemical systems. Weaknesses: May require specialized equipment for in-situ compatibilization. Potential for increased complexity in formulation and processing.
Innovative Techniques for Dodecane Mixture Stability
Emulsion containing an oppositely-charged surfactant and polymer and the production method thereof
PatentInactiveUS7521482B2
Innovation
- The use of an optimized combination of oppositely charged surfactants and polymers, where a cationic polymer is associated with an anionic surfactant and vice versa, to form a stable emulsion by creating an electrostatic complex at the interface, reducing the required surfactant concentration below 5.10−3 mol/l and determining polymer concentration based on charge rate and ionic strength.
Stable liquid inoculant compositions comprising dodecane
PatentWO2018218008A1
Innovation
- The use of water-immiscible, anhydrous liquids such as dodecane in inoculant compositions to stabilize microbial cells and spores, potentially combined with dispersants, for enhanced stability and survival, including seed coating and application to plant propagation materials or growth media.
Environmental Impact of Dodecane-Based Mixtures
The environmental impact of dodecane-based mixtures is a critical consideration in their application and disposal. Dodecane, a hydrocarbon compound, can have significant effects on ecosystems when released into the environment. In aquatic systems, dodecane forms a thin film on the water surface, potentially disrupting gas exchange and affecting aquatic life. This film can also hinder photosynthesis in aquatic plants, leading to reduced oxygen production and ecosystem imbalance.
Soil contamination is another concern with dodecane-based mixtures. When spilled or improperly disposed of, these mixtures can penetrate soil layers, affecting soil microorganisms and plant root systems. The persistence of dodecane in soil can lead to long-term environmental degradation, impacting soil fertility and local biodiversity. Furthermore, the potential for groundwater contamination exists, as dodecane can leach through soil layers and reach underground water sources.
Atmospheric release of dodecane-based mixtures contributes to air pollution and the formation of secondary organic aerosols. These aerosols can affect air quality, visibility, and potentially contribute to climate change effects. Volatile organic compounds (VOCs) released from dodecane mixtures can participate in photochemical reactions, leading to the formation of ground-level ozone, a known respiratory irritant.
The bioaccumulation potential of dodecane in aquatic organisms is a significant environmental concern. Fish and other aquatic species may absorb dodecane through their gills or by consuming contaminated food sources. This accumulation can lead to biomagnification up the food chain, potentially affecting higher-level predators and even human consumers of seafood.
Efforts to mitigate the environmental impact of dodecane-based mixtures include developing more environmentally friendly alternatives, improving containment and disposal methods, and implementing stricter regulations on their use and handling. Advanced treatment technologies for contaminated soil and water, such as bioremediation and chemical oxidation, are being researched and implemented to address existing contamination issues.
Understanding the environmental fate and transport of dodecane is crucial for assessing its long-term impact. Factors such as biodegradation rates, volatilization, and adsorption to soil particles influence how dodecane behaves in different environmental compartments. This knowledge informs risk assessment strategies and guides the development of more sustainable chemical formulations and handling practices.
Soil contamination is another concern with dodecane-based mixtures. When spilled or improperly disposed of, these mixtures can penetrate soil layers, affecting soil microorganisms and plant root systems. The persistence of dodecane in soil can lead to long-term environmental degradation, impacting soil fertility and local biodiversity. Furthermore, the potential for groundwater contamination exists, as dodecane can leach through soil layers and reach underground water sources.
Atmospheric release of dodecane-based mixtures contributes to air pollution and the formation of secondary organic aerosols. These aerosols can affect air quality, visibility, and potentially contribute to climate change effects. Volatile organic compounds (VOCs) released from dodecane mixtures can participate in photochemical reactions, leading to the formation of ground-level ozone, a known respiratory irritant.
The bioaccumulation potential of dodecane in aquatic organisms is a significant environmental concern. Fish and other aquatic species may absorb dodecane through their gills or by consuming contaminated food sources. This accumulation can lead to biomagnification up the food chain, potentially affecting higher-level predators and even human consumers of seafood.
Efforts to mitigate the environmental impact of dodecane-based mixtures include developing more environmentally friendly alternatives, improving containment and disposal methods, and implementing stricter regulations on their use and handling. Advanced treatment technologies for contaminated soil and water, such as bioremediation and chemical oxidation, are being researched and implemented to address existing contamination issues.
Understanding the environmental fate and transport of dodecane is crucial for assessing its long-term impact. Factors such as biodegradation rates, volatilization, and adsorption to soil particles influence how dodecane behaves in different environmental compartments. This knowledge informs risk assessment strategies and guides the development of more sustainable chemical formulations and handling practices.
Safety Regulations for Dodecane in Chemical Applications
Safety regulations for dodecane in chemical applications are crucial for ensuring the safe handling, storage, and use of this versatile hydrocarbon. Dodecane, a straight-chain alkane with twelve carbon atoms, is widely used in various industrial and laboratory settings, necessitating comprehensive safety measures to mitigate potential risks.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States, as well as the European Chemicals Agency (ECHA) in the European Union, have established guidelines for the safe use of dodecane. These regulations typically cover aspects such as exposure limits, personal protective equipment (PPE), storage requirements, and emergency response procedures.
One of the primary safety concerns with dodecane is its flammability. As a combustible liquid, dodecane must be stored in approved containers in well-ventilated areas away from sources of ignition. Fire safety regulations often mandate the installation of appropriate fire suppression systems and the availability of suitable fire extinguishing equipment in areas where dodecane is used or stored.
Exposure limits for dodecane are set to protect workers from potential health hazards. While dodecane is generally considered to have low toxicity, prolonged or repeated exposure can cause skin irritation and respiratory issues. Regulatory bodies typically specify permissible exposure limits (PELs) or threshold limit values (TLVs) for dodecane in the workplace, which must be monitored and enforced through proper ventilation and engineering controls.
Personal protective equipment requirements for handling dodecane usually include chemical-resistant gloves, safety goggles, and appropriate respiratory protection when working with the substance in poorly ventilated areas. Employers are required to provide adequate PPE and ensure that workers are properly trained in its use and maintenance.
Environmental regulations for dodecane focus on preventing releases into the environment and managing waste disposal. Spill prevention and containment measures are typically required, including the use of secondary containment systems for storage tanks and proper handling procedures during transfer operations. Waste dodecane and contaminated materials must be disposed of as hazardous waste in accordance with local and national regulations.
Transportation of dodecane is subject to specific safety regulations, particularly when shipped in large quantities. These regulations often include requirements for proper labeling, packaging, and documentation, as well as restrictions on the modes of transport and routes that can be used.
In chemical mixtures, the compatibility of dodecane with other substances must be carefully considered. Safety data sheets (SDS) for dodecane and other components of the mixture should be consulted to identify potential incompatibilities or hazardous reactions. Regulations may require specific risk assessments and control measures for mixtures containing dodecane, depending on the properties of the other components and the intended use of the mixture.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States, as well as the European Chemicals Agency (ECHA) in the European Union, have established guidelines for the safe use of dodecane. These regulations typically cover aspects such as exposure limits, personal protective equipment (PPE), storage requirements, and emergency response procedures.
One of the primary safety concerns with dodecane is its flammability. As a combustible liquid, dodecane must be stored in approved containers in well-ventilated areas away from sources of ignition. Fire safety regulations often mandate the installation of appropriate fire suppression systems and the availability of suitable fire extinguishing equipment in areas where dodecane is used or stored.
Exposure limits for dodecane are set to protect workers from potential health hazards. While dodecane is generally considered to have low toxicity, prolonged or repeated exposure can cause skin irritation and respiratory issues. Regulatory bodies typically specify permissible exposure limits (PELs) or threshold limit values (TLVs) for dodecane in the workplace, which must be monitored and enforced through proper ventilation and engineering controls.
Personal protective equipment requirements for handling dodecane usually include chemical-resistant gloves, safety goggles, and appropriate respiratory protection when working with the substance in poorly ventilated areas. Employers are required to provide adequate PPE and ensure that workers are properly trained in its use and maintenance.
Environmental regulations for dodecane focus on preventing releases into the environment and managing waste disposal. Spill prevention and containment measures are typically required, including the use of secondary containment systems for storage tanks and proper handling procedures during transfer operations. Waste dodecane and contaminated materials must be disposed of as hazardous waste in accordance with local and national regulations.
Transportation of dodecane is subject to specific safety regulations, particularly when shipped in large quantities. These regulations often include requirements for proper labeling, packaging, and documentation, as well as restrictions on the modes of transport and routes that can be used.
In chemical mixtures, the compatibility of dodecane with other substances must be carefully considered. Safety data sheets (SDS) for dodecane and other components of the mixture should be consulted to identify potential incompatibilities or hazardous reactions. Regulations may require specific risk assessments and control measures for mixtures containing dodecane, depending on the properties of the other components and the intended use of the mixture.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!