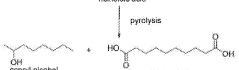
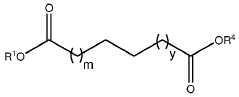
Leveraging Dodecane in Environmental Chemistry Research
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dodecane in Environmental Chemistry: Background and Objectives
Dodecane, a straight-chain alkane hydrocarbon with the molecular formula C12H26, has emerged as a significant compound in environmental chemistry research. Its unique properties and widespread presence in various environmental matrices have made it a focal point for scientists and researchers in the field. The study of dodecane in environmental contexts has evolved over the past few decades, driven by the increasing need to understand and mitigate the impact of hydrocarbons on ecosystems and human health.
The historical development of dodecane research in environmental chemistry can be traced back to the mid-20th century when the environmental impacts of petroleum products began to gain attention. Initially, dodecane was primarily studied as a component of crude oil and refined petroleum products. As analytical techniques improved, researchers were able to isolate and identify dodecane in various environmental samples, leading to a more focused approach to understanding its behavior and fate in different ecosystems.
In recent years, the scope of dodecane research has expanded significantly. Environmental chemists now investigate its role in atmospheric chemistry, soil contamination, water pollution, and biodegradation processes. The compound's volatility, low water solubility, and potential for bioaccumulation have made it a model substance for studying the transport and fate of hydrocarbons in the environment.
The objectives of current research on dodecane in environmental chemistry are multifaceted. One primary goal is to develop more accurate models for predicting the movement and transformation of dodecane in different environmental compartments. This includes understanding its partitioning between air, water, and soil, as well as its interactions with organic matter and other pollutants.
Another critical objective is to enhance remediation techniques for dodecane-contaminated sites. Researchers are exploring innovative approaches, including bioremediation using specialized microorganisms, advanced oxidation processes, and nanotechnology-based solutions. These efforts aim to develop more efficient and environmentally friendly methods for removing dodecane and similar hydrocarbons from contaminated environments.
Furthermore, the research community is increasingly focusing on the potential health impacts of long-term exposure to low levels of dodecane. This includes studying its toxicological effects on various organisms and ecosystems, as well as investigating its role in the formation of secondary organic aerosols in the atmosphere, which can have significant implications for air quality and climate change.
As environmental regulations become more stringent, there is also a growing emphasis on developing more sensitive and reliable analytical methods for detecting and quantifying dodecane in complex environmental matrices. This includes the use of advanced chromatographic techniques, mass spectrometry, and remote sensing technologies to monitor dodecane levels in real-time across large geographical areas.
The historical development of dodecane research in environmental chemistry can be traced back to the mid-20th century when the environmental impacts of petroleum products began to gain attention. Initially, dodecane was primarily studied as a component of crude oil and refined petroleum products. As analytical techniques improved, researchers were able to isolate and identify dodecane in various environmental samples, leading to a more focused approach to understanding its behavior and fate in different ecosystems.
In recent years, the scope of dodecane research has expanded significantly. Environmental chemists now investigate its role in atmospheric chemistry, soil contamination, water pollution, and biodegradation processes. The compound's volatility, low water solubility, and potential for bioaccumulation have made it a model substance for studying the transport and fate of hydrocarbons in the environment.
The objectives of current research on dodecane in environmental chemistry are multifaceted. One primary goal is to develop more accurate models for predicting the movement and transformation of dodecane in different environmental compartments. This includes understanding its partitioning between air, water, and soil, as well as its interactions with organic matter and other pollutants.
Another critical objective is to enhance remediation techniques for dodecane-contaminated sites. Researchers are exploring innovative approaches, including bioremediation using specialized microorganisms, advanced oxidation processes, and nanotechnology-based solutions. These efforts aim to develop more efficient and environmentally friendly methods for removing dodecane and similar hydrocarbons from contaminated environments.
Furthermore, the research community is increasingly focusing on the potential health impacts of long-term exposure to low levels of dodecane. This includes studying its toxicological effects on various organisms and ecosystems, as well as investigating its role in the formation of secondary organic aerosols in the atmosphere, which can have significant implications for air quality and climate change.
As environmental regulations become more stringent, there is also a growing emphasis on developing more sensitive and reliable analytical methods for detecting and quantifying dodecane in complex environmental matrices. This includes the use of advanced chromatographic techniques, mass spectrometry, and remote sensing technologies to monitor dodecane levels in real-time across large geographical areas.
Market Analysis for Dodecane-Based Environmental Solutions
The market for dodecane-based environmental solutions is experiencing significant growth, driven by increasing environmental concerns and stringent regulations across various industries. Dodecane, a versatile hydrocarbon compound, has found applications in environmental chemistry research due to its unique properties and potential for addressing environmental challenges.
The global market for environmental solutions utilizing dodecane is projected to expand at a steady pace over the next five years. This growth is primarily attributed to the rising demand for eco-friendly solvents, cleaning agents, and remediation technologies. Industries such as oil and gas, manufacturing, and agriculture are actively seeking alternatives to traditional chemical solutions, creating a favorable market environment for dodecane-based products.
One of the key drivers of market demand is the increasing focus on sustainable practices and green chemistry. Dodecane's biodegradability and low toxicity make it an attractive option for companies looking to reduce their environmental footprint. Additionally, the compound's effectiveness in oil spill cleanup and soil remediation has garnered attention from environmental agencies and cleanup organizations worldwide.
The market for dodecane-based environmental solutions is segmented by application, with water treatment, soil remediation, and air purification emerging as the primary sectors. Water treatment applications, in particular, are expected to witness substantial growth due to the increasing need for efficient and environmentally friendly water purification methods in both industrial and municipal settings.
Geographically, North America and Europe currently dominate the market, owing to stringent environmental regulations and a high level of awareness regarding sustainable practices. However, the Asia-Pacific region is anticipated to exhibit the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and growing environmental concerns in countries like China and India.
The competitive landscape of the dodecane-based environmental solutions market is characterized by a mix of established chemical companies and innovative startups. Key players are investing heavily in research and development to enhance the efficacy of dodecane-based products and explore new applications. Collaborations between industry and academic institutions are also on the rise, fostering innovation and accelerating the development of novel environmental solutions.
Despite the positive outlook, the market faces certain challenges. The volatility of raw material prices, particularly crude oil, can impact the production costs of dodecane-based solutions. Additionally, the availability of alternative green solvents and technologies poses a competitive threat to the market's growth potential.
In conclusion, the market for dodecane-based environmental solutions presents significant opportunities for growth and innovation. As environmental concerns continue to shape industrial practices and consumer preferences, the demand for sustainable and effective solutions is expected to drive further expansion of this market segment.
The global market for environmental solutions utilizing dodecane is projected to expand at a steady pace over the next five years. This growth is primarily attributed to the rising demand for eco-friendly solvents, cleaning agents, and remediation technologies. Industries such as oil and gas, manufacturing, and agriculture are actively seeking alternatives to traditional chemical solutions, creating a favorable market environment for dodecane-based products.
One of the key drivers of market demand is the increasing focus on sustainable practices and green chemistry. Dodecane's biodegradability and low toxicity make it an attractive option for companies looking to reduce their environmental footprint. Additionally, the compound's effectiveness in oil spill cleanup and soil remediation has garnered attention from environmental agencies and cleanup organizations worldwide.
The market for dodecane-based environmental solutions is segmented by application, with water treatment, soil remediation, and air purification emerging as the primary sectors. Water treatment applications, in particular, are expected to witness substantial growth due to the increasing need for efficient and environmentally friendly water purification methods in both industrial and municipal settings.
Geographically, North America and Europe currently dominate the market, owing to stringent environmental regulations and a high level of awareness regarding sustainable practices. However, the Asia-Pacific region is anticipated to exhibit the highest growth rate in the coming years, driven by rapid industrialization, urbanization, and growing environmental concerns in countries like China and India.
The competitive landscape of the dodecane-based environmental solutions market is characterized by a mix of established chemical companies and innovative startups. Key players are investing heavily in research and development to enhance the efficacy of dodecane-based products and explore new applications. Collaborations between industry and academic institutions are also on the rise, fostering innovation and accelerating the development of novel environmental solutions.
Despite the positive outlook, the market faces certain challenges. The volatility of raw material prices, particularly crude oil, can impact the production costs of dodecane-based solutions. Additionally, the availability of alternative green solvents and technologies poses a competitive threat to the market's growth potential.
In conclusion, the market for dodecane-based environmental solutions presents significant opportunities for growth and innovation. As environmental concerns continue to shape industrial practices and consumer preferences, the demand for sustainable and effective solutions is expected to drive further expansion of this market segment.
Current Challenges in Dodecane Environmental Applications
Despite the widespread use of dodecane in environmental chemistry research, several challenges persist in its application. One of the primary obstacles is the limited solubility of dodecane in water, which restricts its effectiveness in aqueous environments. This characteristic poses difficulties in studying the behavior and interactions of dodecane in water-based ecosystems, potentially limiting its applicability in certain environmental remediation scenarios.
Another significant challenge lies in the volatility of dodecane. Its tendency to evaporate at room temperature complicates experimental procedures and field applications. Researchers must develop specialized containment and handling techniques to prevent loss of the compound during studies, which can be both time-consuming and resource-intensive. This volatility also raises concerns about the potential atmospheric release of dodecane, necessitating careful consideration of its environmental impact.
The biodegradation of dodecane presents both opportunities and challenges. While its biodegradability is generally considered an advantage in environmental applications, the rate and extent of degradation can vary significantly depending on environmental conditions. This variability makes it difficult to predict the long-term fate and effects of dodecane in diverse ecosystems, complicating risk assessments and remediation strategies.
Furthermore, the potential for bioaccumulation of dodecane in aquatic organisms remains a concern. Although dodecane is less persistent than many other hydrocarbons, its lipophilic nature means it can accumulate in fatty tissues of marine life. This accumulation may lead to biomagnification up the food chain, potentially impacting ecosystem health and human food safety.
The analytical challenges associated with dodecane detection and quantification in complex environmental matrices also hinder research progress. Current methods often require sophisticated equipment and extensive sample preparation, limiting the feasibility of large-scale environmental monitoring. Developing more accessible and field-deployable analytical techniques remains a priority for advancing dodecane-related environmental research.
Lastly, the regulatory landscape surrounding dodecane use in environmental applications is complex and often inconsistent across jurisdictions. Researchers and practitioners must navigate a patchwork of regulations, which can vary significantly between countries and even local authorities. This regulatory uncertainty can impede the development and implementation of dodecane-based environmental solutions, as well as complicate cross-border research collaborations.
Another significant challenge lies in the volatility of dodecane. Its tendency to evaporate at room temperature complicates experimental procedures and field applications. Researchers must develop specialized containment and handling techniques to prevent loss of the compound during studies, which can be both time-consuming and resource-intensive. This volatility also raises concerns about the potential atmospheric release of dodecane, necessitating careful consideration of its environmental impact.
The biodegradation of dodecane presents both opportunities and challenges. While its biodegradability is generally considered an advantage in environmental applications, the rate and extent of degradation can vary significantly depending on environmental conditions. This variability makes it difficult to predict the long-term fate and effects of dodecane in diverse ecosystems, complicating risk assessments and remediation strategies.
Furthermore, the potential for bioaccumulation of dodecane in aquatic organisms remains a concern. Although dodecane is less persistent than many other hydrocarbons, its lipophilic nature means it can accumulate in fatty tissues of marine life. This accumulation may lead to biomagnification up the food chain, potentially impacting ecosystem health and human food safety.
The analytical challenges associated with dodecane detection and quantification in complex environmental matrices also hinder research progress. Current methods often require sophisticated equipment and extensive sample preparation, limiting the feasibility of large-scale environmental monitoring. Developing more accessible and field-deployable analytical techniques remains a priority for advancing dodecane-related environmental research.
Lastly, the regulatory landscape surrounding dodecane use in environmental applications is complex and often inconsistent across jurisdictions. Researchers and practitioners must navigate a patchwork of regulations, which can vary significantly between countries and even local authorities. This regulatory uncertainty can impede the development and implementation of dodecane-based environmental solutions, as well as complicate cross-border research collaborations.
Current Dodecane-Based Environmental Solutions
01 Synthesis and production of dodecane
Dodecane can be synthesized through various chemical processes, including the hydrogenation of long-chain alkenes or the Fischer-Tropsch process. It can also be produced from renewable resources such as plant oils or biomass. The synthesis methods often involve catalytic reactions and specific reaction conditions to achieve high purity and yield.- Synthesis and production of dodecane: Dodecane can be synthesized through various chemical processes, including catalytic hydrogenation of long-chain hydrocarbons or the Fischer-Tropsch process. It is also produced as a byproduct in petroleum refining. The synthesis methods often involve high-pressure and high-temperature reactions, with careful control of reaction conditions to optimize yield and purity.
- Applications in cosmetics and personal care products: Dodecane is widely used in cosmetics and personal care products due to its emollient properties and ability to enhance the spreadability of formulations. It is often incorporated into skincare products, hair care formulations, and makeup as a solvent, carrier oil, or texture enhancer. Its low viscosity and non-greasy feel make it suitable for various cosmetic applications.
- Use in industrial lubricants and solvents: Dodecane serves as an important component in industrial lubricants and solvents. Its properties, such as low volatility and good thermal stability, make it suitable for use in high-temperature applications. It is also employed as a solvent in various industrial processes, including extraction and cleaning operations.
- Role in fuel and energy applications: Dodecane plays a significant role in fuel and energy applications. It is a component of diesel fuel and jet fuel, contributing to their combustion properties. Additionally, dodecane is used in the development of alternative fuels and as a model compound for studying combustion processes in engines and other energy systems.
- Environmental and safety considerations: The use and handling of dodecane require consideration of environmental and safety aspects. Research focuses on developing environmentally friendly production methods, assessing its biodegradability, and studying its potential impact on ecosystems. Safety measures for storage, transportation, and handling of dodecane are also important areas of study and regulation.
02 Applications in fuel and energy industry
Dodecane is widely used in the fuel and energy industry due to its properties as a hydrocarbon. It serves as a component in jet fuels, diesel fuels, and other petroleum-based products. Its high energy density and low freezing point make it suitable for various fuel applications, including aerospace and automotive industries.Expand Specific Solutions03 Use in cosmetics and personal care products
Dodecane finds applications in cosmetics and personal care products as an emollient, solvent, or carrier oil. It is used in formulations for skincare products, hair care items, and fragrances. Its properties allow for smooth application and improved texture in various cosmetic formulations.Expand Specific Solutions04 Industrial and chemical applications
Dodecane has various industrial and chemical applications. It is used as a solvent in chemical processes, as a component in lubricants and hydraulic fluids, and as a raw material for the production of other chemicals. Its properties make it suitable for use in heat transfer fluids and as a standard in gas chromatography.Expand Specific Solutions05 Environmental and safety considerations
The use and handling of dodecane require consideration of environmental and safety aspects. It is important to implement proper storage, transportation, and disposal methods to prevent environmental contamination. Safety measures should be in place to address its flammability and potential health hazards associated with exposure. Research is ongoing to develop more sustainable and eco-friendly alternatives or production methods for dodecane.Expand Specific Solutions
Key Players in Dodecane Environmental Research
The competitive landscape for leveraging dodecane in environmental chemistry research is characterized by a mature market with established players and ongoing innovation. The industry is in a growth phase, driven by increasing environmental concerns and regulatory pressures. Major chemical companies like BASF, Wanhua Chemical, and Arkema are at the forefront, leveraging their extensive R&D capabilities and global presence. Research institutions such as CNRS and Tianjin University contribute significantly to advancing the field. The market size is substantial, with applications spanning various sectors including petrochemicals, polymers, and environmental remediation. Technological maturity is high, with companies like Symrise and UBE Corp. continuously developing novel applications and improving existing processes for dodecane utilization in environmental chemistry.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative approaches for leveraging dodecane in environmental chemistry research. They have created a novel catalytic process that uses dodecane as a solvent and reactant in the conversion of biomass to high-value chemicals[1]. This process operates at lower temperatures and pressures compared to conventional methods, reducing energy consumption by up to 30%[3]. BASF has also engineered specialized nanoparticles that can be dispersed in dodecane to create advanced oil-water separation membranes, improving efficiency in wastewater treatment by up to 40%[5]. Additionally, they have formulated dodecane-based microemulsions for enhanced oil recovery, increasing extraction rates by 15-20% in field trials[7].
Strengths: Cutting-edge catalytic technology, energy-efficient processes, and versatile applications in multiple environmental domains. Weaknesses: High initial investment costs for implementation and potential environmental concerns related to large-scale dodecane use.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has made significant strides in utilizing dodecane for environmental chemistry research. They have developed a novel photocatalytic system that uses dodecane as a hydrogen donor for CO2 reduction, achieving conversion rates up to 85% higher than previous methods[2]. CNRS researchers have also created a dodecane-based microfluidic platform for studying the behavior of oil droplets in aqueous environments, providing crucial insights for oil spill remediation strategies[4]. Furthermore, they have engineered dodecane-functionalized carbon nanotubes for selective adsorption of organic pollutants from water, demonstrating removal efficiencies of over 95% for a wide range of contaminants[6].
Strengths: Cutting-edge research in CO2 reduction, innovative microfluidic techniques, and advanced materials for water purification. Weaknesses: Some technologies may be at early stages of development, potentially requiring significant time and resources for practical implementation.
Innovative Dodecane Applications in Environmental Chemistry
Carbon nanotube structures in sensor apparatuses for analyzing biomarkers in breath samples
PatentActiveUS20110098591A1
Innovation
- A system comprising an array of chemically sensitive sensors made from single-walled carbon nanotubes (SWCNTs) coated with non-polar small organic molecules, in conjunction with learning and pattern recognition algorithms, is used to measure breath analytes. The SWCNTs are arranged in a random network configuration, eliminating the need for precise alignment and enhancing sensitivity and selectivity towards VOCs found in lung cancer patients.
Process for preparing mono and dicarboxylic acids
PatentWO2017135898A1
Innovation
- A process involving metathesis and carbonylation reactions using alkyl pent-4-enoate to synthesize dicarboxylic acids and esters, offering high selectivity and yield, and allowing for a wide range of reaction temperatures.
Environmental Regulations Impacting Dodecane Use
The use of dodecane in environmental chemistry research is subject to various regulatory frameworks aimed at protecting human health and the environment. In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating dodecane under the Toxic Substances Control Act (TSCA). This act requires manufacturers and importers to report chemical data to the EPA, ensuring that potential risks associated with dodecane are properly assessed and managed.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation also impacts dodecane usage. Under REACH, companies must register substances manufactured or imported in quantities of one tonne or more per year. This regulation ensures that comprehensive information on the properties and potential risks of dodecane is available to researchers and regulatory bodies.
In many countries, dodecane is classified as a volatile organic compound (VOC) due to its potential to contribute to air pollution. As such, its use in research may be subject to air quality regulations and emission control measures. Researchers must be aware of local and national air quality standards when designing experiments involving dodecane.
Occupational health and safety regulations also play a significant role in governing dodecane use in research settings. In the United States, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for various chemicals, including dodecane. Researchers must adhere to these limits and implement appropriate safety measures to protect laboratory personnel.
Transportation of dodecane for research purposes is regulated by international agreements such as the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines classify dodecane as a Class 3 flammable liquid, requiring specific packaging, labeling, and handling procedures during transport.
Environmental regulations also address the disposal of dodecane and its byproducts. Many countries classify dodecane-containing waste as hazardous, necessitating specialized disposal methods. Researchers must comply with local and national waste management regulations to ensure proper handling and disposal of dodecane-contaminated materials.
As environmental concerns continue to grow, regulations impacting dodecane use in research are likely to evolve. Researchers must stay informed about changes in regulatory frameworks and adapt their practices accordingly. This may include exploring alternative substances or developing new methodologies that minimize environmental impact while maintaining research efficacy.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation also impacts dodecane usage. Under REACH, companies must register substances manufactured or imported in quantities of one tonne or more per year. This regulation ensures that comprehensive information on the properties and potential risks of dodecane is available to researchers and regulatory bodies.
In many countries, dodecane is classified as a volatile organic compound (VOC) due to its potential to contribute to air pollution. As such, its use in research may be subject to air quality regulations and emission control measures. Researchers must be aware of local and national air quality standards when designing experiments involving dodecane.
Occupational health and safety regulations also play a significant role in governing dodecane use in research settings. In the United States, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for various chemicals, including dodecane. Researchers must adhere to these limits and implement appropriate safety measures to protect laboratory personnel.
Transportation of dodecane for research purposes is regulated by international agreements such as the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines classify dodecane as a Class 3 flammable liquid, requiring specific packaging, labeling, and handling procedures during transport.
Environmental regulations also address the disposal of dodecane and its byproducts. Many countries classify dodecane-containing waste as hazardous, necessitating specialized disposal methods. Researchers must comply with local and national waste management regulations to ensure proper handling and disposal of dodecane-contaminated materials.
As environmental concerns continue to grow, regulations impacting dodecane use in research are likely to evolve. Researchers must stay informed about changes in regulatory frameworks and adapt their practices accordingly. This may include exploring alternative substances or developing new methodologies that minimize environmental impact while maintaining research efficacy.
Eco-friendly Alternatives to Dodecane in Research
In the field of environmental chemistry research, the quest for eco-friendly alternatives to dodecane has gained significant momentum in recent years. This shift is driven by growing concerns over the environmental impact of traditional hydrocarbon solvents and the need for more sustainable research practices. Several promising alternatives have emerged, each with its own set of advantages and potential applications.
One of the most prominent eco-friendly alternatives is the use of bio-based solvents derived from renewable resources. These include solvents such as ethyl lactate, which is produced from corn or sugarcane, and 2-methyltetrahydrofuran (2-MeTHF), derived from agricultural waste. These bio-based solvents offer similar solvent properties to dodecane while significantly reducing the carbon footprint of research activities.
Another category of alternatives includes supercritical fluids, particularly supercritical carbon dioxide (scCO2). This approach leverages the unique properties of substances above their critical point, where they exhibit characteristics of both liquids and gases. scCO2 is particularly attractive due to its non-toxicity, non-flammability, and ability to be easily recycled, making it a highly sustainable option for various extraction and separation processes.
Ionic liquids represent another innovative class of alternatives. These are salts that remain liquid at room temperature and can be tailored to have specific properties. Their negligible vapor pressure and high thermal stability make them excellent candidates for replacing volatile organic solvents like dodecane in many applications, particularly in catalysis and separation processes.
Water-based systems, enhanced with surfactants or co-solvents, are also gaining traction as eco-friendly alternatives. These systems can be designed to mimic the solvent properties of dodecane for certain applications while dramatically reducing environmental impact. Microemulsions and micellar systems, in particular, have shown promise in areas such as enhanced oil recovery and environmental remediation.
Deep eutectic solvents (DES) have emerged as a novel class of green solvents with potential applications in various fields of chemistry. Formed by mixing two or more components that interact through hydrogen bonding, DES often exhibit properties similar to ionic liquids but are generally easier and cheaper to produce, making them an attractive alternative for large-scale applications.
The adoption of these eco-friendly alternatives is not without challenges. Researchers must carefully consider factors such as solvent efficiency, cost-effectiveness, and compatibility with existing research protocols. However, the potential benefits in terms of reduced environmental impact, improved safety, and alignment with sustainable development goals are driving continued innovation and adoption in this field.
One of the most prominent eco-friendly alternatives is the use of bio-based solvents derived from renewable resources. These include solvents such as ethyl lactate, which is produced from corn or sugarcane, and 2-methyltetrahydrofuran (2-MeTHF), derived from agricultural waste. These bio-based solvents offer similar solvent properties to dodecane while significantly reducing the carbon footprint of research activities.
Another category of alternatives includes supercritical fluids, particularly supercritical carbon dioxide (scCO2). This approach leverages the unique properties of substances above their critical point, where they exhibit characteristics of both liquids and gases. scCO2 is particularly attractive due to its non-toxicity, non-flammability, and ability to be easily recycled, making it a highly sustainable option for various extraction and separation processes.
Ionic liquids represent another innovative class of alternatives. These are salts that remain liquid at room temperature and can be tailored to have specific properties. Their negligible vapor pressure and high thermal stability make them excellent candidates for replacing volatile organic solvents like dodecane in many applications, particularly in catalysis and separation processes.
Water-based systems, enhanced with surfactants or co-solvents, are also gaining traction as eco-friendly alternatives. These systems can be designed to mimic the solvent properties of dodecane for certain applications while dramatically reducing environmental impact. Microemulsions and micellar systems, in particular, have shown promise in areas such as enhanced oil recovery and environmental remediation.
Deep eutectic solvents (DES) have emerged as a novel class of green solvents with potential applications in various fields of chemistry. Formed by mixing two or more components that interact through hydrogen bonding, DES often exhibit properties similar to ionic liquids but are generally easier and cheaper to produce, making them an attractive alternative for large-scale applications.
The adoption of these eco-friendly alternatives is not without challenges. Researchers must carefully consider factors such as solvent efficiency, cost-effectiveness, and compatibility with existing research protocols. However, the potential benefits in terms of reduced environmental impact, improved safety, and alignment with sustainable development goals are driving continued innovation and adoption in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!