Characterizing growth dynamics of malachite under industrial synthesis conditions
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Synthesis Background and Objectives
Malachite, a copper carbonate hydroxide mineral with the chemical formula Cu2CO3(OH)2, has been a subject of significant interest in both natural and industrial contexts. The synthesis of malachite under industrial conditions has gained particular attention due to its wide-ranging applications in various fields, including pigments, catalysts, and advanced materials.
The historical background of malachite synthesis dates back to ancient times when it was primarily used as a pigment in art and decoration. However, the modern industrial synthesis of malachite has evolved significantly, driven by the need for controlled production of high-quality materials with specific properties. This evolution has led to a deeper understanding of the growth dynamics and the factors influencing malachite formation.
In recent years, the characterization of malachite growth dynamics under industrial synthesis conditions has become a critical area of research. This focus stems from the increasing demand for tailored malachite properties in advanced applications, such as nanotechnology, environmental remediation, and energy storage. Understanding the growth mechanisms allows for precise control over particle size, morphology, and crystallinity, which directly impact the material's performance in these applications.
The primary objective of studying malachite growth dynamics is to optimize synthesis conditions for desired outcomes. This involves investigating the influence of various parameters such as temperature, pH, concentration of reactants, and the presence of additives on the nucleation and growth processes. By elucidating these relationships, researchers aim to develop more efficient and sustainable production methods that can be scaled up for industrial use.
Another crucial aspect of this research is the development of in-situ characterization techniques. These methods allow for real-time monitoring of malachite formation, providing invaluable insights into the kinetics and mechanisms of crystal growth. Such information is essential for fine-tuning synthesis protocols and achieving reproducible results on an industrial scale.
Furthermore, the study of malachite growth dynamics under industrial conditions aims to bridge the gap between laboratory-scale synthesis and large-scale production. This transition often presents challenges related to maintaining product quality, consistency, and yield. By thoroughly understanding the growth dynamics, researchers can address these challenges and develop robust processes suitable for industrial implementation.
In conclusion, the characterization of malachite growth dynamics under industrial synthesis conditions represents a convergence of fundamental materials science and practical industrial needs. This research area not only advances our understanding of crystal growth phenomena but also paves the way for innovative applications and improved manufacturing processes in the realm of copper-based materials.
The historical background of malachite synthesis dates back to ancient times when it was primarily used as a pigment in art and decoration. However, the modern industrial synthesis of malachite has evolved significantly, driven by the need for controlled production of high-quality materials with specific properties. This evolution has led to a deeper understanding of the growth dynamics and the factors influencing malachite formation.
In recent years, the characterization of malachite growth dynamics under industrial synthesis conditions has become a critical area of research. This focus stems from the increasing demand for tailored malachite properties in advanced applications, such as nanotechnology, environmental remediation, and energy storage. Understanding the growth mechanisms allows for precise control over particle size, morphology, and crystallinity, which directly impact the material's performance in these applications.
The primary objective of studying malachite growth dynamics is to optimize synthesis conditions for desired outcomes. This involves investigating the influence of various parameters such as temperature, pH, concentration of reactants, and the presence of additives on the nucleation and growth processes. By elucidating these relationships, researchers aim to develop more efficient and sustainable production methods that can be scaled up for industrial use.
Another crucial aspect of this research is the development of in-situ characterization techniques. These methods allow for real-time monitoring of malachite formation, providing invaluable insights into the kinetics and mechanisms of crystal growth. Such information is essential for fine-tuning synthesis protocols and achieving reproducible results on an industrial scale.
Furthermore, the study of malachite growth dynamics under industrial conditions aims to bridge the gap between laboratory-scale synthesis and large-scale production. This transition often presents challenges related to maintaining product quality, consistency, and yield. By thoroughly understanding the growth dynamics, researchers can address these challenges and develop robust processes suitable for industrial implementation.
In conclusion, the characterization of malachite growth dynamics under industrial synthesis conditions represents a convergence of fundamental materials science and practical industrial needs. This research area not only advances our understanding of crystal growth phenomena but also paves the way for innovative applications and improved manufacturing processes in the realm of copper-based materials.
Industrial Demand for Malachite Production
The industrial demand for malachite production has been steadily increasing due to its diverse applications across various sectors. Malachite, a copper carbonate hydroxide mineral, finds extensive use in the jewelry industry, where its vibrant green color and unique patterns make it a popular choice for ornamental stones and decorative objects. The growing luxury goods market, particularly in emerging economies, has contributed to a surge in demand for high-quality malachite specimens.
In the construction and interior design sectors, malachite has gained traction as a premium material for architectural elements, such as countertops, tiles, and decorative panels. The trend towards sustainable and natural materials in modern design has further boosted the demand for malachite in these applications. Additionally, the art world has seen a renewed interest in malachite as a medium for sculptures and other artistic creations, driving demand among collectors and galleries.
The industrial sector also contributes significantly to the demand for malachite production. Malachite serves as an important ore of copper, and with the global push towards electrification and renewable energy technologies, the demand for copper has been on the rise. This, in turn, has led to increased interest in malachite as a secondary source of copper extraction, particularly in regions where primary copper deposits are becoming depleted.
In the field of materials science, malachite has shown promise in the development of advanced catalysts and adsorbents. Research into its potential applications in environmental remediation, particularly for the removal of heavy metals from wastewater, has sparked interest from the water treatment industry. This emerging market segment presents new opportunities for malachite production and utilization.
The cosmetics and pharmaceutical industries have also begun exploring the potential of malachite in their formulations. Some skincare products now incorporate finely ground malachite for its purported antioxidant properties, while certain alternative medicine practices use malachite-based preparations. Although these applications are still niche, they represent potential growth areas for malachite demand in the future.
As industrial synthesis conditions for malachite production continue to improve, the ability to meet this growing demand becomes more feasible. Understanding the growth dynamics of malachite under controlled conditions is crucial for optimizing production processes, ensuring consistent quality, and potentially reducing costs. This knowledge can lead to more efficient extraction methods and the development of synthetic malachite with properties tailored to specific industrial applications, further expanding its market potential.
In the construction and interior design sectors, malachite has gained traction as a premium material for architectural elements, such as countertops, tiles, and decorative panels. The trend towards sustainable and natural materials in modern design has further boosted the demand for malachite in these applications. Additionally, the art world has seen a renewed interest in malachite as a medium for sculptures and other artistic creations, driving demand among collectors and galleries.
The industrial sector also contributes significantly to the demand for malachite production. Malachite serves as an important ore of copper, and with the global push towards electrification and renewable energy technologies, the demand for copper has been on the rise. This, in turn, has led to increased interest in malachite as a secondary source of copper extraction, particularly in regions where primary copper deposits are becoming depleted.
In the field of materials science, malachite has shown promise in the development of advanced catalysts and adsorbents. Research into its potential applications in environmental remediation, particularly for the removal of heavy metals from wastewater, has sparked interest from the water treatment industry. This emerging market segment presents new opportunities for malachite production and utilization.
The cosmetics and pharmaceutical industries have also begun exploring the potential of malachite in their formulations. Some skincare products now incorporate finely ground malachite for its purported antioxidant properties, while certain alternative medicine practices use malachite-based preparations. Although these applications are still niche, they represent potential growth areas for malachite demand in the future.
As industrial synthesis conditions for malachite production continue to improve, the ability to meet this growing demand becomes more feasible. Understanding the growth dynamics of malachite under controlled conditions is crucial for optimizing production processes, ensuring consistent quality, and potentially reducing costs. This knowledge can lead to more efficient extraction methods and the development of synthetic malachite with properties tailored to specific industrial applications, further expanding its market potential.
Current Challenges in Malachite Growth Characterization
The characterization of malachite growth dynamics under industrial synthesis conditions presents several significant challenges that researchers and industry professionals must address. One of the primary difficulties lies in the complex interplay of various factors influencing malachite formation, including temperature, pressure, pH, and the presence of impurities. These parameters can significantly affect the crystal structure, morphology, and growth rate of malachite, making it challenging to establish consistent and reproducible synthesis conditions.
Another major hurdle is the real-time monitoring of malachite growth processes. Traditional characterization techniques often require sample extraction and ex-situ analysis, which can disrupt the growth environment and potentially alter the crystal formation. This limitation hinders the ability to observe and understand the dynamic changes occurring during malachite synthesis, particularly at the nanoscale level where critical nucleation and growth events take place.
The heterogeneous nature of industrial synthesis environments further complicates the characterization process. Variations in local conditions within large-scale reactors can lead to inconsistencies in malachite growth across different regions, making it difficult to obtain representative data for the entire batch. This spatial heterogeneity poses challenges in scaling up laboratory findings to industrial production levels and ensuring uniform product quality.
Additionally, the time-dependent aspects of malachite growth present unique challenges. The kinetics of crystal formation and the evolution of crystal habits over time require sophisticated time-resolved characterization techniques. However, many existing methods lack the temporal resolution necessary to capture rapid changes in growth dynamics, particularly during the initial stages of nucleation and early crystal development.
The presence of intermediate phases and competing reactions in the synthesis process adds another layer of complexity to malachite growth characterization. These transient species and side reactions can interfere with the primary growth mechanisms, making it challenging to isolate and study the specific factors governing malachite formation. Researchers must develop strategies to differentiate between the various chemical processes occurring simultaneously within the reaction system.
Furthermore, the integration of multiple characterization techniques to obtain a comprehensive understanding of malachite growth dynamics remains a significant challenge. Combining spectroscopic, microscopic, and diffraction methods in a synergistic manner requires advanced instrumentation and data analysis approaches. The development of in-situ and operando characterization methods that can withstand the harsh conditions of industrial synthesis while providing high-resolution, real-time data is an ongoing area of research and development.
Another major hurdle is the real-time monitoring of malachite growth processes. Traditional characterization techniques often require sample extraction and ex-situ analysis, which can disrupt the growth environment and potentially alter the crystal formation. This limitation hinders the ability to observe and understand the dynamic changes occurring during malachite synthesis, particularly at the nanoscale level where critical nucleation and growth events take place.
The heterogeneous nature of industrial synthesis environments further complicates the characterization process. Variations in local conditions within large-scale reactors can lead to inconsistencies in malachite growth across different regions, making it difficult to obtain representative data for the entire batch. This spatial heterogeneity poses challenges in scaling up laboratory findings to industrial production levels and ensuring uniform product quality.
Additionally, the time-dependent aspects of malachite growth present unique challenges. The kinetics of crystal formation and the evolution of crystal habits over time require sophisticated time-resolved characterization techniques. However, many existing methods lack the temporal resolution necessary to capture rapid changes in growth dynamics, particularly during the initial stages of nucleation and early crystal development.
The presence of intermediate phases and competing reactions in the synthesis process adds another layer of complexity to malachite growth characterization. These transient species and side reactions can interfere with the primary growth mechanisms, making it challenging to isolate and study the specific factors governing malachite formation. Researchers must develop strategies to differentiate between the various chemical processes occurring simultaneously within the reaction system.
Furthermore, the integration of multiple characterization techniques to obtain a comprehensive understanding of malachite growth dynamics remains a significant challenge. Combining spectroscopic, microscopic, and diffraction methods in a synergistic manner requires advanced instrumentation and data analysis approaches. The development of in-situ and operando characterization methods that can withstand the harsh conditions of industrial synthesis while providing high-resolution, real-time data is an ongoing area of research and development.
Existing Methods for Malachite Growth Analysis
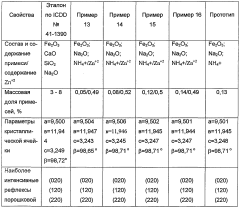
01 Crystal growth mechanisms of malachite
Research on the crystal growth mechanisms of malachite, including factors affecting nucleation, growth rates, and morphology. Studies focus on understanding the kinetics and thermodynamics of malachite formation under various conditions, which is crucial for controlling crystal size and shape in industrial applications.- Crystal growth mechanisms and control: Research on malachite growth dynamics focuses on understanding and controlling crystal growth mechanisms. This includes studying factors that influence nucleation, growth rates, and crystal morphology. Techniques for manipulating growth conditions to achieve desired crystal structures and properties are explored.
- Synthesis methods for malachite nanostructures: Various synthesis methods are developed to produce malachite nanostructures with controlled size, shape, and properties. These methods may include hydrothermal synthesis, sol-gel processes, and template-assisted growth. The focus is on optimizing reaction conditions to achieve specific nanostructure morphologies.
- Environmental factors affecting malachite formation: Studies investigate the impact of environmental factors on malachite growth dynamics. This includes examining the effects of temperature, pH, pressure, and the presence of impurities or additives on crystal formation and growth. Understanding these factors helps in predicting and controlling malachite formation in natural and synthetic environments.
- Applications of malachite growth dynamics: Research explores practical applications of malachite growth dynamics in various fields. This includes using malachite in catalysis, environmental remediation, and as a precursor for other materials. The controlled growth of malachite structures is also investigated for potential use in sensors, electronics, and energy storage devices.
- Modeling and simulation of malachite growth: Computational methods are employed to model and simulate malachite growth dynamics. These models help predict crystal growth patterns, understand the kinetics of formation, and optimize growth conditions. Advanced simulation techniques may incorporate molecular dynamics and machine learning approaches to enhance predictive capabilities.
02 Influence of environmental factors on malachite growth
Investigation of how environmental factors such as temperature, pH, and solution composition affect malachite growth dynamics. This research aims to optimize growth conditions for producing high-quality malachite crystals and to understand the mineral's formation in natural settings.Expand Specific Solutions03 Synthetic methods for malachite production
Development of various synthetic methods for producing malachite, including hydrothermal synthesis, sol-gel processes, and biomimetic approaches. These techniques aim to control the growth dynamics and properties of malachite for applications in materials science and nanotechnology.Expand Specific Solutions04 Malachite nanostructures and their growth dynamics
Exploration of malachite nanostructure formation, including nanorods, nanoparticles, and hierarchical structures. Research focuses on understanding and controlling the growth dynamics at the nanoscale to develop novel materials with unique properties for applications in catalysis, sensing, and environmental remediation.Expand Specific Solutions05 Biomineralization and malachite formation
Study of malachite formation through biomineralization processes, including the role of microorganisms and organic templates in controlling growth dynamics. This research aims to develop bio-inspired approaches for synthesizing malachite with specific morphologies and properties for various applications.Expand Specific Solutions
Key Players in Industrial Malachite Production
The characterization of malachite growth dynamics under industrial synthesis conditions represents a mature field with ongoing research and development. The market for synthetic malachite is relatively small but growing, driven by applications in jewelry, decorative items, and industrial uses. The technology's maturity is evident in the involvement of established players like BASF Corp. and Corning, Inc., which bring extensive expertise in materials science and chemical synthesis. Universities such as Guizhou University and the University of Florida contribute to fundamental research, while specialized companies like Henan Liliang Diamond Co., Ltd. and Element Six Ltd. leverage their experience in synthetic materials to advance malachite production techniques. The competitive landscape is diverse, with a mix of large corporations, academic institutions, and niche players collaborating and competing to optimize synthesis processes and expand applications.
BASF Corp.
Technical Solution: BASF Corp. has developed an advanced synthesis method for malachite growth under industrial conditions. Their approach utilizes a controlled precipitation process in a continuous flow reactor system. This method allows for precise control of pH, temperature, and reactant concentrations, resulting in high-quality malachite crystals with uniform size distribution and morphology[1]. The process incorporates in-situ monitoring techniques, such as real-time particle size analysis and spectroscopic measurements, to optimize growth parameters dynamically. BASF's technology also includes a proprietary post-synthesis treatment that enhances the stability and color intensity of the malachite product[3].
Strengths: Precise control over crystal growth parameters, scalable continuous process, high product quality and consistency. Weaknesses: Potentially higher energy consumption, complex equipment requirements.
Corning, Inc.
Technical Solution: Corning, Inc. has developed an advanced sol-gel based method for synthesizing malachite under industrial conditions. Their approach utilizes a carefully controlled gelation process followed by a unique supercritical drying technique to produce high-surface-area malachite nanostructures[9]. The process incorporates in-situ doping capabilities, allowing for the introduction of various metal ions to modify the electronic and optical properties of the malachite product. Corning's technology also features a proprietary surface functionalization step that enhances the dispersibility and chemical reactivity of the synthesized malachite particles. Additionally, the company has implemented an automated process control system that utilizes spectroscopic feedback to maintain optimal reaction conditions throughout the synthesis[10].
Strengths: High surface area product, tunable properties through doping, excellent control over particle size and morphology. Weaknesses: Potentially higher production costs, specialized equipment requirements for supercritical drying.
Innovative Approaches in Growth Dynamics Study
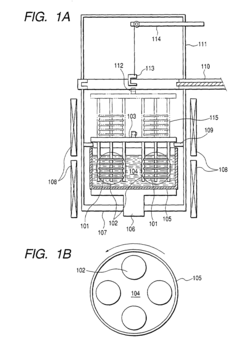
Malachite and method for the production thereof
PatentWO2004076354A1
Innovation
- The process involves evaporating a solution of basic copper carbonate and ammonium carbonate with controlled zinc content, forming polycrystalline malachite with alternating light and dark green layers, and condensing vapor to achieve malachite with enhanced mechanical properties and reduced impurities.
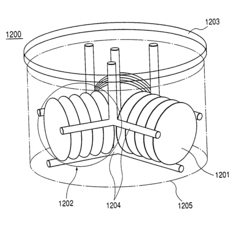
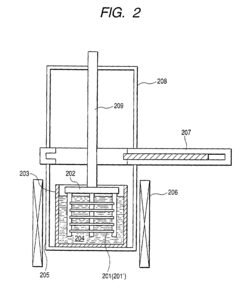
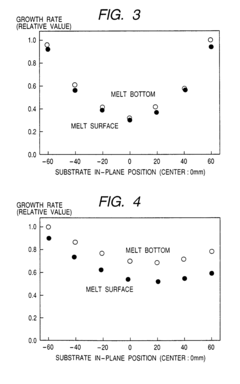
Liquid phase growth process, liquid phase growth system and substrate member production method
PatentInactiveUS7022181B2
Innovation
- The system rotates the crucible independently from the substrate, positioning the substrate off-center to facilitate uniform melt flow and crystal growth, with additional flow-adjusting mechanisms like fins and rectifying plates to enhance melt distribution and prevent contamination.
Environmental Impact of Industrial Malachite Production
The industrial production of malachite, a copper carbonate hydroxide mineral, has significant environmental implications that warrant careful consideration. The synthesis process typically involves the reaction of copper salts with carbonate-containing solutions under controlled conditions, which can lead to various environmental impacts.
One of the primary concerns is the potential for water pollution. The industrial synthesis of malachite often requires large volumes of water, and the resulting wastewater may contain high levels of dissolved copper and other heavy metals. If not properly treated, this effluent can contaminate local water sources, posing risks to aquatic ecosystems and potentially entering the food chain. Additionally, the use of carbonate-rich solutions may alter the pH of water bodies if discharged without adequate neutralization.
Air quality is another area of environmental impact. Depending on the specific industrial processes employed, the production of malachite may release particulate matter and volatile organic compounds (VOCs) into the atmosphere. These emissions can contribute to local air pollution, potentially affecting human health and vegetation in surrounding areas. Furthermore, the energy-intensive nature of some synthesis methods may indirectly contribute to greenhouse gas emissions if fossil fuels are used as the primary energy source.
Soil contamination is a potential long-term consequence of malachite production. Improper disposal of solid waste, including unreacted materials and byproducts, can lead to the accumulation of heavy metals in soil. This can have detrimental effects on soil fertility, microbial communities, and plant growth, potentially impacting local agriculture and ecosystems.
The extraction and processing of raw materials required for malachite synthesis, such as copper ores, also have upstream environmental impacts. These may include habitat destruction, soil erosion, and the release of pollutants associated with mining activities. The transportation of raw materials and finished products contributes to carbon emissions and may pose risks of accidental spills during transit.
To mitigate these environmental impacts, industry stakeholders are increasingly focusing on developing more sustainable production methods. These include closed-loop water systems to minimize water consumption and pollution, improved filtration and treatment technologies for air and water emissions, and the exploration of bio-based or low-impact synthesis routes. Additionally, there is growing interest in recycling and upcycling strategies to reduce the demand for newly synthesized malachite and minimize waste generation.
One of the primary concerns is the potential for water pollution. The industrial synthesis of malachite often requires large volumes of water, and the resulting wastewater may contain high levels of dissolved copper and other heavy metals. If not properly treated, this effluent can contaminate local water sources, posing risks to aquatic ecosystems and potentially entering the food chain. Additionally, the use of carbonate-rich solutions may alter the pH of water bodies if discharged without adequate neutralization.
Air quality is another area of environmental impact. Depending on the specific industrial processes employed, the production of malachite may release particulate matter and volatile organic compounds (VOCs) into the atmosphere. These emissions can contribute to local air pollution, potentially affecting human health and vegetation in surrounding areas. Furthermore, the energy-intensive nature of some synthesis methods may indirectly contribute to greenhouse gas emissions if fossil fuels are used as the primary energy source.
Soil contamination is a potential long-term consequence of malachite production. Improper disposal of solid waste, including unreacted materials and byproducts, can lead to the accumulation of heavy metals in soil. This can have detrimental effects on soil fertility, microbial communities, and plant growth, potentially impacting local agriculture and ecosystems.
The extraction and processing of raw materials required for malachite synthesis, such as copper ores, also have upstream environmental impacts. These may include habitat destruction, soil erosion, and the release of pollutants associated with mining activities. The transportation of raw materials and finished products contributes to carbon emissions and may pose risks of accidental spills during transit.
To mitigate these environmental impacts, industry stakeholders are increasingly focusing on developing more sustainable production methods. These include closed-loop water systems to minimize water consumption and pollution, improved filtration and treatment technologies for air and water emissions, and the exploration of bio-based or low-impact synthesis routes. Additionally, there is growing interest in recycling and upcycling strategies to reduce the demand for newly synthesized malachite and minimize waste generation.
Quality Control Measures in Synthetic Malachite
Quality control measures play a crucial role in ensuring the consistency and reliability of synthetic malachite production. The industrial synthesis of malachite requires careful monitoring and control of various parameters to achieve desired product characteristics. One of the primary quality control measures involves the precise regulation of temperature and pH throughout the synthesis process. These factors significantly influence the growth dynamics and final properties of the malachite crystals.
Regular sampling and analysis of the reaction mixture are essential components of quality control. This includes monitoring the concentration of reactants, such as copper ions and carbonate species, to maintain optimal conditions for malachite formation. Advanced analytical techniques, such as X-ray diffraction (XRD) and scanning electron microscopy (SEM), are employed to assess the crystalline structure and morphology of the synthesized malachite. These methods provide valuable insights into the growth patterns and help identify any deviations from the desired product specifications.
Particle size distribution is another critical quality parameter in synthetic malachite production. Laser diffraction techniques are commonly used to measure and control the size range of malachite particles. This ensures uniformity in the final product and helps meet specific application requirements. Additionally, colorimetry and spectrophotometry are utilized to evaluate the color intensity and purity of the synthesized malachite, as these properties are essential for its use in pigments and decorative applications.
Impurity control is a vital aspect of quality assurance in malachite synthesis. Inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS) are employed to detect and quantify trace impurities that may affect the product's performance or aesthetic qualities. Stringent purification processes, such as recrystallization and selective precipitation, are implemented to minimize contaminants and ensure high-purity malachite production.
To maintain consistent quality across batches, standardized operating procedures (SOPs) are developed and strictly followed. These SOPs encompass all aspects of the synthesis process, from raw material selection to final product packaging. Regular calibration and maintenance of equipment used in the synthesis and analysis processes are also integral to quality control measures. This ensures accurate and reliable results throughout the production cycle.
Implementing statistical process control (SPC) techniques allows for continuous monitoring and improvement of the malachite synthesis process. By analyzing trends and patterns in quality data, manufacturers can identify potential issues early and make necessary adjustments to maintain product consistency. Furthermore, the establishment of a comprehensive quality management system, often in compliance with ISO 9001 standards, helps ensure that all quality control measures are systematically implemented and documented.
Regular sampling and analysis of the reaction mixture are essential components of quality control. This includes monitoring the concentration of reactants, such as copper ions and carbonate species, to maintain optimal conditions for malachite formation. Advanced analytical techniques, such as X-ray diffraction (XRD) and scanning electron microscopy (SEM), are employed to assess the crystalline structure and morphology of the synthesized malachite. These methods provide valuable insights into the growth patterns and help identify any deviations from the desired product specifications.
Particle size distribution is another critical quality parameter in synthetic malachite production. Laser diffraction techniques are commonly used to measure and control the size range of malachite particles. This ensures uniformity in the final product and helps meet specific application requirements. Additionally, colorimetry and spectrophotometry are utilized to evaluate the color intensity and purity of the synthesized malachite, as these properties are essential for its use in pigments and decorative applications.
Impurity control is a vital aspect of quality assurance in malachite synthesis. Inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS) are employed to detect and quantify trace impurities that may affect the product's performance or aesthetic qualities. Stringent purification processes, such as recrystallization and selective precipitation, are implemented to minimize contaminants and ensure high-purity malachite production.
To maintain consistent quality across batches, standardized operating procedures (SOPs) are developed and strictly followed. These SOPs encompass all aspects of the synthesis process, from raw material selection to final product packaging. Regular calibration and maintenance of equipment used in the synthesis and analysis processes are also integral to quality control measures. This ensures accurate and reliable results throughout the production cycle.
Implementing statistical process control (SPC) techniques allows for continuous monitoring and improvement of the malachite synthesis process. By analyzing trends and patterns in quality data, manufacturers can identify potential issues early and make necessary adjustments to maintain product consistency. Furthermore, the establishment of a comprehensive quality management system, often in compliance with ISO 9001 standards, helps ensure that all quality control measures are systematically implemented and documented.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!