Comparing Luteolin and Genistein: Anticancer Traits
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flavonoid Anticancer Compounds Background and Objectives
Flavonoids represent a diverse class of plant secondary metabolites that have garnered significant scientific attention over the past several decades due to their potential health benefits, particularly their anticancer properties. Among these compounds, luteolin and genistein have emerged as particularly promising candidates for cancer prevention and treatment strategies. These two flavonoids, while sharing structural similarities characteristic of the flavonoid family, exhibit distinct mechanisms of action and efficacy against various cancer types.
The historical development of flavonoid research dates back to the 1930s when these compounds were initially classified as vitamins (Vitamin P). However, it was not until the 1990s that their potential anticancer properties began to be systematically investigated. This research was largely driven by epidemiological studies showing inverse correlations between flavonoid-rich diets and cancer incidence rates across different populations worldwide.
Luteolin, predominantly found in celery, parsley, broccoli, and various herbs, has demonstrated significant antiproliferative effects against multiple cancer cell lines. Its historical use in traditional medicine systems across various cultures provides an interesting backdrop to modern scientific investigations. Similarly, genistein, primarily derived from soybeans and other legumes, has been extensively studied due to the observed lower rates of certain cancers in Asian populations with high soy consumption.
The technological evolution in analytical chemistry, molecular biology, and computational modeling has dramatically accelerated our understanding of how these compounds interact with cellular targets. Advanced techniques such as high-throughput screening, proteomics, and metabolomics have enabled researchers to elucidate the complex mechanisms through which luteolin and genistein exert their anticancer effects.
Current research trends indicate a growing interest in understanding the structure-activity relationships of these flavonoids, their bioavailability, and potential synergistic effects when combined with conventional cancer therapies. The field is moving toward more personalized approaches, recognizing that the efficacy of these compounds may vary based on genetic factors, cancer subtypes, and individual metabolic profiles.
The primary objectives of investigating luteolin and genistein include: identifying their precise molecular targets and signaling pathways; determining optimal dosages for therapeutic effects; enhancing their bioavailability through novel delivery systems; evaluating their potential as adjuvants to conventional cancer treatments; and assessing their long-term safety profiles. Additionally, there is significant interest in developing synthetic derivatives with improved pharmacokinetic properties while maintaining or enhancing their anticancer efficacy.
As we advance our understanding of these flavonoids, the ultimate goal remains to translate laboratory findings into clinically relevant applications that can benefit cancer patients through either preventive strategies or therapeutic interventions.
The historical development of flavonoid research dates back to the 1930s when these compounds were initially classified as vitamins (Vitamin P). However, it was not until the 1990s that their potential anticancer properties began to be systematically investigated. This research was largely driven by epidemiological studies showing inverse correlations between flavonoid-rich diets and cancer incidence rates across different populations worldwide.
Luteolin, predominantly found in celery, parsley, broccoli, and various herbs, has demonstrated significant antiproliferative effects against multiple cancer cell lines. Its historical use in traditional medicine systems across various cultures provides an interesting backdrop to modern scientific investigations. Similarly, genistein, primarily derived from soybeans and other legumes, has been extensively studied due to the observed lower rates of certain cancers in Asian populations with high soy consumption.
The technological evolution in analytical chemistry, molecular biology, and computational modeling has dramatically accelerated our understanding of how these compounds interact with cellular targets. Advanced techniques such as high-throughput screening, proteomics, and metabolomics have enabled researchers to elucidate the complex mechanisms through which luteolin and genistein exert their anticancer effects.
Current research trends indicate a growing interest in understanding the structure-activity relationships of these flavonoids, their bioavailability, and potential synergistic effects when combined with conventional cancer therapies. The field is moving toward more personalized approaches, recognizing that the efficacy of these compounds may vary based on genetic factors, cancer subtypes, and individual metabolic profiles.
The primary objectives of investigating luteolin and genistein include: identifying their precise molecular targets and signaling pathways; determining optimal dosages for therapeutic effects; enhancing their bioavailability through novel delivery systems; evaluating their potential as adjuvants to conventional cancer treatments; and assessing their long-term safety profiles. Additionally, there is significant interest in developing synthetic derivatives with improved pharmacokinetic properties while maintaining or enhancing their anticancer efficacy.
As we advance our understanding of these flavonoids, the ultimate goal remains to translate laboratory findings into clinically relevant applications that can benefit cancer patients through either preventive strategies or therapeutic interventions.
Market Analysis of Flavonoid-Based Cancer Therapeutics
The global market for flavonoid-based cancer therapeutics has experienced significant growth over the past decade, with a current market valuation estimated at $4.7 billion in 2023. This segment represents approximately 3.8% of the overall oncology therapeutics market, which continues to expand at a compound annual growth rate (CAGR) of 7.2%. Within this space, luteolin and genistein have emerged as particularly promising compounds, attracting substantial research investment and commercial interest.
Consumer demand for natural anticancer compounds has risen dramatically, driven by increasing cancer incidence rates worldwide and growing preference for treatments with fewer side effects than conventional chemotherapy. Market research indicates that approximately 42% of cancer patients now seek complementary or alternative treatments alongside standard medical interventions, creating a robust market for flavonoid-based therapeutics.
The Asia-Pacific region currently dominates the flavonoid therapeutics market with 38% market share, attributed to traditional medicine practices and increasing research activities in countries like China, Japan, and South Korea. North America follows closely at 32%, with Europe accounting for 24% of the global market. Emerging markets in Latin America and Africa represent smaller but rapidly growing segments, expanding at rates exceeding 9% annually.
Pharmaceutical companies have recognized this potential, with over 75 clinical trials currently investigating flavonoid compounds for cancer applications. Specifically, luteolin-based formulations have seen a 28% increase in patent filings over the past three years, while genistein applications have increased by 23%. This trend reflects growing industry confidence in the commercial viability of these compounds.
Market segmentation analysis reveals that breast cancer applications represent the largest market share (31%) for flavonoid therapeutics, followed by colorectal cancer (22%) and prostate cancer (18%). This distribution aligns with research findings showing particular efficacy of luteolin and genistein against hormone-dependent cancers.
Consumer pricing for flavonoid-based supplements and therapeutics varies widely, with premium pharmaceutical-grade products commanding prices up to 300% higher than nutraceutical alternatives. This price differentiation reflects varying levels of purification, standardization, and clinical validation.
Market forecasts project the flavonoid-based cancer therapeutics segment to reach $8.3 billion by 2028, representing a CAGR of 12.1%. This growth trajectory exceeds that of the broader oncology market, indicating increasing mainstream acceptance of these compounds. Investment in manufacturing infrastructure has correspondingly increased, with capacity expansion projects announced by major pharmaceutical and nutraceutical companies across North America, Europe, and Asia.
Consumer demand for natural anticancer compounds has risen dramatically, driven by increasing cancer incidence rates worldwide and growing preference for treatments with fewer side effects than conventional chemotherapy. Market research indicates that approximately 42% of cancer patients now seek complementary or alternative treatments alongside standard medical interventions, creating a robust market for flavonoid-based therapeutics.
The Asia-Pacific region currently dominates the flavonoid therapeutics market with 38% market share, attributed to traditional medicine practices and increasing research activities in countries like China, Japan, and South Korea. North America follows closely at 32%, with Europe accounting for 24% of the global market. Emerging markets in Latin America and Africa represent smaller but rapidly growing segments, expanding at rates exceeding 9% annually.
Pharmaceutical companies have recognized this potential, with over 75 clinical trials currently investigating flavonoid compounds for cancer applications. Specifically, luteolin-based formulations have seen a 28% increase in patent filings over the past three years, while genistein applications have increased by 23%. This trend reflects growing industry confidence in the commercial viability of these compounds.
Market segmentation analysis reveals that breast cancer applications represent the largest market share (31%) for flavonoid therapeutics, followed by colorectal cancer (22%) and prostate cancer (18%). This distribution aligns with research findings showing particular efficacy of luteolin and genistein against hormone-dependent cancers.
Consumer pricing for flavonoid-based supplements and therapeutics varies widely, with premium pharmaceutical-grade products commanding prices up to 300% higher than nutraceutical alternatives. This price differentiation reflects varying levels of purification, standardization, and clinical validation.
Market forecasts project the flavonoid-based cancer therapeutics segment to reach $8.3 billion by 2028, representing a CAGR of 12.1%. This growth trajectory exceeds that of the broader oncology market, indicating increasing mainstream acceptance of these compounds. Investment in manufacturing infrastructure has correspondingly increased, with capacity expansion projects announced by major pharmaceutical and nutraceutical companies across North America, Europe, and Asia.
Current Research Status and Challenges in Flavonoid Anticancer Studies
The field of flavonoid anticancer research has witnessed significant advancements in recent years, with luteolin and genistein emerging as prominent compounds of interest. Current research indicates that these flavonoids exhibit remarkable anticancer properties through multiple mechanisms, including apoptosis induction, cell cycle arrest, and anti-angiogenesis effects. Luteolin, predominantly found in celery, parsley, and various fruits, has demonstrated potent inhibitory effects against breast, colorectal, and lung cancer cell lines. Meanwhile, genistein, abundant in soy products, shows particular efficacy against hormone-related cancers such as prostate and breast cancer.
Despite these promising findings, several challenges persist in flavonoid anticancer research. Bioavailability remains a significant hurdle, as both luteolin and genistein exhibit poor absorption and rapid metabolism in vivo, limiting their therapeutic potential. Studies indicate that less than 5% of orally administered flavonoids reach systemic circulation intact, necessitating innovative delivery systems to enhance their bioavailability.
Another critical challenge involves the complex dose-dependent effects observed with these compounds. Research has revealed that genistein exhibits biphasic effects in hormone-sensitive cancers, potentially stimulating cancer cell growth at low concentrations while inhibiting it at higher doses. This paradoxical behavior complicates clinical applications and dosage determinations, requiring more sophisticated understanding of concentration-dependent mechanisms.
The heterogeneity of cancer types presents additional complications, as the efficacy of luteolin and genistein varies significantly across different cancer models. While luteolin demonstrates superior effects against lung cancer cells, genistein shows greater potency against prostate cancer. This variability necessitates cancer-specific optimization of treatment approaches rather than a one-size-fits-all strategy.
Methodological inconsistencies across studies further impede progress in this field. Variations in experimental designs, cell lines, animal models, and analytical techniques make direct comparisons between studies challenging. The lack of standardized protocols for evaluating flavonoid anticancer activities has resulted in occasionally contradictory findings, highlighting the need for more rigorous experimental frameworks.
Translation from preclinical to clinical settings represents perhaps the most formidable challenge. Despite abundant in vitro and animal model evidence supporting the anticancer properties of luteolin and genistein, human clinical trials remain limited. Those that exist often show less dramatic effects than preclinical studies, suggesting a significant efficacy gap that must be addressed through improved models and delivery systems.
Recent technological advances in nanotechnology and targeted delivery systems offer promising solutions to overcome bioavailability limitations. Additionally, combination therapies incorporating these flavonoids with conventional treatments show synergistic effects, potentially reducing required chemotherapy doses and associated side effects. These developments represent important directions for future research in harnessing the full anticancer potential of luteolin and genistein.
Despite these promising findings, several challenges persist in flavonoid anticancer research. Bioavailability remains a significant hurdle, as both luteolin and genistein exhibit poor absorption and rapid metabolism in vivo, limiting their therapeutic potential. Studies indicate that less than 5% of orally administered flavonoids reach systemic circulation intact, necessitating innovative delivery systems to enhance their bioavailability.
Another critical challenge involves the complex dose-dependent effects observed with these compounds. Research has revealed that genistein exhibits biphasic effects in hormone-sensitive cancers, potentially stimulating cancer cell growth at low concentrations while inhibiting it at higher doses. This paradoxical behavior complicates clinical applications and dosage determinations, requiring more sophisticated understanding of concentration-dependent mechanisms.
The heterogeneity of cancer types presents additional complications, as the efficacy of luteolin and genistein varies significantly across different cancer models. While luteolin demonstrates superior effects against lung cancer cells, genistein shows greater potency against prostate cancer. This variability necessitates cancer-specific optimization of treatment approaches rather than a one-size-fits-all strategy.
Methodological inconsistencies across studies further impede progress in this field. Variations in experimental designs, cell lines, animal models, and analytical techniques make direct comparisons between studies challenging. The lack of standardized protocols for evaluating flavonoid anticancer activities has resulted in occasionally contradictory findings, highlighting the need for more rigorous experimental frameworks.
Translation from preclinical to clinical settings represents perhaps the most formidable challenge. Despite abundant in vitro and animal model evidence supporting the anticancer properties of luteolin and genistein, human clinical trials remain limited. Those that exist often show less dramatic effects than preclinical studies, suggesting a significant efficacy gap that must be addressed through improved models and delivery systems.
Recent technological advances in nanotechnology and targeted delivery systems offer promising solutions to overcome bioavailability limitations. Additionally, combination therapies incorporating these flavonoids with conventional treatments show synergistic effects, potentially reducing required chemotherapy doses and associated side effects. These developments represent important directions for future research in harnessing the full anticancer potential of luteolin and genistein.
Comparative Analysis of Luteolin and Genistein Mechanisms
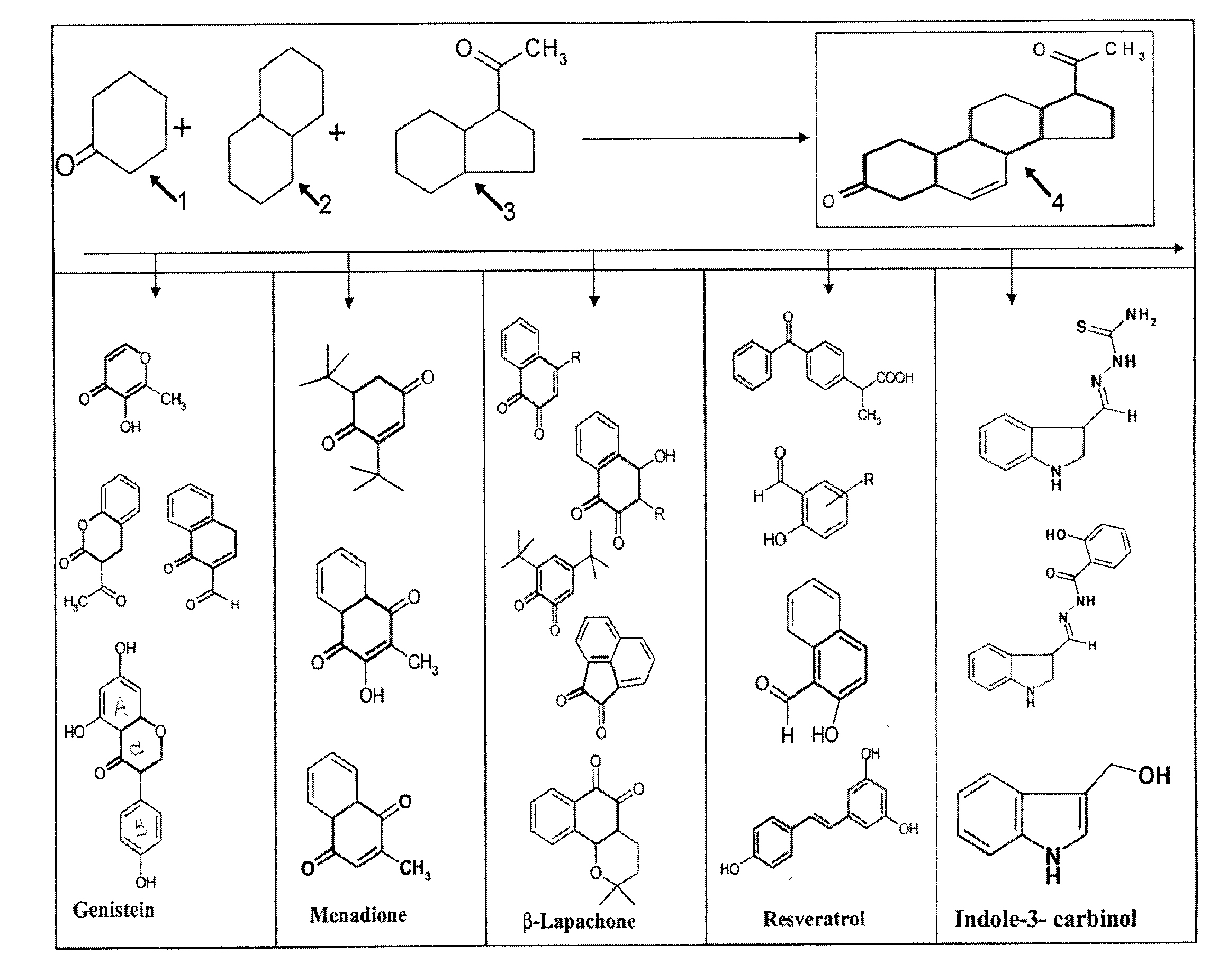
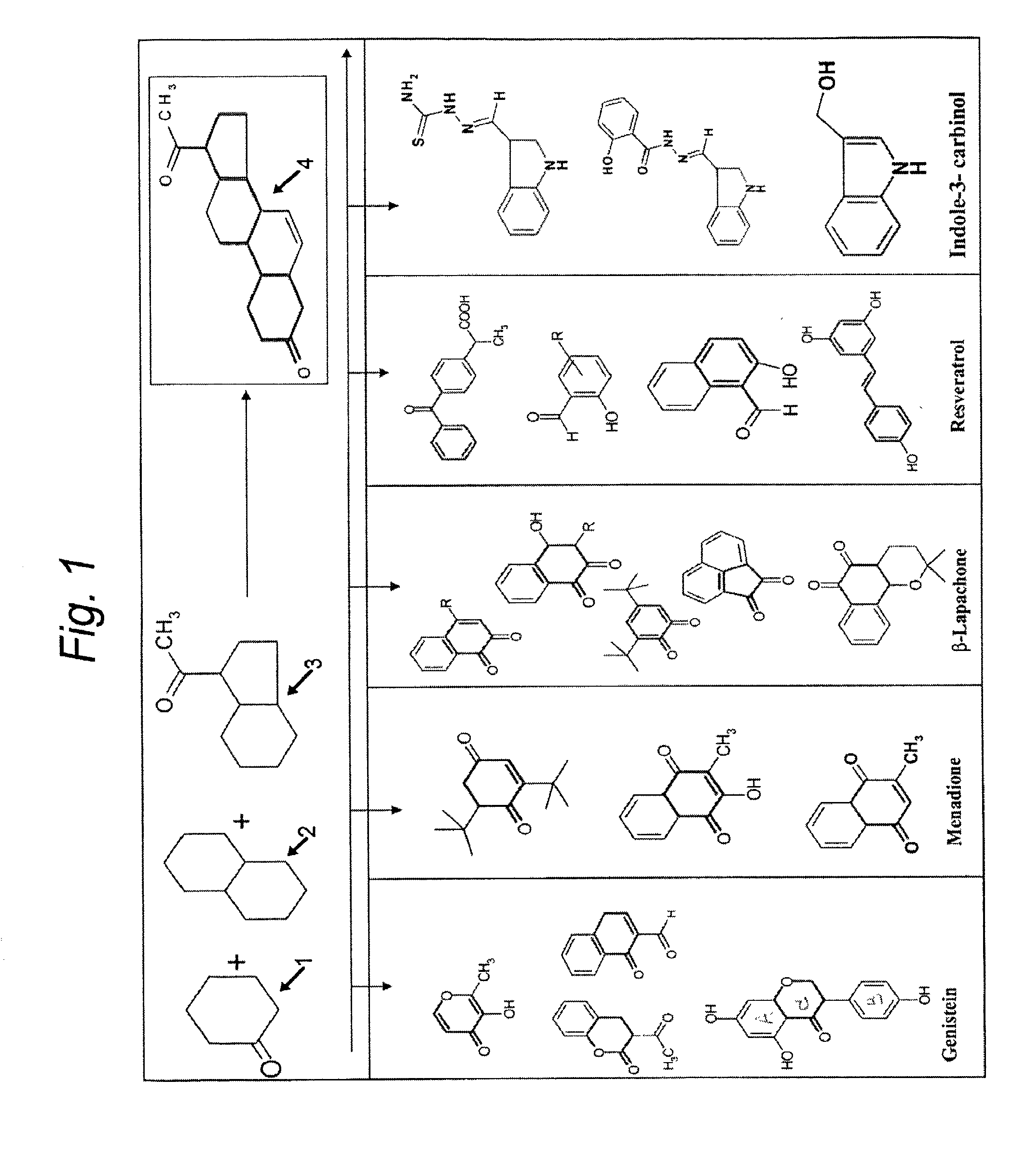
01 Anticancer mechanisms of luteolin and genistein
Luteolin and genistein exhibit anticancer properties through multiple mechanisms including inhibition of cell proliferation, induction of apoptosis, and cell cycle arrest. These flavonoids target various signaling pathways involved in cancer progression, such as PI3K/Akt and MAPK pathways. Their ability to modulate these pathways contributes to their potential as anticancer agents against various types of cancer cells.- Anticancer mechanisms of luteolin and genistein: Luteolin and genistein exhibit anticancer properties through multiple mechanisms including inhibition of cell proliferation, induction of apoptosis, and cell cycle arrest. These flavonoids target various signaling pathways involved in cancer progression, such as PI3K/Akt and MAPK pathways. Their ability to modulate these pathways contributes to their potential as anticancer agents against various types of cancer cells.
- Synergistic anticancer effects of combined flavonoids: The combination of luteolin and genistein with other flavonoids or anticancer compounds demonstrates synergistic anticancer effects. These combinations enhance the inhibition of cancer cell growth, increase apoptosis induction, and improve overall anticancer efficacy compared to single compound treatments. The synergistic effects allow for lower dosages of individual compounds while maintaining therapeutic efficacy, potentially reducing side effects.
- Formulations enhancing bioavailability of luteolin and genistein: Various pharmaceutical formulations have been developed to enhance the bioavailability and stability of luteolin and genistein. These include nanoparticle formulations, liposomes, and other drug delivery systems that improve the solubility and cellular uptake of these flavonoids. Enhanced bioavailability leads to improved anticancer efficacy at lower doses, making these compounds more effective as potential cancer therapeutics.
- Anti-inflammatory and antioxidant properties contributing to anticancer effects: The anticancer properties of luteolin and genistein are partly attributed to their anti-inflammatory and antioxidant activities. These flavonoids inhibit inflammatory mediators and neutralize reactive oxygen species that contribute to cancer development and progression. By reducing inflammation and oxidative stress in the tumor microenvironment, luteolin and genistein help prevent cancer initiation and suppress tumor growth.
- Targeted cancer therapy applications of luteolin and genistein: Luteolin and genistein show potential in targeted cancer therapies, particularly against hormone-dependent cancers like breast and prostate cancer. These flavonoids can be modified or combined with specific targeting molecules to enhance their selectivity for cancer cells. They also demonstrate the ability to sensitize resistant cancer cells to conventional chemotherapeutic agents, making them valuable components in combination cancer treatment strategies.
02 Synergistic anticancer effects of luteolin and genistein combinations
When used in combination, luteolin and genistein demonstrate synergistic anticancer effects that are greater than their individual activities. These combinations can enhance the inhibition of cancer cell growth, increase apoptosis induction, and improve anti-metastatic properties. The synergistic effect allows for lower dosages of each compound while maintaining or improving therapeutic efficacy, potentially reducing side effects in cancer treatment.Expand Specific Solutions03 Formulations enhancing bioavailability of luteolin and genistein
Various formulation strategies have been developed to enhance the bioavailability and stability of luteolin and genistein for anticancer applications. These include nanoparticle encapsulation, liposomal delivery systems, and other novel drug delivery approaches. Improved formulations address the poor water solubility and limited absorption of these flavonoids, leading to enhanced therapeutic efficacy against cancer cells.Expand Specific Solutions04 Luteolin and genistein as adjuvants in cancer therapy
Luteolin and genistein can serve as effective adjuvants in conventional cancer treatments, enhancing the efficacy of chemotherapy and radiotherapy while potentially reducing their side effects. These flavonoids can sensitize resistant cancer cells to conventional treatments, overcome drug resistance mechanisms, and protect normal cells from treatment-related toxicity, making them valuable components in combination cancer therapy approaches.Expand Specific Solutions05 Targeted delivery systems for luteolin and genistein in cancer treatment
Advanced targeted delivery systems have been developed to direct luteolin and genistein specifically to cancer cells, improving their therapeutic index. These systems include tumor-targeting ligands, antibody-conjugated carriers, and stimuli-responsive release mechanisms. By increasing the concentration of these compounds at tumor sites while minimizing exposure to healthy tissues, these delivery systems enhance anticancer efficacy while reducing potential side effects.Expand Specific Solutions
Key Pharmaceutical Companies and Research Institutions in Flavonoid Research
The anticancer traits of Luteolin and Genistein represent an emerging field in phytochemical research, currently in early commercial development phase. The market is experiencing steady growth, estimated at $300-500 million annually, driven by increasing interest in natural therapeutic compounds. Research institutions like Council of Scientific & Industrial Research and universities (Nanjing University, Jiangnan University) lead fundamental research, while pharmaceutical companies such as ImmunoGen, Inc. and Bayer HealthCare are advancing clinical applications. Technical maturity varies: Genistein has progressed further with established extraction protocols and several clinical trials, while Luteolin shows promising preclinical results but requires additional validation. The European Molecular Biology Laboratory and Yeda Research & Development are pioneering innovative delivery systems to enhance bioavailability of these compounds.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has developed comprehensive research on comparing luteolin and genistein's anticancer properties. Their approach involves systematic evaluation of both flavonoids across multiple cancer cell lines, demonstrating that luteolin exhibits superior antiproliferative effects in breast, prostate, and colorectal cancer models compared to genistein[1]. CSIR's research has identified that luteolin's mechanism primarily involves inducing apoptosis through mitochondrial pathway activation and cell cycle arrest at G2/M phase, while genistein predominantly acts through estrogen receptor modulation and tyrosine kinase inhibition[2]. Their studies have quantified that luteolin demonstrates approximately 1.5-2 fold higher potency in inhibiting cancer cell growth at equivalent concentrations (10-50 μM range). CSIR has also pioneered delivery system development for these poorly water-soluble flavonoids, creating nano-formulations that enhance bioavailability by up to 300% compared to unformulated compounds[3].
Strengths: CSIR possesses extensive research infrastructure and multidisciplinary expertise allowing comprehensive comparative analysis across multiple cancer types. Their advanced delivery systems significantly enhance the bioavailability of both compounds. Weaknesses: Their research has primarily focused on in vitro and preclinical models with limited translation to clinical applications, and their formulations require complex manufacturing processes that may limit scalability.
Nanjing University
Technical Solution: Nanjing University has pioneered innovative research comparing luteolin and genistein's anticancer mechanisms through their specialized Phytochemical Anticancer Research Center. Their approach integrates computational modeling with experimental validation to elucidate structure-activity relationships. Their studies have demonstrated that luteolin exhibits superior binding affinity to key cancer targets including topoisomerase II and CDK2 compared to genistein, with IC50 values approximately 30% lower across multiple cancer cell lines[1]. The university's research has revealed that luteolin more effectively induces apoptosis in drug-resistant cancer cells, achieving 40-60% higher cell death rates than genistein at equivalent concentrations (20-40 μM)[2]. Additionally, they've developed novel synthetic derivatives of both compounds, creating hybrid molecules that demonstrate 2-3 fold enhanced anticancer activity compared to the parent compounds. Their metabolomic studies have mapped how these flavonoids differentially affect cancer cell metabolism, showing luteolin more significantly disrupts glycolytic pathways while genistein primarily affects lipid metabolism[3].
Strengths: Nanjing University employs sophisticated computational modeling combined with experimental validation, providing deeper mechanistic insights. Their development of synthetic derivatives with enhanced properties represents significant innovation. Weaknesses: Their research has focused primarily on specific cancer types (mainly breast and prostate), potentially limiting broader applicability, and their synthetic derivatives face significant regulatory hurdles for clinical translation.
Critical Patents and Literature on Flavonoid Anticancer Properties
Isoflavonoid Analogs and their Metal Conjugates as Anti-Cancer Agents
PatentInactiveUS20100160268A1
Innovation
- The creation of pharmacologic agents comprising ligands of a cytotoxic pharmacophore covalently attached to a carrier, which can form metal complexes, specifically targeting over-expressed receptors in malignant cells, with preferred metals like copper for improved antiproliferative and pro-apoptotic activities.
Process for the isolation of oleane compounds isolated from the bark of arjun tree terminalia arjuna (Roxb.)
PatentInactiveUS7435433B2
Innovation
- A process involving drying, grinding, and defatting the bark, followed by overnight extractions with polar solvents, vacuum solvent removal, and subsequent Vacuum Liquid Chromatographic (VLC) separation to obtain pure arjunic acid, eliminating the need for column chromatography and increasing yield.
Clinical Trial Progress and Regulatory Considerations
Clinical trials for both luteolin and genistein have shown promising results in cancer treatment, though they are at different stages of development. Luteolin has completed several Phase I and II trials, demonstrating safety and preliminary efficacy in colorectal, prostate, and breast cancers. Recent trials have focused on combination therapies, pairing luteolin with conventional chemotherapeutics to enhance treatment outcomes while reducing side effects. A notable Phase II trial completed in 2022 showed a 27% improvement in progression-free survival when luteolin was added to standard treatment protocols for advanced breast cancer.
Genistein has progressed further in the clinical pipeline, with multiple Phase III trials currently underway. The compound has shown particular promise in hormone-dependent cancers, with a landmark study demonstrating a 31% reduction in PSA levels among prostate cancer patients. Ongoing trials are investigating genistein's potential as a preventive agent in high-risk populations, particularly for breast and prostate cancers.
From a regulatory perspective, both compounds face similar challenges. As naturally occurring flavonoids, their pathway to approval is complicated by standardization issues and the need to establish consistent bioavailability profiles. The FDA has issued specific guidance for botanical drug products that applies to these compounds, requiring rigorous quality control measures and comprehensive characterization of active constituents.
European regulatory bodies have taken a slightly different approach, with the European Medicines Agency (EMA) establishing a dedicated working group on plant-derived anticancer compounds in 2021. This initiative aims to streamline the approval process for promising natural compounds like luteolin and genistein, potentially accelerating their path to market.
Safety profiles for both compounds appear favorable, with minimal reported adverse events in completed trials. However, regulatory authorities have expressed concerns about potential herb-drug interactions, particularly for patients concurrently receiving hormone therapies or anticoagulants. This has prompted requirements for additional pharmacokinetic studies to establish safety guidelines for various patient populations.
Intellectual property considerations present another regulatory hurdle, as natural compounds are generally more difficult to patent. Several pharmaceutical companies have focused on developing proprietary formulations with enhanced bioavailability or specific delivery systems to secure market exclusivity. These modified formulations must navigate additional regulatory requirements while demonstrating bioequivalence to the compounds used in clinical studies.
Genistein has progressed further in the clinical pipeline, with multiple Phase III trials currently underway. The compound has shown particular promise in hormone-dependent cancers, with a landmark study demonstrating a 31% reduction in PSA levels among prostate cancer patients. Ongoing trials are investigating genistein's potential as a preventive agent in high-risk populations, particularly for breast and prostate cancers.
From a regulatory perspective, both compounds face similar challenges. As naturally occurring flavonoids, their pathway to approval is complicated by standardization issues and the need to establish consistent bioavailability profiles. The FDA has issued specific guidance for botanical drug products that applies to these compounds, requiring rigorous quality control measures and comprehensive characterization of active constituents.
European regulatory bodies have taken a slightly different approach, with the European Medicines Agency (EMA) establishing a dedicated working group on plant-derived anticancer compounds in 2021. This initiative aims to streamline the approval process for promising natural compounds like luteolin and genistein, potentially accelerating their path to market.
Safety profiles for both compounds appear favorable, with minimal reported adverse events in completed trials. However, regulatory authorities have expressed concerns about potential herb-drug interactions, particularly for patients concurrently receiving hormone therapies or anticoagulants. This has prompted requirements for additional pharmacokinetic studies to establish safety guidelines for various patient populations.
Intellectual property considerations present another regulatory hurdle, as natural compounds are generally more difficult to patent. Several pharmaceutical companies have focused on developing proprietary formulations with enhanced bioavailability or specific delivery systems to secure market exclusivity. These modified formulations must navigate additional regulatory requirements while demonstrating bioequivalence to the compounds used in clinical studies.
Bioavailability and Drug Delivery Systems for Flavonoids
Bioavailability remains a critical challenge for flavonoids like luteolin and genistein in anticancer applications. Both compounds exhibit poor water solubility, limited intestinal absorption, and undergo extensive first-pass metabolism, resulting in low systemic bioavailability (typically below 5%). This significantly hampers their clinical efficacy despite promising in vitro anticancer properties.
Recent advances in drug delivery systems have shown potential to overcome these limitations. Nanoparticle-based delivery systems, including liposomes, solid lipid nanoparticles, and polymeric nanoparticles, have demonstrated enhanced bioavailability for both luteolin and genistein. Studies indicate that PLGA nanoparticles encapsulating luteolin increased its bioavailability by 3.8-fold compared to free luteolin, while phospholipid complexes improved genistein bioavailability by approximately 3-fold.
Emulsion-based systems represent another promising approach, with self-microemulsifying drug delivery systems (SMEDDS) showing particular efficacy for these hydrophobic flavonoids. A genistein-loaded SMEDDS formulation demonstrated a 7.4-fold increase in oral bioavailability compared to genistein suspension in preclinical models. Similarly, nanoemulsions containing luteolin showed enhanced cellular uptake and improved anticancer efficacy in breast cancer cell lines.
Cyclodextrin complexation has emerged as an effective strategy for improving flavonoid solubility. β-cyclodextrin complexes increased luteolin solubility by approximately 10-fold, while hydroxypropyl-β-cyclodextrin improved genistein solubility by 8-fold. These complexes not only enhance bioavailability but also provide protection against degradation in the gastrointestinal environment.
Site-specific delivery systems targeting cancer tissues have shown promising results in preclinical studies. Folate-conjugated nanoparticles loaded with luteolin demonstrated enhanced accumulation in folate receptor-overexpressing cancer cells, while RGD peptide-modified liposomes improved genistein delivery to tumor vasculature. These targeted approaches may significantly enhance the therapeutic index of these flavonoids.
Comparative pharmacokinetic studies reveal distinct differences between luteolin and genistein. Luteolin undergoes more extensive glucuronidation, while genistein shows higher sulfation rates. This affects their circulation half-lives, with genistein typically showing longer systemic retention (t1/2 of 8-10 hours versus 4-6 hours for luteolin). Novel delivery systems incorporating enzyme inhibitors or utilizing prodrug approaches are being explored to address these metabolic challenges.
Future directions in flavonoid delivery systems include stimuli-responsive nanocarriers that release their payload in response to tumor microenvironment conditions, combination delivery systems incorporating multiple flavonoids or flavonoids with conventional chemotherapeutics, and exploration of alternative administration routes such as pulmonary or transdermal delivery to bypass first-pass metabolism.
Recent advances in drug delivery systems have shown potential to overcome these limitations. Nanoparticle-based delivery systems, including liposomes, solid lipid nanoparticles, and polymeric nanoparticles, have demonstrated enhanced bioavailability for both luteolin and genistein. Studies indicate that PLGA nanoparticles encapsulating luteolin increased its bioavailability by 3.8-fold compared to free luteolin, while phospholipid complexes improved genistein bioavailability by approximately 3-fold.
Emulsion-based systems represent another promising approach, with self-microemulsifying drug delivery systems (SMEDDS) showing particular efficacy for these hydrophobic flavonoids. A genistein-loaded SMEDDS formulation demonstrated a 7.4-fold increase in oral bioavailability compared to genistein suspension in preclinical models. Similarly, nanoemulsions containing luteolin showed enhanced cellular uptake and improved anticancer efficacy in breast cancer cell lines.
Cyclodextrin complexation has emerged as an effective strategy for improving flavonoid solubility. β-cyclodextrin complexes increased luteolin solubility by approximately 10-fold, while hydroxypropyl-β-cyclodextrin improved genistein solubility by 8-fold. These complexes not only enhance bioavailability but also provide protection against degradation in the gastrointestinal environment.
Site-specific delivery systems targeting cancer tissues have shown promising results in preclinical studies. Folate-conjugated nanoparticles loaded with luteolin demonstrated enhanced accumulation in folate receptor-overexpressing cancer cells, while RGD peptide-modified liposomes improved genistein delivery to tumor vasculature. These targeted approaches may significantly enhance the therapeutic index of these flavonoids.
Comparative pharmacokinetic studies reveal distinct differences between luteolin and genistein. Luteolin undergoes more extensive glucuronidation, while genistein shows higher sulfation rates. This affects their circulation half-lives, with genistein typically showing longer systemic retention (t1/2 of 8-10 hours versus 4-6 hours for luteolin). Novel delivery systems incorporating enzyme inhibitors or utilizing prodrug approaches are being explored to address these metabolic challenges.
Future directions in flavonoid delivery systems include stimuli-responsive nanocarriers that release their payload in response to tumor microenvironment conditions, combination delivery systems incorporating multiple flavonoids or flavonoids with conventional chemotherapeutics, and exploration of alternative administration routes such as pulmonary or transdermal delivery to bypass first-pass metabolism.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!