Comparing the Stability of Geometric Isomers in Diels-Alder Reactions
AUG 1, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isomer Stability in DA Reactions: Background and Objectives
The Diels-Alder reaction, discovered in 1928 by Otto Diels and Kurt Alder, has been a cornerstone in organic synthesis for nearly a century. This [4+2] cycloaddition reaction between a conjugated diene and a dienophile has revolutionized the field of synthetic chemistry, enabling the creation of complex cyclic compounds with remarkable stereoselectivity and atom economy.
In recent years, the focus on geometric isomers in Diels-Alder reactions has gained significant attention due to its profound impact on reaction outcomes and product stability. Geometric isomers, also known as cis-trans isomers, are compounds with the same molecular formula but different spatial arrangements of atoms. These isomers can exhibit vastly different physical and chemical properties, making their stability a crucial factor in Diels-Alder reactions.
The stability of geometric isomers in Diels-Alder reactions is influenced by various factors, including steric hindrance, electronic effects, and thermodynamic considerations. Understanding these factors is essential for predicting reaction outcomes and designing efficient synthetic strategies. As the field of organic synthesis continues to evolve, there is a growing need for a comprehensive analysis of isomer stability in Diels-Alder reactions.
The primary objective of this technical research report is to provide a thorough comparison of the stability of geometric isomers in Diels-Alder reactions. By examining the underlying principles and recent advancements in this area, we aim to shed light on the factors that govern isomer stability and their implications for synthetic applications.
This report will explore the historical context of Diels-Alder reactions and the role of geometric isomers in organic synthesis. We will delve into the fundamental concepts of stereochemistry and conformational analysis that underpin our understanding of isomer stability. Additionally, we will examine the latest research findings and experimental techniques used to investigate and quantify the stability of geometric isomers in Diels-Alder reactions.
By analyzing the current state of knowledge and identifying key research trends, this report seeks to provide valuable insights for researchers, synthetic chemists, and industry professionals working in the field of organic synthesis. The findings presented here will contribute to the ongoing efforts to optimize Diels-Alder reactions and expand their applications in various domains, including pharmaceuticals, materials science, and natural product synthesis.
In recent years, the focus on geometric isomers in Diels-Alder reactions has gained significant attention due to its profound impact on reaction outcomes and product stability. Geometric isomers, also known as cis-trans isomers, are compounds with the same molecular formula but different spatial arrangements of atoms. These isomers can exhibit vastly different physical and chemical properties, making their stability a crucial factor in Diels-Alder reactions.
The stability of geometric isomers in Diels-Alder reactions is influenced by various factors, including steric hindrance, electronic effects, and thermodynamic considerations. Understanding these factors is essential for predicting reaction outcomes and designing efficient synthetic strategies. As the field of organic synthesis continues to evolve, there is a growing need for a comprehensive analysis of isomer stability in Diels-Alder reactions.
The primary objective of this technical research report is to provide a thorough comparison of the stability of geometric isomers in Diels-Alder reactions. By examining the underlying principles and recent advancements in this area, we aim to shed light on the factors that govern isomer stability and their implications for synthetic applications.
This report will explore the historical context of Diels-Alder reactions and the role of geometric isomers in organic synthesis. We will delve into the fundamental concepts of stereochemistry and conformational analysis that underpin our understanding of isomer stability. Additionally, we will examine the latest research findings and experimental techniques used to investigate and quantify the stability of geometric isomers in Diels-Alder reactions.
By analyzing the current state of knowledge and identifying key research trends, this report seeks to provide valuable insights for researchers, synthetic chemists, and industry professionals working in the field of organic synthesis. The findings presented here will contribute to the ongoing efforts to optimize Diels-Alder reactions and expand their applications in various domains, including pharmaceuticals, materials science, and natural product synthesis.
Applications and Market Demand for DA Reaction Products
The Diels-Alder (DA) reaction has become a cornerstone in organic synthesis, with its products finding extensive applications across various industries. The market demand for DA reaction products is driven by their versatility and the unique structural features they offer.
In the pharmaceutical industry, DA reaction products serve as key intermediates in the synthesis of complex drug molecules. The ability to create specific stereoisomers through DA reactions is particularly valuable in drug development, where the spatial arrangement of atoms can significantly impact a compound's biological activity. This has led to increased demand for DA-derived structures in the design of new therapeutic agents, especially in areas such as cancer treatment and neurological disorders.
The polymer industry also heavily relies on DA reaction products. The reversible nature of some DA reactions has opened up possibilities for self-healing materials and recyclable polymers. These advanced materials are gaining traction in automotive, aerospace, and consumer electronics sectors, where durability and sustainability are paramount. The market for such smart materials is projected to grow substantially in the coming years, driven by increasing environmental concerns and stringent regulations on waste reduction.
In the field of agrochemicals, DA reaction products contribute to the development of more effective and environmentally friendly pesticides and herbicides. The ability to fine-tune molecular structures through DA reactions allows for the creation of compounds with improved target specificity and reduced environmental impact. This aligns with the growing demand for sustainable agricultural practices and has led to increased investment in DA-based agrochemical research.
The flavor and fragrance industry also benefits from DA reaction products. The cyclic structures formed in these reactions often possess unique olfactory properties, making them valuable in the creation of novel scents and flavors. As consumer preferences evolve towards more complex and natural-seeming aromas, the demand for DA-derived compounds in this sector continues to rise.
In the materials science sector, DA reactions play a crucial role in the development of advanced functional materials. From organic semiconductors for flexible electronics to novel catalysts for green chemistry applications, the structural diversity offered by DA products is driving innovation. The growing focus on nanotechnology and smart materials is expected to further boost the demand for DA-derived structures in this field.
The market for DA reaction products is also influenced by the broader trend towards green chemistry. The atom economy and potential for solvent-free conditions in many DA reactions align well with sustainability goals. This has led to increased interest from industries seeking to reduce their environmental footprint while maintaining product performance.
In the pharmaceutical industry, DA reaction products serve as key intermediates in the synthesis of complex drug molecules. The ability to create specific stereoisomers through DA reactions is particularly valuable in drug development, where the spatial arrangement of atoms can significantly impact a compound's biological activity. This has led to increased demand for DA-derived structures in the design of new therapeutic agents, especially in areas such as cancer treatment and neurological disorders.
The polymer industry also heavily relies on DA reaction products. The reversible nature of some DA reactions has opened up possibilities for self-healing materials and recyclable polymers. These advanced materials are gaining traction in automotive, aerospace, and consumer electronics sectors, where durability and sustainability are paramount. The market for such smart materials is projected to grow substantially in the coming years, driven by increasing environmental concerns and stringent regulations on waste reduction.
In the field of agrochemicals, DA reaction products contribute to the development of more effective and environmentally friendly pesticides and herbicides. The ability to fine-tune molecular structures through DA reactions allows for the creation of compounds with improved target specificity and reduced environmental impact. This aligns with the growing demand for sustainable agricultural practices and has led to increased investment in DA-based agrochemical research.
The flavor and fragrance industry also benefits from DA reaction products. The cyclic structures formed in these reactions often possess unique olfactory properties, making them valuable in the creation of novel scents and flavors. As consumer preferences evolve towards more complex and natural-seeming aromas, the demand for DA-derived compounds in this sector continues to rise.
In the materials science sector, DA reactions play a crucial role in the development of advanced functional materials. From organic semiconductors for flexible electronics to novel catalysts for green chemistry applications, the structural diversity offered by DA products is driving innovation. The growing focus on nanotechnology and smart materials is expected to further boost the demand for DA-derived structures in this field.
The market for DA reaction products is also influenced by the broader trend towards green chemistry. The atom economy and potential for solvent-free conditions in many DA reactions align well with sustainability goals. This has led to increased interest from industries seeking to reduce their environmental footprint while maintaining product performance.
Current Challenges in Geometric Isomer Stability
The stability of geometric isomers in Diels-Alder reactions presents several significant challenges in contemporary organic chemistry. One of the primary issues is the difficulty in predicting and controlling the stereochemistry of the products. The reaction's outcome often depends on subtle differences in the spatial arrangement of atoms, making it challenging to anticipate which isomer will predominate.
Another challenge lies in the energetic differences between geometric isomers. These differences can be quite small, sometimes on the order of a few kilocalories per mole, making it difficult to reliably separate or selectively produce one isomer over another. This becomes particularly problematic when attempting to synthesize specific compounds for pharmaceutical or materials science applications.
The influence of reaction conditions on isomer stability adds another layer of complexity. Factors such as temperature, pressure, and solvent can significantly affect the relative stability of geometric isomers. Researchers often struggle to find optimal conditions that favor the formation of the desired isomer while minimizing unwanted side products.
Furthermore, the dynamic nature of some geometric isomers poses challenges in their isolation and characterization. Some isomers may interconvert rapidly under certain conditions, making it difficult to study their individual properties or to utilize them in subsequent reactions.
The presence of multiple functional groups in complex molecules can also complicate the analysis of geometric isomer stability. Interactions between different parts of the molecule can lead to unexpected stabilization or destabilization effects, making it challenging to apply general principles of isomer stability across diverse molecular structures.
Another significant challenge is the development of reliable computational methods for predicting isomer stability. While quantum mechanical calculations have improved dramatically in recent years, accurately modeling the subtle energetic differences between geometric isomers, especially in complex reaction environments, remains a formidable task.
Lastly, the challenge of scalability in the production of specific geometric isomers is a major concern for industrial applications. Methods that work well on a laboratory scale may not be feasible or economically viable when scaled up to industrial production levels, necessitating the development of new synthetic strategies and separation techniques.
Another challenge lies in the energetic differences between geometric isomers. These differences can be quite small, sometimes on the order of a few kilocalories per mole, making it difficult to reliably separate or selectively produce one isomer over another. This becomes particularly problematic when attempting to synthesize specific compounds for pharmaceutical or materials science applications.
The influence of reaction conditions on isomer stability adds another layer of complexity. Factors such as temperature, pressure, and solvent can significantly affect the relative stability of geometric isomers. Researchers often struggle to find optimal conditions that favor the formation of the desired isomer while minimizing unwanted side products.
Furthermore, the dynamic nature of some geometric isomers poses challenges in their isolation and characterization. Some isomers may interconvert rapidly under certain conditions, making it difficult to study their individual properties or to utilize them in subsequent reactions.
The presence of multiple functional groups in complex molecules can also complicate the analysis of geometric isomer stability. Interactions between different parts of the molecule can lead to unexpected stabilization or destabilization effects, making it challenging to apply general principles of isomer stability across diverse molecular structures.
Another significant challenge is the development of reliable computational methods for predicting isomer stability. While quantum mechanical calculations have improved dramatically in recent years, accurately modeling the subtle energetic differences between geometric isomers, especially in complex reaction environments, remains a formidable task.
Lastly, the challenge of scalability in the production of specific geometric isomers is a major concern for industrial applications. Methods that work well on a laboratory scale may not be feasible or economically viable when scaled up to industrial production levels, necessitating the development of new synthetic strategies and separation techniques.
Existing Methods for Isomer Stability Analysis
01 Stereochemistry in Diels-Alder reactions
Diels-Alder reactions often result in the formation of geometric isomers. The stereochemistry of the products is influenced by the orientation of the reactants and the reaction conditions. Understanding and controlling the stereochemistry is crucial for obtaining desired isomers with specific properties and stability.- Stereochemistry in Diels-Alder reactions: Diels-Alder reactions often result in the formation of geometric isomers. The stereochemistry of the products is influenced by the orientation of the reactants during the cycloaddition process. The stability of these isomers can vary depending on factors such as steric hindrance and electronic effects.
- Endo vs. exo selectivity: In Diels-Alder reactions, the formation of endo and exo products is common. The endo product is often kinetically favored due to secondary orbital interactions, while the exo product may be thermodynamically more stable. The relative stability of these isomers can affect the overall reaction outcome and product distribution.
- Influence of substituents on isomer stability: The presence and nature of substituents on the diene and dienophile can significantly impact the stability of geometric isomers formed in Diels-Alder reactions. Bulky substituents may favor certain conformations, while electronic effects can influence the overall energetics of the isomers.
- Temperature effects on isomer distribution: The temperature at which Diels-Alder reactions are conducted can affect the distribution of geometric isomers. Higher temperatures may favor thermodynamically stable products, while lower temperatures might preserve kinetically favored isomers. This temperature dependence can be exploited to control the stereochemical outcome of the reaction.
- Isomer interconversion and equilibrium: In some cases, geometric isomers formed in Diels-Alder reactions may undergo interconversion. The rate and extent of this interconversion depend on the energy barriers between isomers and the reaction conditions. Understanding these equilibrium processes is crucial for predicting and controlling the final isomer distribution and overall stability of the products.
02 Stability of endo and exo isomers
In Diels-Alder reactions, endo and exo isomers can be formed. The stability of these isomers can vary, with the endo isomer often being kinetically favored but thermodynamically less stable than the exo isomer. Factors such as temperature and solvent can affect the ratio of endo to exo products and their relative stabilities.Expand Specific Solutions03 Influence of substituents on isomer stability
The presence and nature of substituents on the diene and dienophile can significantly impact the stability of the resulting geometric isomers. Electron-withdrawing or electron-donating groups can affect the electronic distribution and, consequently, the stability of the cycloadduct isomers.Expand Specific Solutions04 Catalysts and reaction conditions affecting isomer formation
Various catalysts and reaction conditions can be employed to influence the formation and stability of geometric isomers in Diels-Alder reactions. These factors can alter the reaction kinetics and thermodynamics, leading to preferential formation of specific isomers or affecting their stability post-reaction.Expand Specific Solutions05 Analytical methods for isomer characterization
Characterization of geometric isomers resulting from Diels-Alder reactions is crucial for understanding their stability and properties. Various analytical techniques, such as NMR spectroscopy, X-ray crystallography, and chromatographic methods, can be employed to identify and quantify the isomers formed and assess their relative stabilities.Expand Specific Solutions
Key Research Groups and Industrial Players
The field of geometric isomer stability in Diels-Alder reactions is in a mature stage of development, with established principles and ongoing research. The market size is relatively small, primarily focused on academic and industrial research applications. Technologically, the field is well-developed, with companies like Bristol Myers Squibb, Novartis, and Eisai R&D Management leading in pharmaceutical applications. Academic institutions such as New York University and the German Cancer Research Center contribute significantly to fundamental research. While not a large commercial market, this area remains crucial for advancing organic synthesis methodologies and drug discovery processes.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed a comprehensive approach to studying the stability of geometric isomers in Diels-Alder reactions, focusing on their application in drug discovery. Their method combines advanced spectroscopic techniques, including NMR and X-ray crystallography, with computational modeling to elucidate the structural factors influencing isomer stability[2]. They have also implemented a novel microfluidic platform for rapid screening of reaction conditions, allowing for real-time monitoring of isomer formation and interconversion[4]. This integrated approach has led to the discovery of several promising drug candidates with improved pharmacological properties.
Strengths: Comprehensive analytical techniques, innovative microfluidic screening platform, and direct application to drug discovery. Weaknesses: Potential scalability issues for large-scale synthesis and limited applicability to non-pharmaceutical compounds.
Novartis AG
Technical Solution: Novartis AG has developed advanced computational methods to predict and analyze the stability of geometric isomers in Diels-Alder reactions. Their approach combines quantum mechanical calculations with machine learning algorithms to accurately model the energetics and kinetics of these reactions[1]. This method allows for rapid screening of potential reactants and products, enabling the design of more efficient synthetic routes. Novartis has also implemented a high-throughput experimental platform to validate and refine their computational predictions, significantly accelerating the discovery of novel compounds with desired properties[3].
Strengths: Cutting-edge computational methods, integration of machine learning, and high-throughput experimental validation. Weaknesses: High computational costs and potential limitations in accurately modeling complex reaction environments.
Innovative Approaches to Stability Prediction
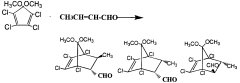
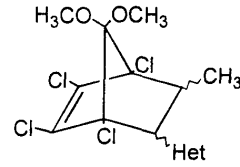
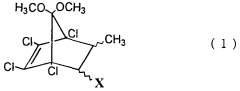
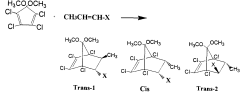
Novel bicycloheptene derivatives and process for the preparation thereof
PatentWO2000066525A1
Innovation
- A novel method involving a Diels-Alder reaction between 2-butenoic acid and 5,5-dimethoxy-1,2,3,4-tetrachlorocyclopentadiene, conducted without a solvent at controlled temperatures, followed by recrystallization to isolate the desired isomers, which enhances the production yield and separation of trans-1 and cis isomers.
Diels-Alder reaction in ionic liquid medium
PatentInactiveFR2757850B1
Innovation
- The Diels-Alder reaction is conducted in a liquid organic-inorganic salt medium, specifically using quaternary ammonium and/or quaternary phosphonium salts with non-coordinating anions, which accelerates the reaction and enhances stereoselectivity, allowing easy separation of reagents and products.
Computational Tools for Isomer Stability Assessment
Computational tools have become indispensable in assessing the stability of geometric isomers in Diels-Alder reactions. These tools provide researchers with powerful means to predict and analyze isomer stability without the need for extensive laboratory experiments. One of the most widely used computational methods is density functional theory (DFT), which offers a balance between accuracy and computational efficiency.
DFT calculations can provide valuable insights into the electronic structure and energetics of different isomers. By comparing the calculated ground state energies of various geometric isomers, researchers can predict their relative stabilities. Additionally, DFT can be used to compute activation energies and reaction barriers, which are crucial for understanding the kinetics of Diels-Alder reactions and the formation of specific isomers.
Another important computational tool is molecular dynamics (MD) simulations. MD simulations allow researchers to study the dynamic behavior of molecules over time, providing information about conformational changes and the stability of different isomers under various conditions. This approach is particularly useful for investigating the effects of temperature and solvent on isomer stability in Diels-Alder reactions.
Quantum chemical calculations, such as those based on coupled cluster theory or Møller-Plesset perturbation theory, offer higher accuracy for small to medium-sized systems. These methods can provide benchmark results for validating less computationally expensive approaches and are particularly useful for studying subtle differences in isomer stability.
Machine learning algorithms have also emerged as powerful tools for predicting isomer stability. By training on large datasets of known Diels-Alder reactions and isomer stabilities, these algorithms can quickly estimate the stability of new isomers with reasonable accuracy. This approach is especially valuable for high-throughput screening of potential reaction products.
Computational tools also include specialized software packages designed for analyzing and visualizing molecular structures and properties. These packages often integrate various computational methods and provide user-friendly interfaces for researchers to set up calculations, analyze results, and generate visual representations of molecular orbitals, electron densities, and other relevant properties.
In conclusion, the array of computational tools available for assessing isomer stability in Diels-Alder reactions has greatly enhanced our ability to predict and understand these complex chemical processes. By combining different computational approaches, researchers can gain a comprehensive understanding of isomer stability and guide experimental efforts in the synthesis of desired products.
DFT calculations can provide valuable insights into the electronic structure and energetics of different isomers. By comparing the calculated ground state energies of various geometric isomers, researchers can predict their relative stabilities. Additionally, DFT can be used to compute activation energies and reaction barriers, which are crucial for understanding the kinetics of Diels-Alder reactions and the formation of specific isomers.
Another important computational tool is molecular dynamics (MD) simulations. MD simulations allow researchers to study the dynamic behavior of molecules over time, providing information about conformational changes and the stability of different isomers under various conditions. This approach is particularly useful for investigating the effects of temperature and solvent on isomer stability in Diels-Alder reactions.
Quantum chemical calculations, such as those based on coupled cluster theory or Møller-Plesset perturbation theory, offer higher accuracy for small to medium-sized systems. These methods can provide benchmark results for validating less computationally expensive approaches and are particularly useful for studying subtle differences in isomer stability.
Machine learning algorithms have also emerged as powerful tools for predicting isomer stability. By training on large datasets of known Diels-Alder reactions and isomer stabilities, these algorithms can quickly estimate the stability of new isomers with reasonable accuracy. This approach is especially valuable for high-throughput screening of potential reaction products.
Computational tools also include specialized software packages designed for analyzing and visualizing molecular structures and properties. These packages often integrate various computational methods and provide user-friendly interfaces for researchers to set up calculations, analyze results, and generate visual representations of molecular orbitals, electron densities, and other relevant properties.
In conclusion, the array of computational tools available for assessing isomer stability in Diels-Alder reactions has greatly enhanced our ability to predict and understand these complex chemical processes. By combining different computational approaches, researchers can gain a comprehensive understanding of isomer stability and guide experimental efforts in the synthesis of desired products.
Environmental Factors Affecting Isomer Stability
Environmental factors play a crucial role in determining the stability of geometric isomers in Diels-Alder reactions. Temperature is one of the most significant factors affecting isomer stability. Higher temperatures generally favor the formation of thermodynamically stable products, while lower temperatures may lead to kinetically controlled reactions, resulting in different isomer distributions.
Solvent polarity is another critical environmental factor influencing isomer stability. Polar solvents can stabilize charged or polar transition states, potentially altering the reaction pathway and favoring specific isomers. Conversely, non-polar solvents may promote different stereochemical outcomes due to their inability to stabilize polar intermediates.
Pressure is an often-overlooked environmental factor that can impact isomer stability in Diels-Alder reactions. High-pressure conditions can accelerate reaction rates and potentially favor the formation of more compact transition states, leading to different isomer distributions compared to reactions conducted at atmospheric pressure.
The presence of catalysts or Lewis acids in the reaction environment can significantly influence isomer stability. These additives can coordinate with reactants, altering their electronic properties and potentially favoring the formation of specific isomers. Catalysts can also lower activation energies, allowing reactions to proceed under milder conditions and potentially affecting isomer distributions.
pH is another environmental factor that can affect isomer stability, particularly in aqueous systems. Changes in pH can alter the protonation state of reactants or intermediates, potentially leading to different reaction pathways and isomer distributions. This factor is especially relevant in biological systems where Diels-Alder reactions may occur under physiological conditions.
Light exposure can also impact isomer stability, particularly for photosensitive compounds. Certain isomers may undergo photoisomerization or photodegradation, altering the overall isomer distribution in the reaction mixture. This factor is especially important when considering the long-term stability of reaction products.
The presence of stabilizing or destabilizing agents in the reaction environment can significantly influence isomer stability. For example, radical scavengers may prevent unwanted side reactions that could lead to isomer interconversion or degradation. Conversely, oxidizing or reducing agents may promote isomerization processes, affecting the final product distribution.
Solvent polarity is another critical environmental factor influencing isomer stability. Polar solvents can stabilize charged or polar transition states, potentially altering the reaction pathway and favoring specific isomers. Conversely, non-polar solvents may promote different stereochemical outcomes due to their inability to stabilize polar intermediates.
Pressure is an often-overlooked environmental factor that can impact isomer stability in Diels-Alder reactions. High-pressure conditions can accelerate reaction rates and potentially favor the formation of more compact transition states, leading to different isomer distributions compared to reactions conducted at atmospheric pressure.
The presence of catalysts or Lewis acids in the reaction environment can significantly influence isomer stability. These additives can coordinate with reactants, altering their electronic properties and potentially favoring the formation of specific isomers. Catalysts can also lower activation energies, allowing reactions to proceed under milder conditions and potentially affecting isomer distributions.
pH is another environmental factor that can affect isomer stability, particularly in aqueous systems. Changes in pH can alter the protonation state of reactants or intermediates, potentially leading to different reaction pathways and isomer distributions. This factor is especially relevant in biological systems where Diels-Alder reactions may occur under physiological conditions.
Light exposure can also impact isomer stability, particularly for photosensitive compounds. Certain isomers may undergo photoisomerization or photodegradation, altering the overall isomer distribution in the reaction mixture. This factor is especially important when considering the long-term stability of reaction products.
The presence of stabilizing or destabilizing agents in the reaction environment can significantly influence isomer stability. For example, radical scavengers may prevent unwanted side reactions that could lead to isomer interconversion or degradation. Conversely, oxidizing or reducing agents may promote isomerization processes, affecting the final product distribution.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!