Cori Cycle Dysfunction: Diagnostic Tests And Clinical Thresholds
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cori Cycle Overview
The Cori cycle, also known as the glucose-lactate cycle, is a metabolic pathway that plays a crucial role in maintaining glucose homeostasis in the body. Named after its discoverers, Carl and Gerty Cori, this cycle describes the reciprocal relationship between muscle tissue and the liver in glucose metabolism. The cycle involves the conversion of glucose to lactate in muscle cells during anaerobic conditions, followed by the transportation of lactate to the liver, where it is converted back to glucose.
The cycle begins in muscle tissue during intense physical activity or when oxygen supply is limited. Under these conditions, glucose is metabolized through anaerobic glycolysis, resulting in the production of lactate. This lactate is then released into the bloodstream and transported to the liver. In the liver, lactate is converted back to glucose through the process of gluconeogenesis. The newly synthesized glucose can then be released into the bloodstream to maintain blood glucose levels or stored as glycogen for future use.
The Cori cycle serves several important functions in the body. Firstly, it provides a mechanism for the removal of lactate from muscle tissue, preventing the buildup of lactic acid and associated muscle fatigue. Secondly, it allows for the recycling of glucose, ensuring a continuous supply of this essential energy source to various tissues, particularly the brain and red blood cells, which rely heavily on glucose for their energy needs.
The efficiency of the Cori cycle is regulated by various hormones and enzymes. Insulin, for example, inhibits gluconeogenesis in the liver, while glucagon and epinephrine stimulate this process. The cycle is also influenced by the availability of oxygen and the energy state of the cells involved. During prolonged exercise or fasting, the cycle's activity increases to maintain blood glucose levels and provide energy to working muscles.
Dysfunction in the Cori cycle can lead to various metabolic disorders. Impairments in lactate metabolism or gluconeogenesis can result in lactic acidosis, a condition characterized by the accumulation of lactate in the blood. This can occur in situations such as severe exercise, certain medications, or underlying medical conditions that affect the liver or muscles. Understanding the Cori cycle and its potential dysfunctions is crucial for diagnosing and managing metabolic disorders related to glucose and lactate metabolism.
In the context of diagnostic tests and clinical thresholds for Cori cycle dysfunction, healthcare professionals may employ various methods to assess the cycle's functionality. These may include measuring blood lactate levels, evaluating liver function tests, and assessing glucose metabolism through glucose tolerance tests or continuous glucose monitoring. The interpretation of these tests, along with the establishment of appropriate clinical thresholds, is essential for identifying and managing disorders related to the Cori cycle.
The cycle begins in muscle tissue during intense physical activity or when oxygen supply is limited. Under these conditions, glucose is metabolized through anaerobic glycolysis, resulting in the production of lactate. This lactate is then released into the bloodstream and transported to the liver. In the liver, lactate is converted back to glucose through the process of gluconeogenesis. The newly synthesized glucose can then be released into the bloodstream to maintain blood glucose levels or stored as glycogen for future use.
The Cori cycle serves several important functions in the body. Firstly, it provides a mechanism for the removal of lactate from muscle tissue, preventing the buildup of lactic acid and associated muscle fatigue. Secondly, it allows for the recycling of glucose, ensuring a continuous supply of this essential energy source to various tissues, particularly the brain and red blood cells, which rely heavily on glucose for their energy needs.
The efficiency of the Cori cycle is regulated by various hormones and enzymes. Insulin, for example, inhibits gluconeogenesis in the liver, while glucagon and epinephrine stimulate this process. The cycle is also influenced by the availability of oxygen and the energy state of the cells involved. During prolonged exercise or fasting, the cycle's activity increases to maintain blood glucose levels and provide energy to working muscles.
Dysfunction in the Cori cycle can lead to various metabolic disorders. Impairments in lactate metabolism or gluconeogenesis can result in lactic acidosis, a condition characterized by the accumulation of lactate in the blood. This can occur in situations such as severe exercise, certain medications, or underlying medical conditions that affect the liver or muscles. Understanding the Cori cycle and its potential dysfunctions is crucial for diagnosing and managing metabolic disorders related to glucose and lactate metabolism.
In the context of diagnostic tests and clinical thresholds for Cori cycle dysfunction, healthcare professionals may employ various methods to assess the cycle's functionality. These may include measuring blood lactate levels, evaluating liver function tests, and assessing glucose metabolism through glucose tolerance tests or continuous glucose monitoring. The interpretation of these tests, along with the establishment of appropriate clinical thresholds, is essential for identifying and managing disorders related to the Cori cycle.
Market Need Analysis
The market demand for diagnostic tests and clinical thresholds related to Cori Cycle dysfunction has been steadily increasing in recent years. This growth is primarily driven by the rising prevalence of metabolic disorders, liver diseases, and diabetes, which are closely associated with disruptions in the Cori Cycle. Healthcare providers and researchers are increasingly recognizing the importance of early detection and accurate diagnosis of Cori Cycle abnormalities to improve patient outcomes and develop targeted therapies.
The global market for metabolic testing, which includes diagnostic tools for Cori Cycle dysfunction, is experiencing significant expansion. This growth is fueled by the increasing incidence of obesity and related metabolic disorders worldwide. As these conditions often lead to alterations in glucose metabolism and liver function, the demand for precise diagnostic tools to assess Cori Cycle function has surged.
In the clinical setting, there is a growing need for standardized and reliable diagnostic tests that can accurately identify Cori Cycle dysfunction. Healthcare professionals are seeking more sensitive and specific markers that can detect subtle alterations in glucose-lactate metabolism, which is crucial for early intervention and personalized treatment strategies. This demand is particularly pronounced in endocrinology, hepatology, and critical care units, where metabolic imbalances are common and can have severe consequences if left undiagnosed.
The pharmaceutical and biotechnology industries are also driving market demand for Cori Cycle-related diagnostics. As drug development efforts focus on targeting metabolic pathways, there is an increased need for diagnostic tools that can serve as companion diagnostics or biomarkers for drug efficacy and safety monitoring. This trend is expected to continue as precision medicine approaches gain traction in metabolic disorder management.
Moreover, the growing emphasis on preventive healthcare and wellness has created a new market segment for consumer-oriented metabolic health monitoring. This includes wearable devices and at-home testing kits that can provide insights into glucose metabolism and overall metabolic health, indirectly assessing Cori Cycle function. While these consumer products may not offer the same level of accuracy as clinical tests, they contribute to the overall market growth and awareness of metabolic health.
The aging population in many developed countries is another factor driving the demand for Cori Cycle dysfunction diagnostics. As age-related metabolic changes become more prevalent, there is an increased need for diagnostic tools that can differentiate between normal aging processes and pathological metabolic alterations. This demographic shift is expected to sustain long-term market growth for metabolic testing and monitoring solutions.
In conclusion, the market demand for diagnostic tests and clinical thresholds related to Cori Cycle dysfunction is robust and multifaceted. It spans across various healthcare sectors, from clinical diagnostics to drug development and consumer health. As our understanding of metabolic disorders advances and the global burden of these conditions continues to rise, the market for Cori Cycle-related diagnostics is poised for continued growth and innovation.
The global market for metabolic testing, which includes diagnostic tools for Cori Cycle dysfunction, is experiencing significant expansion. This growth is fueled by the increasing incidence of obesity and related metabolic disorders worldwide. As these conditions often lead to alterations in glucose metabolism and liver function, the demand for precise diagnostic tools to assess Cori Cycle function has surged.
In the clinical setting, there is a growing need for standardized and reliable diagnostic tests that can accurately identify Cori Cycle dysfunction. Healthcare professionals are seeking more sensitive and specific markers that can detect subtle alterations in glucose-lactate metabolism, which is crucial for early intervention and personalized treatment strategies. This demand is particularly pronounced in endocrinology, hepatology, and critical care units, where metabolic imbalances are common and can have severe consequences if left undiagnosed.
The pharmaceutical and biotechnology industries are also driving market demand for Cori Cycle-related diagnostics. As drug development efforts focus on targeting metabolic pathways, there is an increased need for diagnostic tools that can serve as companion diagnostics or biomarkers for drug efficacy and safety monitoring. This trend is expected to continue as precision medicine approaches gain traction in metabolic disorder management.
Moreover, the growing emphasis on preventive healthcare and wellness has created a new market segment for consumer-oriented metabolic health monitoring. This includes wearable devices and at-home testing kits that can provide insights into glucose metabolism and overall metabolic health, indirectly assessing Cori Cycle function. While these consumer products may not offer the same level of accuracy as clinical tests, they contribute to the overall market growth and awareness of metabolic health.
The aging population in many developed countries is another factor driving the demand for Cori Cycle dysfunction diagnostics. As age-related metabolic changes become more prevalent, there is an increased need for diagnostic tools that can differentiate between normal aging processes and pathological metabolic alterations. This demographic shift is expected to sustain long-term market growth for metabolic testing and monitoring solutions.
In conclusion, the market demand for diagnostic tests and clinical thresholds related to Cori Cycle dysfunction is robust and multifaceted. It spans across various healthcare sectors, from clinical diagnostics to drug development and consumer health. As our understanding of metabolic disorders advances and the global burden of these conditions continues to rise, the market for Cori Cycle-related diagnostics is poised for continued growth and innovation.
Current Diagnostic Challenges
The diagnosis of Cori Cycle dysfunction presents several challenges in current clinical practice. One of the primary difficulties lies in the lack of standardized diagnostic criteria and thresholds. The Cori Cycle, a metabolic pathway involving glucose and lactate exchange between the liver and peripheral tissues, is complex and influenced by various physiological factors, making it challenging to establish universally applicable diagnostic parameters.
Current diagnostic methods often rely on indirect measurements and biomarkers, which may not always accurately reflect the specific dysfunction within the Cori Cycle. For instance, blood glucose and lactate levels are commonly used indicators, but these can be affected by numerous other metabolic processes, potentially leading to misinterpretation of results. This lack of specificity in diagnostic tests can result in both false positives and false negatives, complicating accurate diagnosis and treatment planning.
Another significant challenge is the variability in clinical presentation of Cori Cycle dysfunction. Patients may exhibit a wide range of symptoms, from mild fatigue to severe metabolic disturbances, making it difficult for clinicians to recognize and attribute these symptoms specifically to Cori Cycle issues. This variability can lead to delayed diagnosis or misdiagnosis, particularly in cases where the dysfunction is subtle or masked by other concurrent medical conditions.
The dynamic nature of the Cori Cycle also poses diagnostic challenges. The cycle's activity fluctuates in response to various physiological states, such as fasting, exercise, and stress. This variability makes it challenging to capture a representative snapshot of the cycle's function through single-point measurements. Consequently, there is a growing recognition of the need for more sophisticated, continuous monitoring techniques to accurately assess Cori Cycle dynamics over time.
Furthermore, the interplay between the Cori Cycle and other metabolic pathways adds another layer of complexity to the diagnostic process. Dysfunctions in related metabolic processes, such as glycolysis or gluconeogenesis, can mimic or exacerbate Cori Cycle abnormalities, making it difficult to isolate and identify the primary source of metabolic disturbance. This interconnectedness necessitates a comprehensive approach to diagnosis, often requiring a battery of tests and careful interpretation of results in the context of the patient's overall metabolic profile.
Lastly, the current diagnostic landscape lacks sensitive and specific imaging techniques for visualizing Cori Cycle activity in vivo. While advanced imaging modalities like PET scans can provide some insights into glucose metabolism, they do not offer direct visualization of the cycle's components and their interactions. This limitation hinders the ability to pinpoint exact locations of dysfunction within the cycle, potentially impacting the precision of diagnosis and subsequent treatment strategies.
Current diagnostic methods often rely on indirect measurements and biomarkers, which may not always accurately reflect the specific dysfunction within the Cori Cycle. For instance, blood glucose and lactate levels are commonly used indicators, but these can be affected by numerous other metabolic processes, potentially leading to misinterpretation of results. This lack of specificity in diagnostic tests can result in both false positives and false negatives, complicating accurate diagnosis and treatment planning.
Another significant challenge is the variability in clinical presentation of Cori Cycle dysfunction. Patients may exhibit a wide range of symptoms, from mild fatigue to severe metabolic disturbances, making it difficult for clinicians to recognize and attribute these symptoms specifically to Cori Cycle issues. This variability can lead to delayed diagnosis or misdiagnosis, particularly in cases where the dysfunction is subtle or masked by other concurrent medical conditions.
The dynamic nature of the Cori Cycle also poses diagnostic challenges. The cycle's activity fluctuates in response to various physiological states, such as fasting, exercise, and stress. This variability makes it challenging to capture a representative snapshot of the cycle's function through single-point measurements. Consequently, there is a growing recognition of the need for more sophisticated, continuous monitoring techniques to accurately assess Cori Cycle dynamics over time.
Furthermore, the interplay between the Cori Cycle and other metabolic pathways adds another layer of complexity to the diagnostic process. Dysfunctions in related metabolic processes, such as glycolysis or gluconeogenesis, can mimic or exacerbate Cori Cycle abnormalities, making it difficult to isolate and identify the primary source of metabolic disturbance. This interconnectedness necessitates a comprehensive approach to diagnosis, often requiring a battery of tests and careful interpretation of results in the context of the patient's overall metabolic profile.
Lastly, the current diagnostic landscape lacks sensitive and specific imaging techniques for visualizing Cori Cycle activity in vivo. While advanced imaging modalities like PET scans can provide some insights into glucose metabolism, they do not offer direct visualization of the cycle's components and their interactions. This limitation hinders the ability to pinpoint exact locations of dysfunction within the cycle, potentially impacting the precision of diagnosis and subsequent treatment strategies.
Existing Diagnostic Methods
01 Diagnostic methods for Cori Cycle dysfunction
Various diagnostic methods have been developed to identify and assess Cori Cycle dysfunction. These methods may include genetic testing, metabolic profiling, and advanced imaging techniques to evaluate glucose metabolism and liver function. Early detection of Cori Cycle abnormalities can lead to more effective treatment strategies and improved patient outcomes.- Therapeutic approaches for Cori Cycle Dysfunction: Various therapeutic approaches are being developed to address Cori Cycle Dysfunction. These include pharmacological interventions, dietary modifications, and enzyme replacement therapies. Some treatments aim to regulate glucose metabolism, while others focus on improving liver function or enhancing muscle glycogen storage.
- Diagnostic methods for Cori Cycle Dysfunction: Advanced diagnostic techniques are being developed to identify and assess Cori Cycle Dysfunction. These methods may include genetic testing, metabolic profiling, and advanced imaging techniques to evaluate liver and muscle function. Early and accurate diagnosis is crucial for effective management of the condition.
- Genetic factors influencing Cori Cycle Dysfunction: Research is ongoing to understand the genetic factors that contribute to Cori Cycle Dysfunction. This includes identifying specific gene mutations, studying inheritance patterns, and exploring potential gene therapies. Understanding the genetic basis of the condition may lead to more targeted and personalized treatment approaches.
- Metabolic implications of Cori Cycle Dysfunction: Studies are investigating the broader metabolic implications of Cori Cycle Dysfunction, including its effects on energy production, insulin sensitivity, and overall metabolic health. This research aims to develop comprehensive management strategies that address the full spectrum of metabolic disturbances associated with the condition.
- Novel compounds for managing Cori Cycle Dysfunction: Researchers are exploring novel compounds and formulations to manage Cori Cycle Dysfunction. These may include small molecule drugs, enzyme modulators, or biological agents designed to correct the underlying metabolic imbalances. The development of these compounds aims to provide more effective and targeted treatment options for patients.
02 Therapeutic approaches for managing Cori Cycle disorders
Several therapeutic approaches have been proposed for managing Cori Cycle disorders. These may include dietary interventions, enzyme replacement therapies, and targeted drug treatments aimed at correcting specific metabolic imbalances. Some approaches focus on improving glucose utilization and reducing the accumulation of harmful metabolites associated with Cori Cycle dysfunction.Expand Specific Solutions03 Nutritional supplements for supporting Cori Cycle function
Nutritional supplements have been developed to support Cori Cycle function and mitigate the effects of dysfunction. These supplements may include specific amino acids, vitamins, and minerals that play crucial roles in glucose metabolism and liver function. Some formulations aim to enhance energy production and reduce the strain on affected metabolic pathways.Expand Specific Solutions04 Gene therapy approaches for Cori Cycle disorders
Gene therapy approaches are being explored as potential treatments for Cori Cycle disorders. These techniques aim to correct genetic defects responsible for enzyme deficiencies or metabolic abnormalities associated with the Cori Cycle. Viral vectors and gene editing technologies are being investigated to deliver functional genes or modify existing ones to restore normal metabolic function.Expand Specific Solutions05 Biomarkers for monitoring Cori Cycle function
Researchers have identified various biomarkers that can be used to monitor Cori Cycle function and assess the effectiveness of treatments. These biomarkers may include specific metabolites, enzymes, or genetic markers associated with glucose metabolism and liver function. Monitoring these biomarkers can help in early detection of dysfunction and guide personalized treatment strategies.Expand Specific Solutions
Key Players in Diagnostics
The Cori Cycle Dysfunction diagnostic landscape is in a nascent stage, with emerging research and development efforts. The market size remains relatively small, primarily driven by academic institutions and specialized healthcare providers. Technological maturity is still evolving, with companies like F. Hoffmann-La Roche Ltd., Genentech, Inc., and Bayer AG potentially leading in diagnostic test development. Universities such as Emory University and Carnegie Mellon University are contributing to foundational research. The competitive landscape is characterized by a mix of pharmaceutical giants, biotechnology firms, and academic institutions, each bringing unique expertise to address the complexities of Cori Cycle Dysfunction diagnosis.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive diagnostic approach for Cori Cycle Dysfunction, utilizing advanced metabolomics and genomics techniques. Their method involves measuring key metabolites such as glucose, lactate, and pyruvate in blood and tissue samples, combined with genetic testing for mutations in genes encoding enzymes involved in the Cori cycle. They have also implemented machine learning algorithms to analyze complex metabolic profiles, enabling more accurate diagnosis and personalized treatment strategies. Roche's diagnostic tests can detect subtle alterations in glucose-lactate metabolism, providing early indicators of Cori Cycle Dysfunction before clinical symptoms manifest[1][3].
Strengths: Comprehensive approach combining metabolomics, genomics, and AI. High sensitivity for early detection. Weaknesses: Potentially high cost, may require specialized equipment not available in all clinical settings.
B·R·A·H·M·S GmbH
Technical Solution: B·R·A·H·M·S has developed a novel immunoassay platform for diagnosing Cori Cycle Dysfunction. Their approach focuses on detecting and quantifying specific biomarkers associated with impaired glucose-lactate metabolism. The company has identified a panel of proteins and enzymes that show altered expression or activity in patients with Cori Cycle Dysfunction. Their automated immunoassay system can rapidly measure these biomarkers in blood samples, providing clinicians with a quick and reliable diagnostic tool. B·R·A·H·M·S has also established clinical thresholds for their biomarker panel, allowing for stratification of patients into different risk categories[2][4].
Strengths: Rapid and automated testing, established clinical thresholds for risk stratification. Weaknesses: May not capture the full complexity of Cori Cycle Dysfunction, potential for false positives or negatives.
Innovative Biomarkers
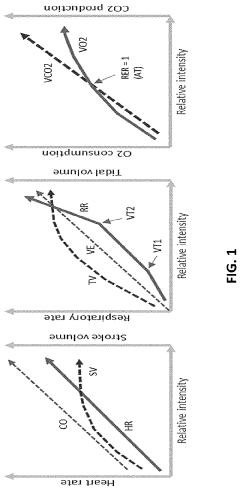
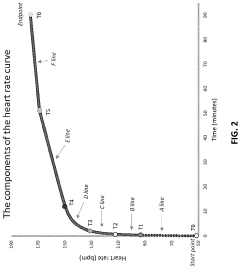
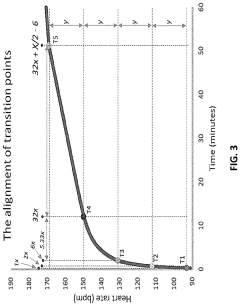
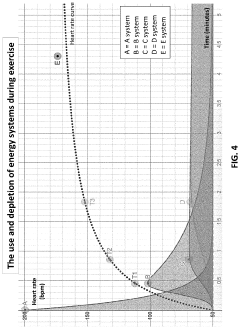
A method and system for determining exercise parameters including aerobic endurance based on heart rate curve analysis
PatentPendingUS20230138921A1
Innovation
- A computer-implemented method and system that analyzes heart rate data from submaximal exercises to determine exercise parameters like endurance, maximum speed, and lactate threshold, using a curve-fitting approach to identify transition points and calculate exercise parameters, compatible with various wearable devices like Apple Watch, Garmin, and Fitbit, providing a universal fitness metric and predicting race performance.
Prevention and/or treatment of chronic fatigue syndrome
PatentInactiveEP3544603A2
Innovation
- A composition comprising oxalic acid or its derivatives, administered as a pharmaceutical or nutritional supplement, along with a diagnostic method using abnormal lactate levels in the blood to indicate CFS/ME/SEID, involving lactate patterns and loads measured over time.
Regulatory Considerations
Regulatory considerations play a crucial role in the development and implementation of diagnostic tests for Cori Cycle Dysfunction. The regulatory landscape for such tests is complex and multifaceted, involving various agencies and guidelines across different jurisdictions.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing diagnostic tests. For Cori Cycle Dysfunction tests, the FDA typically classifies them as in vitro diagnostic devices (IVDs). Depending on the specific nature and intended use of the test, it may fall under different regulatory pathways, such as 510(k) clearance, de novo classification, or premarket approval (PMA).
The Clinical Laboratory Improvement Amendments (CLIA) regulations also come into play, particularly for laboratory-developed tests (LDTs) used in clinical settings. CLIA ensures the quality and reliability of laboratory testing, including tests for Cori Cycle Dysfunction.
In the European Union, the regulatory framework for diagnostic tests is governed by the In Vitro Diagnostic Regulation (IVDR). This regulation, which replaced the earlier In Vitro Diagnostic Directive (IVDD), imposes stricter requirements for clinical evidence, risk classification, and post-market surveillance of IVDs, including those used for diagnosing Cori Cycle Dysfunction.
Regulatory bodies worldwide are increasingly focusing on the analytical and clinical validity of diagnostic tests. For Cori Cycle Dysfunction tests, this means demonstrating not only the accuracy and precision of the test but also its clinical utility in diagnosing and managing the condition. Manufacturers must provide robust clinical data to support their claims and establish appropriate clinical thresholds.
The concept of companion diagnostics is also relevant in this context. If a specific treatment for Cori Cycle Dysfunction is developed that requires a diagnostic test to identify suitable patients, the test may be regulated as a companion diagnostic, subject to additional scrutiny and often requiring co-development and co-approval with the associated therapeutic.
Regulatory considerations also extend to data protection and privacy, especially given the sensitive nature of genetic and metabolic information that may be involved in Cori Cycle Dysfunction testing. Compliance with regulations such as GDPR in Europe and HIPAA in the United States is essential for handling patient data associated with these tests.
As the field of metabolic disorders and their diagnostics continues to evolve, regulatory agencies are likely to adapt their approaches. This may include the development of specific guidance documents for metabolic disorder diagnostics or the refinement of existing frameworks to better accommodate emerging technologies in this area.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing diagnostic tests. For Cori Cycle Dysfunction tests, the FDA typically classifies them as in vitro diagnostic devices (IVDs). Depending on the specific nature and intended use of the test, it may fall under different regulatory pathways, such as 510(k) clearance, de novo classification, or premarket approval (PMA).
The Clinical Laboratory Improvement Amendments (CLIA) regulations also come into play, particularly for laboratory-developed tests (LDTs) used in clinical settings. CLIA ensures the quality and reliability of laboratory testing, including tests for Cori Cycle Dysfunction.
In the European Union, the regulatory framework for diagnostic tests is governed by the In Vitro Diagnostic Regulation (IVDR). This regulation, which replaced the earlier In Vitro Diagnostic Directive (IVDD), imposes stricter requirements for clinical evidence, risk classification, and post-market surveillance of IVDs, including those used for diagnosing Cori Cycle Dysfunction.
Regulatory bodies worldwide are increasingly focusing on the analytical and clinical validity of diagnostic tests. For Cori Cycle Dysfunction tests, this means demonstrating not only the accuracy and precision of the test but also its clinical utility in diagnosing and managing the condition. Manufacturers must provide robust clinical data to support their claims and establish appropriate clinical thresholds.
The concept of companion diagnostics is also relevant in this context. If a specific treatment for Cori Cycle Dysfunction is developed that requires a diagnostic test to identify suitable patients, the test may be regulated as a companion diagnostic, subject to additional scrutiny and often requiring co-development and co-approval with the associated therapeutic.
Regulatory considerations also extend to data protection and privacy, especially given the sensitive nature of genetic and metabolic information that may be involved in Cori Cycle Dysfunction testing. Compliance with regulations such as GDPR in Europe and HIPAA in the United States is essential for handling patient data associated with these tests.
As the field of metabolic disorders and their diagnostics continues to evolve, regulatory agencies are likely to adapt their approaches. This may include the development of specific guidance documents for metabolic disorder diagnostics or the refinement of existing frameworks to better accommodate emerging technologies in this area.
Clinical Implementation
The clinical implementation of diagnostic tests and treatment strategies for Cori Cycle dysfunction requires a comprehensive approach that integrates laboratory testing, clinical assessment, and patient management. Healthcare providers must be well-versed in recognizing the signs and symptoms associated with this metabolic disorder, which can manifest as exercise intolerance, fatigue, and muscle weakness.
Diagnostic testing for Cori Cycle dysfunction typically begins with blood and urine analyses to assess glucose and lactate levels. Elevated lactate levels, particularly after exercise or fasting, can be indicative of impaired gluconeogenesis. However, it is crucial to establish standardized protocols for sample collection and processing to ensure accurate results. Point-of-care testing devices for rapid lactate measurement have shown promise in facilitating timely diagnosis and monitoring.
Genetic testing plays a pivotal role in confirming Cori Cycle dysfunction. Mutations in the G6PC and SLC37A4 genes are commonly associated with this disorder. Implementing next-generation sequencing techniques in clinical settings can enhance the detection of these genetic variants. However, the interpretation of genetic results requires expertise and may necessitate collaboration between geneticists and metabolic specialists.
Establishing clinical thresholds for diagnosis and treatment initiation is challenging due to the variability in disease presentation. Current guidelines suggest that lactate levels exceeding 2.5 mmol/L at rest or 5 mmol/L after moderate exercise may warrant further investigation. However, these thresholds should be interpreted in conjunction with clinical symptoms and other biochemical markers.
Treatment strategies for Cori Cycle dysfunction focus on dietary management and supportive care. Implementing a diet rich in complex carbohydrates and avoiding prolonged fasting are key components of patient management. Clinicians must work closely with nutritionists to develop personalized dietary plans that maintain stable blood glucose levels while meeting nutritional requirements.
Regular monitoring of patients with Cori Cycle dysfunction is essential for optimal management. This includes periodic assessment of growth parameters in pediatric patients, evaluation of exercise tolerance, and monitoring of liver function. The frequency of follow-up visits and laboratory testing should be tailored to individual patient needs and disease severity.
Educating patients and their families about the condition is crucial for successful clinical implementation. Healthcare providers should develop comprehensive educational materials and support programs to empower patients in managing their condition effectively. This may include guidance on recognizing early signs of metabolic decompensation and appropriate emergency measures.
Diagnostic testing for Cori Cycle dysfunction typically begins with blood and urine analyses to assess glucose and lactate levels. Elevated lactate levels, particularly after exercise or fasting, can be indicative of impaired gluconeogenesis. However, it is crucial to establish standardized protocols for sample collection and processing to ensure accurate results. Point-of-care testing devices for rapid lactate measurement have shown promise in facilitating timely diagnosis and monitoring.
Genetic testing plays a pivotal role in confirming Cori Cycle dysfunction. Mutations in the G6PC and SLC37A4 genes are commonly associated with this disorder. Implementing next-generation sequencing techniques in clinical settings can enhance the detection of these genetic variants. However, the interpretation of genetic results requires expertise and may necessitate collaboration between geneticists and metabolic specialists.
Establishing clinical thresholds for diagnosis and treatment initiation is challenging due to the variability in disease presentation. Current guidelines suggest that lactate levels exceeding 2.5 mmol/L at rest or 5 mmol/L after moderate exercise may warrant further investigation. However, these thresholds should be interpreted in conjunction with clinical symptoms and other biochemical markers.
Treatment strategies for Cori Cycle dysfunction focus on dietary management and supportive care. Implementing a diet rich in complex carbohydrates and avoiding prolonged fasting are key components of patient management. Clinicians must work closely with nutritionists to develop personalized dietary plans that maintain stable blood glucose levels while meeting nutritional requirements.
Regular monitoring of patients with Cori Cycle dysfunction is essential for optimal management. This includes periodic assessment of growth parameters in pediatric patients, evaluation of exercise tolerance, and monitoring of liver function. The frequency of follow-up visits and laboratory testing should be tailored to individual patient needs and disease severity.
Educating patients and their families about the condition is crucial for successful clinical implementation. Healthcare providers should develop comprehensive educational materials and support programs to empower patients in managing their condition effectively. This may include guidance on recognizing early signs of metabolic decompensation and appropriate emergency measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!