How To Use NMR/Tracer Methods To Quantify Cori Cycle Fluxes
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR/Tracer Methods for Cori Cycle Flux Quantification
The Cori cycle, also known as the glucose-lactate cycle, plays a crucial role in glucose homeostasis and energy metabolism. Quantifying the fluxes within this cycle is essential for understanding metabolic disorders and developing targeted therapies. Nuclear Magnetic Resonance (NMR) and tracer methods have emerged as powerful tools for measuring these fluxes with high precision and accuracy.
NMR spectroscopy offers a non-invasive approach to study metabolic processes in real-time. By utilizing isotopically labeled substrates, such as 13C-glucose, researchers can track the movement of carbon atoms through various metabolic pathways, including the Cori cycle. The high resolution and sensitivity of NMR allow for the detection of subtle changes in metabolite concentrations and flux rates.
Tracer methods, often used in conjunction with NMR, involve the administration of labeled compounds to track their metabolic fate. In the context of the Cori cycle, isotopically labeled glucose or lactate can be used to measure the rates of gluconeogenesis and glycolysis. These methods provide valuable insights into the dynamic interplay between glucose production and utilization.
The integration of NMR and tracer techniques has led to the development of sophisticated flux analysis methods. One such approach is 13C isotopomer analysis, which examines the distribution of 13C labels in metabolic intermediates to infer flux rates. This method allows for the simultaneous measurement of multiple fluxes within the Cori cycle and related pathways.
Another powerful technique is dynamic nuclear polarization (DNP)-enhanced NMR, which dramatically increases the sensitivity of NMR measurements. This method enables the detection of metabolites at physiological concentrations and allows for real-time monitoring of metabolic fluxes in vivo. DNP-NMR has been successfully applied to study hepatic glucose metabolism and the Cori cycle in animal models.
Advances in NMR hardware and pulse sequences have further improved the ability to quantify Cori cycle fluxes. High-field NMR spectrometers, combined with specialized probes and cryogenic cooling systems, offer enhanced spectral resolution and sensitivity. Additionally, the development of rapid acquisition techniques, such as non-uniform sampling and compressed sensing, has reduced experiment times and improved temporal resolution.
The application of these NMR and tracer methods to quantify Cori cycle fluxes has provided valuable insights into metabolic disorders such as diabetes and liver diseases. By measuring the rates of glucose production and utilization, researchers can assess the impact of various interventions on glucose homeostasis and develop more effective treatment strategies.
NMR spectroscopy offers a non-invasive approach to study metabolic processes in real-time. By utilizing isotopically labeled substrates, such as 13C-glucose, researchers can track the movement of carbon atoms through various metabolic pathways, including the Cori cycle. The high resolution and sensitivity of NMR allow for the detection of subtle changes in metabolite concentrations and flux rates.
Tracer methods, often used in conjunction with NMR, involve the administration of labeled compounds to track their metabolic fate. In the context of the Cori cycle, isotopically labeled glucose or lactate can be used to measure the rates of gluconeogenesis and glycolysis. These methods provide valuable insights into the dynamic interplay between glucose production and utilization.
The integration of NMR and tracer techniques has led to the development of sophisticated flux analysis methods. One such approach is 13C isotopomer analysis, which examines the distribution of 13C labels in metabolic intermediates to infer flux rates. This method allows for the simultaneous measurement of multiple fluxes within the Cori cycle and related pathways.
Another powerful technique is dynamic nuclear polarization (DNP)-enhanced NMR, which dramatically increases the sensitivity of NMR measurements. This method enables the detection of metabolites at physiological concentrations and allows for real-time monitoring of metabolic fluxes in vivo. DNP-NMR has been successfully applied to study hepatic glucose metabolism and the Cori cycle in animal models.
Advances in NMR hardware and pulse sequences have further improved the ability to quantify Cori cycle fluxes. High-field NMR spectrometers, combined with specialized probes and cryogenic cooling systems, offer enhanced spectral resolution and sensitivity. Additionally, the development of rapid acquisition techniques, such as non-uniform sampling and compressed sensing, has reduced experiment times and improved temporal resolution.
The application of these NMR and tracer methods to quantify Cori cycle fluxes has provided valuable insights into metabolic disorders such as diabetes and liver diseases. By measuring the rates of glucose production and utilization, researchers can assess the impact of various interventions on glucose homeostasis and develop more effective treatment strategies.
Clinical Relevance of Cori Cycle Flux Measurements
The clinical relevance of Cori cycle flux measurements extends far beyond basic metabolic research, offering valuable insights into various physiological and pathological states. Quantifying Cori cycle fluxes provides a window into whole-body glucose homeostasis and liver function, making it a powerful diagnostic and monitoring tool in clinical settings.
In diabetes management, Cori cycle flux measurements can reveal the extent of hepatic glucose production and peripheral glucose utilization. This information is crucial for tailoring treatment strategies and assessing the efficacy of interventions. Abnormal Cori cycle activity often precedes overt hyperglycemia, potentially serving as an early marker for diabetes risk or progression.
For patients with liver diseases, such as cirrhosis or hepatitis, Cori cycle flux measurements offer a non-invasive means to evaluate hepatic function. Alterations in cycle fluxes can indicate compromised liver metabolism, helping clinicians gauge disease severity and monitor treatment responses. This approach is particularly valuable when liver biopsy is contraindicated or impractical.
In critical care settings, Cori cycle flux measurements can guide nutritional support strategies. By understanding the balance between glucose production and utilization, clinicians can optimize parenteral nutrition protocols, potentially improving outcomes in critically ill patients. This is especially relevant in conditions like sepsis, where metabolic derangements are common.
Cancer metabolism research has also benefited from Cori cycle flux measurements. Many tumors exhibit altered glucose metabolism, and quantifying Cori cycle activity can provide insights into tumor behavior and treatment responses. This information may help in developing targeted therapies and monitoring treatment efficacy.
In the field of exercise physiology, Cori cycle flux measurements offer a deeper understanding of energy metabolism during physical activity. This knowledge can be applied to optimize training regimens for athletes and develop exercise interventions for patients with metabolic disorders.
The non-invasive nature of NMR/Tracer methods for quantifying Cori cycle fluxes makes them particularly suitable for longitudinal studies in clinical populations. This allows for the monitoring of disease progression or treatment effects over time, providing valuable data for personalized medicine approaches.
Furthermore, Cori cycle flux measurements can contribute to the development of novel biomarkers for metabolic health. By correlating flux data with other clinical parameters, researchers may identify new indicators of metabolic dysfunction or disease risk, potentially leading to improved diagnostic and prognostic tools.
In diabetes management, Cori cycle flux measurements can reveal the extent of hepatic glucose production and peripheral glucose utilization. This information is crucial for tailoring treatment strategies and assessing the efficacy of interventions. Abnormal Cori cycle activity often precedes overt hyperglycemia, potentially serving as an early marker for diabetes risk or progression.
For patients with liver diseases, such as cirrhosis or hepatitis, Cori cycle flux measurements offer a non-invasive means to evaluate hepatic function. Alterations in cycle fluxes can indicate compromised liver metabolism, helping clinicians gauge disease severity and monitor treatment responses. This approach is particularly valuable when liver biopsy is contraindicated or impractical.
In critical care settings, Cori cycle flux measurements can guide nutritional support strategies. By understanding the balance between glucose production and utilization, clinicians can optimize parenteral nutrition protocols, potentially improving outcomes in critically ill patients. This is especially relevant in conditions like sepsis, where metabolic derangements are common.
Cancer metabolism research has also benefited from Cori cycle flux measurements. Many tumors exhibit altered glucose metabolism, and quantifying Cori cycle activity can provide insights into tumor behavior and treatment responses. This information may help in developing targeted therapies and monitoring treatment efficacy.
In the field of exercise physiology, Cori cycle flux measurements offer a deeper understanding of energy metabolism during physical activity. This knowledge can be applied to optimize training regimens for athletes and develop exercise interventions for patients with metabolic disorders.
The non-invasive nature of NMR/Tracer methods for quantifying Cori cycle fluxes makes them particularly suitable for longitudinal studies in clinical populations. This allows for the monitoring of disease progression or treatment effects over time, providing valuable data for personalized medicine approaches.
Furthermore, Cori cycle flux measurements can contribute to the development of novel biomarkers for metabolic health. By correlating flux data with other clinical parameters, researchers may identify new indicators of metabolic dysfunction or disease risk, potentially leading to improved diagnostic and prognostic tools.
Current Challenges in Metabolic Flux Analysis
Metabolic flux analysis (MFA) has become an indispensable tool for understanding cellular metabolism and its regulation. However, several challenges persist in accurately quantifying metabolic fluxes, particularly in complex biological systems such as the Cori cycle. The Cori cycle, a metabolic pathway involving the liver and skeletal muscles, plays a crucial role in glucose homeostasis and energy metabolism.
One of the primary challenges in metabolic flux analysis of the Cori cycle is the dynamic nature of the system. The fluxes within the cycle can change rapidly in response to physiological conditions, making it difficult to capture accurate measurements at specific time points. This temporal variability necessitates the development of more sophisticated experimental designs and analytical methods to account for these dynamic changes.
Another significant challenge is the compartmentalization of metabolic processes within different tissues and cellular organelles. The Cori cycle involves interactions between the liver and skeletal muscles, with metabolites shuttling between these organs. Traditional MFA techniques often struggle to distinguish between compartment-specific fluxes, leading to potential misinterpretations of metabolic activities.
The complexity of metabolic networks and the presence of multiple parallel pathways also pose challenges in flux quantification. The Cori cycle interacts with various other metabolic pathways, including glycolysis, gluconeogenesis, and the citric acid cycle. Disentangling the contributions of these interconnected pathways to overall flux distributions requires advanced mathematical modeling and experimental approaches.
Furthermore, the low concentrations of certain metabolic intermediates in the Cori cycle present analytical challenges. Some key metabolites may be present at levels below the detection limits of conventional analytical techniques, necessitating the development of more sensitive and specific methods for their quantification.
The heterogeneity of cell populations within tissues involved in the Cori cycle adds another layer of complexity to flux analysis. Different cell types within the liver or skeletal muscles may exhibit distinct metabolic profiles, making it challenging to obtain representative flux measurements for the entire tissue.
Lastly, the integration of data from multiple analytical platforms, such as NMR and mass spectrometry, remains a significant challenge in metabolic flux analysis. Each technique has its strengths and limitations, and combining data from different sources requires sophisticated computational approaches to ensure consistency and accuracy in flux estimations.
One of the primary challenges in metabolic flux analysis of the Cori cycle is the dynamic nature of the system. The fluxes within the cycle can change rapidly in response to physiological conditions, making it difficult to capture accurate measurements at specific time points. This temporal variability necessitates the development of more sophisticated experimental designs and analytical methods to account for these dynamic changes.
Another significant challenge is the compartmentalization of metabolic processes within different tissues and cellular organelles. The Cori cycle involves interactions between the liver and skeletal muscles, with metabolites shuttling between these organs. Traditional MFA techniques often struggle to distinguish between compartment-specific fluxes, leading to potential misinterpretations of metabolic activities.
The complexity of metabolic networks and the presence of multiple parallel pathways also pose challenges in flux quantification. The Cori cycle interacts with various other metabolic pathways, including glycolysis, gluconeogenesis, and the citric acid cycle. Disentangling the contributions of these interconnected pathways to overall flux distributions requires advanced mathematical modeling and experimental approaches.
Furthermore, the low concentrations of certain metabolic intermediates in the Cori cycle present analytical challenges. Some key metabolites may be present at levels below the detection limits of conventional analytical techniques, necessitating the development of more sensitive and specific methods for their quantification.
The heterogeneity of cell populations within tissues involved in the Cori cycle adds another layer of complexity to flux analysis. Different cell types within the liver or skeletal muscles may exhibit distinct metabolic profiles, making it challenging to obtain representative flux measurements for the entire tissue.
Lastly, the integration of data from multiple analytical platforms, such as NMR and mass spectrometry, remains a significant challenge in metabolic flux analysis. Each technique has its strengths and limitations, and combining data from different sources requires sophisticated computational approaches to ensure consistency and accuracy in flux estimations.
Existing NMR/Tracer Protocols for Flux Quantification
01 NMR spectroscopy for metabolic flux analysis
Nuclear Magnetic Resonance (NMR) spectroscopy is used to analyze metabolic fluxes in the Cori cycle. This technique allows for the measurement of isotopically labeled metabolites, providing insights into the rates of glucose-lactate cycling between liver and peripheral tissues.- NMR spectroscopy for metabolic flux analysis: Nuclear Magnetic Resonance (NMR) spectroscopy is used to analyze metabolic fluxes in the Cori cycle. This technique allows for the measurement of isotopically labeled metabolites, providing insights into the rates of glucose-lactate cycling and other metabolic processes.
- Tracer methods for studying Cori cycle dynamics: Tracer methods involve the use of isotopically labeled compounds to track metabolic pathways. These techniques are applied to study the Cori cycle fluxes, allowing researchers to quantify the rates of glucose production and utilization in various physiological states.
- Advanced imaging techniques for metabolic flux analysis: Advanced imaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), are used in conjunction with NMR and tracer methods to visualize and quantify Cori cycle fluxes in real-time within living organisms.
- Data analysis and modeling of Cori cycle fluxes: Sophisticated data analysis techniques and mathematical modeling are employed to interpret the complex data obtained from NMR and tracer studies of the Cori cycle. These methods help in estimating flux rates and understanding the regulation of glucose metabolism.
- Integration of multi-omics data for comprehensive flux analysis: Integration of data from multiple omics approaches, including metabolomics, proteomics, and transcriptomics, with NMR and tracer data to provide a more comprehensive understanding of Cori cycle fluxes and their regulation in various physiological and pathological conditions.
02 Tracer methods for studying Cori cycle dynamics
Tracer methods involve the use of isotopically labeled compounds to track metabolic pathways. In the context of the Cori cycle, these methods can be employed to measure glucose and lactate fluxes between tissues, providing quantitative data on cycle activity.Expand Specific Solutions03 Advanced imaging techniques for metabolic studies
Advanced imaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), can be used in conjunction with NMR and tracer methods to visualize and quantify Cori cycle fluxes in real-time within living organisms.Expand Specific Solutions04 Computational modeling of Cori cycle fluxes
Computational models are developed to integrate data from NMR and tracer studies, allowing for more comprehensive analysis of Cori cycle fluxes. These models can simulate various physiological conditions and predict metabolic responses.Expand Specific Solutions05 Microfluidic devices for metabolic flux analysis
Microfluidic devices are being developed to facilitate high-throughput analysis of metabolic fluxes, including those involved in the Cori cycle. These devices can integrate NMR detection or other analytical methods for rapid and precise measurements of metabolite concentrations and fluxes.Expand Specific Solutions
Key Players in Metabolomics Research
The quantification of Cori cycle fluxes using NMR/Tracer methods is an emerging field in metabolic research, currently in its early development stage. The market size is relatively small but growing, driven by increasing interest in metabolic disorders and diabetes research. Technologically, the approach is still evolving, with varying levels of maturity among key players. Companies like Schlumberger Technologies and Baker Hughes are leveraging their expertise in NMR technology for this application, while research institutions such as the Centre National de la Recherche Scientifique and the Institute of Process Engineering, Chinese Academy of Sciences are advancing the fundamental science. Specialized firms like Spinlock S.R.L. are developing targeted NMR solutions, indicating a gradual shift towards more refined and application-specific technologies in this niche area.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has developed advanced NMR logging tools for quantifying Cori cycle fluxes in subsurface environments. Their technology utilizes pulsed NMR sequences optimized for detecting metabolites involved in the Cori cycle. The system employs sophisticated inversion algorithms to extract flux information from multi-dimensional NMR data[1]. Schlumberger's approach combines downhole NMR measurements with tracer studies, injecting labeled glucose to track its conversion through the cycle. This integrated method provides spatially-resolved flux maps in geological formations, enabling better understanding of microbial activity and carbon cycling in the deep biosphere[2][3].
Strengths: Unparalleled expertise in downhole NMR technology; ability to perform in situ measurements in extreme environments. Weaknesses: High cost; limited to subsurface applications.
Baker Hughes Co.
Technical Solution: Baker Hughes has pioneered the use of NMR and tracer methods for quantifying Cori cycle fluxes in oil and gas reservoirs. Their approach combines advanced NMR logging tools with innovative tracer compounds designed to mimic Cori cycle intermediates. The company's proprietary algorithms analyze the time-dependent distribution of these tracers to extract flux information. Baker Hughes' technology incorporates machine learning techniques to improve flux quantification accuracy and robustness in complex geological settings[4]. Their system can simultaneously measure multiple metabolic pathways, providing a comprehensive view of subsurface microbial activity and its impact on reservoir properties[5].
Strengths: Comprehensive solution integrating NMR, tracers, and AI; applicable to a wide range of reservoir conditions. Weaknesses: Primarily focused on oil and gas industry applications; may require significant customization for other fields.
Innovations in Isotope Labeling Strategies
NMR procedure for determination of mass flow of the single components of a multi-component flow
PatentInactiveEP0496330A2
Innovation
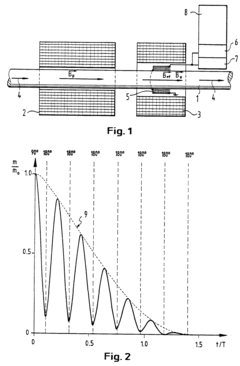
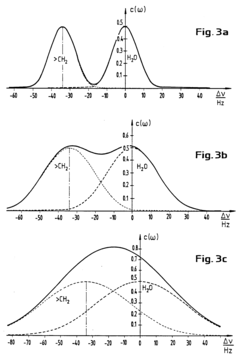
- A nuclear magnetic resonance (NMR) method using a Carr-Purcell-Meiboom-Gill pulse sequence and subsequent spectroscopy to measure nuclear spin echo sequences, combined with Fourier transformation for frequency analysis, allowing for real-time determination of mean velocities and volume fractions, and thus mass flow, in a pipe with a mixture of petroleum, water, and gas.
Method for processing nuclear magnetic resonance (NMR) spectroscopic data
PatentInactiveUS10866295B2
Innovation
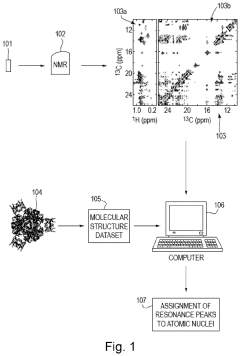
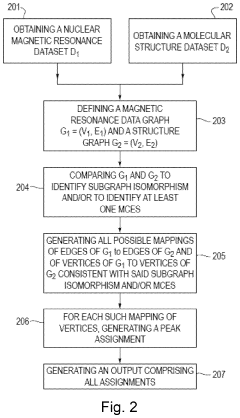
- A graph-matching algorithm that combines structural models with experimental multidimensional magnetic resonance data to accurately identify confident and ambiguous peak assignments by comparing experimental distance restraints with structural models, reducing the need for laborious experiments and providing exact sets of plausible assignments.
Regulatory Considerations for Metabolic Studies
Regulatory considerations play a crucial role in metabolic studies, particularly when using advanced techniques like NMR and tracer methods to quantify Cori cycle fluxes. These studies often involve human subjects and the use of isotopically labeled compounds, necessitating careful adherence to established guidelines and regulations.
One of the primary regulatory bodies overseeing such research is the Food and Drug Administration (FDA). The FDA has specific requirements for the use of stable isotopes in human metabolic studies, including the need for Investigational New Drug (IND) applications for certain isotopically labeled compounds. Researchers must demonstrate the safety and purity of these tracers, as well as provide a comprehensive protocol for their administration and subsequent analysis.
Institutional Review Boards (IRBs) also play a critical role in the regulatory process. They are responsible for reviewing and approving research protocols involving human subjects, ensuring that studies are ethically sound and that participant rights and welfare are protected. For Cori cycle flux studies, IRBs will scrutinize the experimental design, participant selection criteria, and potential risks associated with the administration of labeled compounds and NMR procedures.
The use of NMR spectroscopy in metabolic studies falls under the purview of medical device regulations. While NMR is generally considered a non-invasive technique, researchers must still comply with safety standards related to magnetic field exposure and ensure proper calibration and maintenance of NMR equipment.
Data privacy and protection regulations, such as the General Data Protection Regulation (GDPR) in the European Union or the Health Insurance Portability and Accountability Act (HIPAA) in the United States, are also relevant to metabolic studies. These regulations govern the collection, storage, and handling of personal health information obtained during the research process.
Researchers must also consider regulations surrounding the procurement and handling of biological samples. This includes adherence to good laboratory practices (GLP) and proper documentation of sample collection, storage, and analysis procedures.
International collaborations in metabolic research may introduce additional regulatory complexities. Researchers must navigate differing regulatory frameworks across countries and ensure compliance with import/export regulations for biological samples and isotopically labeled compounds.
Lastly, the reporting and publication of metabolic study results are subject to regulatory oversight. Researchers must adhere to guidelines for clinical trial registration and results reporting, such as those outlined by ClinicalTrials.gov. Transparency in methodology and data sharing practices is increasingly emphasized by regulatory bodies and scientific journals alike.
One of the primary regulatory bodies overseeing such research is the Food and Drug Administration (FDA). The FDA has specific requirements for the use of stable isotopes in human metabolic studies, including the need for Investigational New Drug (IND) applications for certain isotopically labeled compounds. Researchers must demonstrate the safety and purity of these tracers, as well as provide a comprehensive protocol for their administration and subsequent analysis.
Institutional Review Boards (IRBs) also play a critical role in the regulatory process. They are responsible for reviewing and approving research protocols involving human subjects, ensuring that studies are ethically sound and that participant rights and welfare are protected. For Cori cycle flux studies, IRBs will scrutinize the experimental design, participant selection criteria, and potential risks associated with the administration of labeled compounds and NMR procedures.
The use of NMR spectroscopy in metabolic studies falls under the purview of medical device regulations. While NMR is generally considered a non-invasive technique, researchers must still comply with safety standards related to magnetic field exposure and ensure proper calibration and maintenance of NMR equipment.
Data privacy and protection regulations, such as the General Data Protection Regulation (GDPR) in the European Union or the Health Insurance Portability and Accountability Act (HIPAA) in the United States, are also relevant to metabolic studies. These regulations govern the collection, storage, and handling of personal health information obtained during the research process.
Researchers must also consider regulations surrounding the procurement and handling of biological samples. This includes adherence to good laboratory practices (GLP) and proper documentation of sample collection, storage, and analysis procedures.
International collaborations in metabolic research may introduce additional regulatory complexities. Researchers must navigate differing regulatory frameworks across countries and ensure compliance with import/export regulations for biological samples and isotopically labeled compounds.
Lastly, the reporting and publication of metabolic study results are subject to regulatory oversight. Researchers must adhere to guidelines for clinical trial registration and results reporting, such as those outlined by ClinicalTrials.gov. Transparency in methodology and data sharing practices is increasingly emphasized by regulatory bodies and scientific journals alike.
Data Analysis and Modeling Approaches
The quantification of Cori cycle fluxes using NMR/Tracer methods requires sophisticated data analysis and modeling approaches. These techniques involve the integration of experimental data with mathematical models to accurately estimate metabolic fluxes.
One common approach is the use of isotopomer analysis, which involves tracking the distribution of labeled atoms in metabolites. This method utilizes nuclear magnetic resonance (NMR) spectroscopy to measure the relative abundance of different isotopomers. The resulting data is then analyzed using computational models to estimate flux rates through various metabolic pathways, including the Cori cycle.
Metabolic flux analysis (MFA) is another powerful tool for quantifying Cori cycle fluxes. This approach combines experimental measurements with stoichiometric models of metabolism to calculate intracellular fluxes. In the context of the Cori cycle, MFA can be used to estimate the rates of glucose production in the liver and lactate production in peripheral tissues.
Kinetic modeling is also employed to analyze NMR/Tracer data for Cori cycle flux quantification. These models incorporate enzyme kinetics and regulatory mechanisms to simulate the dynamic behavior of metabolic pathways. By fitting experimental data to these models, researchers can estimate flux rates and identify key regulatory points in the Cori cycle.
Machine learning algorithms are increasingly being applied to analyze complex NMR/Tracer datasets. These techniques can identify patterns and relationships in the data that may not be apparent through traditional analysis methods. For example, neural networks and support vector machines have been used to predict metabolic fluxes based on NMR spectral data.
Bayesian inference methods provide a probabilistic framework for estimating Cori cycle fluxes from NMR/Tracer data. These approaches allow for the incorporation of prior knowledge and the quantification of uncertainty in flux estimates. Markov Chain Monte Carlo (MCMC) algorithms are often used to sample from the posterior distribution of flux parameters.
Multicompartmental modeling is particularly relevant for studying the Cori cycle, as it involves interactions between multiple tissues. These models divide the system into distinct compartments (e.g., liver, muscle, blood) and describe the transfer of metabolites between them. By incorporating NMR/Tracer data into these models, researchers can estimate tissue-specific fluxes and inter-organ substrate exchange rates.
One common approach is the use of isotopomer analysis, which involves tracking the distribution of labeled atoms in metabolites. This method utilizes nuclear magnetic resonance (NMR) spectroscopy to measure the relative abundance of different isotopomers. The resulting data is then analyzed using computational models to estimate flux rates through various metabolic pathways, including the Cori cycle.
Metabolic flux analysis (MFA) is another powerful tool for quantifying Cori cycle fluxes. This approach combines experimental measurements with stoichiometric models of metabolism to calculate intracellular fluxes. In the context of the Cori cycle, MFA can be used to estimate the rates of glucose production in the liver and lactate production in peripheral tissues.
Kinetic modeling is also employed to analyze NMR/Tracer data for Cori cycle flux quantification. These models incorporate enzyme kinetics and regulatory mechanisms to simulate the dynamic behavior of metabolic pathways. By fitting experimental data to these models, researchers can estimate flux rates and identify key regulatory points in the Cori cycle.
Machine learning algorithms are increasingly being applied to analyze complex NMR/Tracer datasets. These techniques can identify patterns and relationships in the data that may not be apparent through traditional analysis methods. For example, neural networks and support vector machines have been used to predict metabolic fluxes based on NMR spectral data.
Bayesian inference methods provide a probabilistic framework for estimating Cori cycle fluxes from NMR/Tracer data. These approaches allow for the incorporation of prior knowledge and the quantification of uncertainty in flux estimates. Markov Chain Monte Carlo (MCMC) algorithms are often used to sample from the posterior distribution of flux parameters.
Multicompartmental modeling is particularly relevant for studying the Cori cycle, as it involves interactions between multiple tissues. These models divide the system into distinct compartments (e.g., liver, muscle, blood) and describe the transfer of metabolites between them. By incorporating NMR/Tracer data into these models, researchers can estimate tissue-specific fluxes and inter-organ substrate exchange rates.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!