Cori Cycle And Hypoxia: Metabolic Responses And Interventions
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cori Cycle and Hypoxia Background
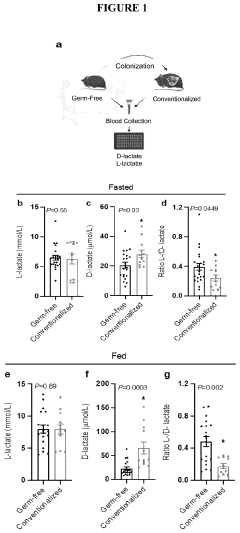
The Cori cycle, also known as the glucose-lactate cycle, is a metabolic pathway that plays a crucial role in maintaining glucose homeostasis during periods of intense exercise or hypoxia. Named after its discoverers, Carl and Gerty Cori, this cycle involves the interconversion of glucose and lactate between the liver and peripheral tissues, particularly skeletal muscles.
In normal physiological conditions, glucose serves as the primary energy source for most cells. However, during hypoxia or intense physical activity, oxygen availability becomes limited, leading to a shift in cellular metabolism. This shift results in increased anaerobic glycolysis and the production of lactate in muscle tissues.
The Cori cycle begins when lactate, produced in muscle cells under anaerobic conditions, is released into the bloodstream. It is then transported to the liver, where it is converted back to glucose through gluconeogenesis. This newly synthesized glucose is subsequently released into the circulation and can be utilized by various tissues, including the brain and muscles.
Hypoxia, a state of reduced oxygen availability in tissues, significantly impacts the Cori cycle and overall metabolic responses. During hypoxic conditions, cells must adapt their metabolism to maintain energy production and cellular function. This adaptation involves a complex interplay of various metabolic pathways and regulatory mechanisms.
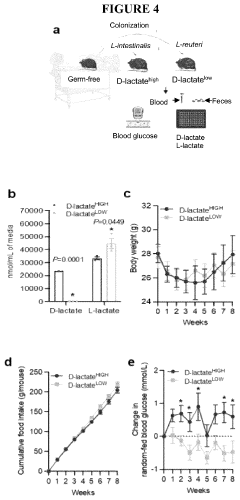
One of the primary metabolic responses to hypoxia is the upregulation of anaerobic glycolysis. This shift allows cells to generate ATP in the absence of sufficient oxygen, albeit less efficiently than through oxidative phosphorylation. The increased reliance on anaerobic glycolysis leads to a higher production of lactate, which in turn activates the Cori cycle to recycle this metabolic byproduct.
The hypoxia-inducible factor (HIF) pathway plays a central role in coordinating cellular responses to low oxygen levels. HIF-1α, a key transcription factor, becomes stabilized under hypoxic conditions and regulates the expression of numerous genes involved in glucose metabolism, angiogenesis, and cell survival.
Understanding the intricate relationship between the Cori cycle and hypoxia is crucial for developing effective interventions in various clinical scenarios, such as ischemic diseases, high-altitude adaptation, and exercise physiology. Research in this field has led to the exploration of novel therapeutic approaches, including metabolic modulation and hypoxia preconditioning, to enhance cellular resilience and improve patient outcomes in hypoxia-related conditions.
In normal physiological conditions, glucose serves as the primary energy source for most cells. However, during hypoxia or intense physical activity, oxygen availability becomes limited, leading to a shift in cellular metabolism. This shift results in increased anaerobic glycolysis and the production of lactate in muscle tissues.
The Cori cycle begins when lactate, produced in muscle cells under anaerobic conditions, is released into the bloodstream. It is then transported to the liver, where it is converted back to glucose through gluconeogenesis. This newly synthesized glucose is subsequently released into the circulation and can be utilized by various tissues, including the brain and muscles.
Hypoxia, a state of reduced oxygen availability in tissues, significantly impacts the Cori cycle and overall metabolic responses. During hypoxic conditions, cells must adapt their metabolism to maintain energy production and cellular function. This adaptation involves a complex interplay of various metabolic pathways and regulatory mechanisms.
One of the primary metabolic responses to hypoxia is the upregulation of anaerobic glycolysis. This shift allows cells to generate ATP in the absence of sufficient oxygen, albeit less efficiently than through oxidative phosphorylation. The increased reliance on anaerobic glycolysis leads to a higher production of lactate, which in turn activates the Cori cycle to recycle this metabolic byproduct.
The hypoxia-inducible factor (HIF) pathway plays a central role in coordinating cellular responses to low oxygen levels. HIF-1α, a key transcription factor, becomes stabilized under hypoxic conditions and regulates the expression of numerous genes involved in glucose metabolism, angiogenesis, and cell survival.
Understanding the intricate relationship between the Cori cycle and hypoxia is crucial for developing effective interventions in various clinical scenarios, such as ischemic diseases, high-altitude adaptation, and exercise physiology. Research in this field has led to the exploration of novel therapeutic approaches, including metabolic modulation and hypoxia preconditioning, to enhance cellular resilience and improve patient outcomes in hypoxia-related conditions.
Clinical Relevance and Applications
The Cori cycle and hypoxia research has significant clinical relevance and applications across various medical fields. In critical care medicine, understanding the Cori cycle's role in glucose metabolism during hypoxic conditions is crucial for managing patients with respiratory distress or circulatory shock. Clinicians can use this knowledge to optimize nutritional support and metabolic interventions, potentially improving patient outcomes in intensive care units.
In the field of sports medicine, insights into the Cori cycle and hypoxic responses are valuable for enhancing athletic performance and recovery. Athletes training at high altitudes or using hypoxic chambers can benefit from tailored nutrition and exercise strategies that account for the metabolic shifts occurring during oxygen-limited conditions. This knowledge can help prevent overtraining and optimize performance gains.
Oncology is another area where this research has important applications. Many solid tumors exhibit hypoxic regions, which can influence cancer cell metabolism and contribute to treatment resistance. Understanding the Cori cycle's role in tumor metabolism under hypoxic conditions can lead to the development of novel therapeutic approaches targeting cancer-specific metabolic vulnerabilities.
In the management of diabetes, the Cori cycle's involvement in glucose homeostasis has implications for treatment strategies. Clinicians can use this knowledge to fine-tune insulin therapy and dietary recommendations, particularly for patients with comorbidities that may affect tissue oxygenation, such as cardiovascular or respiratory diseases.
Neurology and neurosurgery also benefit from insights into the Cori cycle and hypoxia. In conditions like stroke or traumatic brain injury, where cerebral hypoxia is a concern, understanding metabolic adaptations can inform neuroprotective strategies and guide post-injury management to minimize secondary damage and promote recovery.
Cardiovascular medicine leverages this research in the context of ischemic heart disease and heart failure. Clinicians can apply knowledge of hypoxia-induced metabolic changes to develop better strategies for myocardial protection during surgeries or in the management of chronic cardiac conditions.
Lastly, in the field of transplantation medicine, understanding the Cori cycle and hypoxic responses is crucial for organ preservation and post-transplant care. This knowledge can help optimize preservation solutions and develop strategies to mitigate ischemia-reperfusion injury, potentially improving graft survival rates and patient outcomes.
In the field of sports medicine, insights into the Cori cycle and hypoxic responses are valuable for enhancing athletic performance and recovery. Athletes training at high altitudes or using hypoxic chambers can benefit from tailored nutrition and exercise strategies that account for the metabolic shifts occurring during oxygen-limited conditions. This knowledge can help prevent overtraining and optimize performance gains.
Oncology is another area where this research has important applications. Many solid tumors exhibit hypoxic regions, which can influence cancer cell metabolism and contribute to treatment resistance. Understanding the Cori cycle's role in tumor metabolism under hypoxic conditions can lead to the development of novel therapeutic approaches targeting cancer-specific metabolic vulnerabilities.
In the management of diabetes, the Cori cycle's involvement in glucose homeostasis has implications for treatment strategies. Clinicians can use this knowledge to fine-tune insulin therapy and dietary recommendations, particularly for patients with comorbidities that may affect tissue oxygenation, such as cardiovascular or respiratory diseases.
Neurology and neurosurgery also benefit from insights into the Cori cycle and hypoxia. In conditions like stroke or traumatic brain injury, where cerebral hypoxia is a concern, understanding metabolic adaptations can inform neuroprotective strategies and guide post-injury management to minimize secondary damage and promote recovery.
Cardiovascular medicine leverages this research in the context of ischemic heart disease and heart failure. Clinicians can apply knowledge of hypoxia-induced metabolic changes to develop better strategies for myocardial protection during surgeries or in the management of chronic cardiac conditions.
Lastly, in the field of transplantation medicine, understanding the Cori cycle and hypoxic responses is crucial for organ preservation and post-transplant care. This knowledge can help optimize preservation solutions and develop strategies to mitigate ischemia-reperfusion injury, potentially improving graft survival rates and patient outcomes.
Metabolic Adaptations to Hypoxia
Hypoxia, characterized by reduced oxygen availability, triggers a series of metabolic adaptations in the human body to maintain cellular function and survival. These adaptations involve complex biochemical and physiological changes that affect energy production, substrate utilization, and cellular signaling pathways.
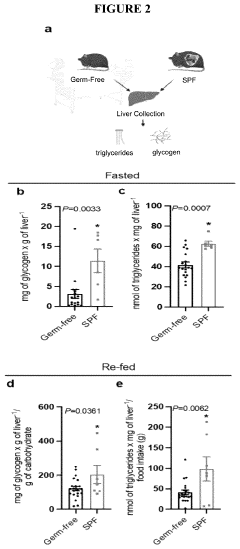
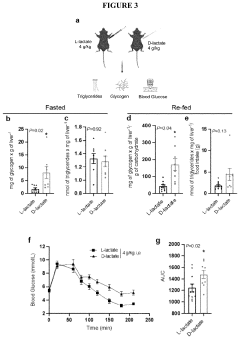
One of the primary metabolic adaptations to hypoxia is the shift from aerobic to anaerobic metabolism. As oxygen levels decrease, cells rely more heavily on glycolysis for ATP production. This shift is mediated by the upregulation of glycolytic enzymes and glucose transporters, allowing for increased glucose uptake and utilization. The increased glycolytic flux leads to a higher production of lactate, which can be shuttled to the liver via the Cori cycle for gluconeogenesis.
The Cori cycle plays a crucial role in hypoxic conditions by facilitating the recycling of lactate produced in skeletal muscles. This process helps maintain blood glucose levels and provides an alternative energy source for tissues that can utilize lactate. The cycle's efficiency is enhanced during hypoxia, with increased activity of key enzymes such as lactate dehydrogenase and pyruvate carboxylase.
Hypoxia-inducible factors (HIFs) are central regulators of the cellular response to low oxygen levels. These transcription factors activate genes involved in angiogenesis, erythropoiesis, and metabolic reprogramming. HIF-1α, in particular, promotes the expression of genes encoding glucose transporters and glycolytic enzymes, further supporting the metabolic shift towards anaerobic glycolysis.
Mitochondrial function is also significantly affected by hypoxia. To minimize oxygen consumption and reduce the production of reactive oxygen species, cells downregulate mitochondrial oxidative phosphorylation. This is achieved through decreased expression of electron transport chain components and increased mitochondrial autophagy. Concurrently, there is an upregulation of mitochondrial glycerol-3-phosphate dehydrogenase, which helps maintain the glycerol-3-phosphate shuttle and supports glycolysis.
Lipid metabolism undergoes notable changes during hypoxia. Fatty acid oxidation is suppressed to conserve oxygen, while lipid synthesis is enhanced. This metabolic shift is mediated by HIF-1α, which promotes the expression of lipogenic enzymes and inhibits carnitine palmitoyltransferase 1 (CPT1), a key enzyme in fatty acid oxidation.
Protein metabolism is also altered in response to hypoxia. There is a general decrease in protein synthesis to conserve energy, accompanied by increased protein breakdown to provide amino acids for gluconeogenesis and other essential processes. However, the synthesis of specific proteins involved in hypoxia adaptation, such as erythropoietin and vascular endothelial growth factor, is upregulated.
One of the primary metabolic adaptations to hypoxia is the shift from aerobic to anaerobic metabolism. As oxygen levels decrease, cells rely more heavily on glycolysis for ATP production. This shift is mediated by the upregulation of glycolytic enzymes and glucose transporters, allowing for increased glucose uptake and utilization. The increased glycolytic flux leads to a higher production of lactate, which can be shuttled to the liver via the Cori cycle for gluconeogenesis.
The Cori cycle plays a crucial role in hypoxic conditions by facilitating the recycling of lactate produced in skeletal muscles. This process helps maintain blood glucose levels and provides an alternative energy source for tissues that can utilize lactate. The cycle's efficiency is enhanced during hypoxia, with increased activity of key enzymes such as lactate dehydrogenase and pyruvate carboxylase.
Hypoxia-inducible factors (HIFs) are central regulators of the cellular response to low oxygen levels. These transcription factors activate genes involved in angiogenesis, erythropoiesis, and metabolic reprogramming. HIF-1α, in particular, promotes the expression of genes encoding glucose transporters and glycolytic enzymes, further supporting the metabolic shift towards anaerobic glycolysis.
Mitochondrial function is also significantly affected by hypoxia. To minimize oxygen consumption and reduce the production of reactive oxygen species, cells downregulate mitochondrial oxidative phosphorylation. This is achieved through decreased expression of electron transport chain components and increased mitochondrial autophagy. Concurrently, there is an upregulation of mitochondrial glycerol-3-phosphate dehydrogenase, which helps maintain the glycerol-3-phosphate shuttle and supports glycolysis.
Lipid metabolism undergoes notable changes during hypoxia. Fatty acid oxidation is suppressed to conserve oxygen, while lipid synthesis is enhanced. This metabolic shift is mediated by HIF-1α, which promotes the expression of lipogenic enzymes and inhibits carnitine palmitoyltransferase 1 (CPT1), a key enzyme in fatty acid oxidation.
Protein metabolism is also altered in response to hypoxia. There is a general decrease in protein synthesis to conserve energy, accompanied by increased protein breakdown to provide amino acids for gluconeogenesis and other essential processes. However, the synthesis of specific proteins involved in hypoxia adaptation, such as erythropoietin and vascular endothelial growth factor, is upregulated.
Current Interventional Strategies
01 Metabolic adaptations in hypoxic conditions
The Cori cycle plays a crucial role in metabolic adaptations during hypoxia. Under low oxygen conditions, cells shift towards anaerobic glycolysis, producing lactate. The Cori cycle helps recycle this lactate in the liver, converting it back to glucose for energy production. This process helps maintain energy homeostasis and supports cellular function in oxygen-deprived environments.- Metabolic adaptations in hypoxic conditions: The Cori cycle plays a crucial role in metabolic adaptations during hypoxia. Under low oxygen conditions, cells shift towards anaerobic glycolysis, producing lactate. The Cori cycle helps recycle this lactate in the liver, converting it back to glucose for energy production. This process helps maintain energy homeostasis and supports cellular function in oxygen-deprived environments.
- Biomarkers for hypoxia and metabolic stress: Identification and measurement of specific biomarkers can indicate hypoxic conditions and associated metabolic stress. These biomarkers may include enzymes involved in the Cori cycle, lactate levels, and other metabolites. Monitoring these biomarkers can provide insights into the severity of hypoxia and the body's metabolic responses, potentially aiding in diagnosis and treatment strategies.
- Therapeutic interventions targeting the Cori cycle: Developing therapeutic strategies that modulate the Cori cycle can potentially mitigate the effects of hypoxia on cellular metabolism. These interventions may involve enhancing lactate clearance, improving glucose utilization, or modulating key enzymes in the cycle. Such approaches could have applications in treating conditions associated with tissue hypoxia, such as ischemic diseases or high-altitude sickness.
- Genetic factors influencing hypoxia response: Genetic variations can affect an individual's response to hypoxic conditions and the efficiency of the Cori cycle. Studying these genetic factors can provide insights into why some individuals are more susceptible to hypoxia-related complications. This knowledge could lead to personalized approaches for preventing or treating hypoxia-induced metabolic disturbances.
- Imaging techniques for assessing metabolic responses to hypoxia: Advanced imaging technologies can be used to visualize and quantify metabolic changes associated with the Cori cycle and hypoxia in real-time. These techniques may include PET scans, MRI spectroscopy, or other molecular imaging methods. Such approaches allow for non-invasive assessment of tissue metabolism under hypoxic conditions, potentially improving diagnosis and monitoring of hypoxia-related disorders.
02 Biomarkers for hypoxia detection
Identification and measurement of specific biomarkers can help detect and monitor hypoxic conditions in the body. These biomarkers may include metabolites involved in the Cori cycle, such as lactate levels, as well as other molecules that are altered in response to low oxygen environments. Monitoring these biomarkers can provide insights into the severity of hypoxia and guide treatment strategies.Expand Specific Solutions03 Therapeutic interventions targeting hypoxia-induced metabolic changes
Understanding the metabolic responses to hypoxia, including the Cori cycle, can lead to the development of targeted therapeutic interventions. These may include drugs or treatments that modulate glucose metabolism, enhance lactate clearance, or improve oxygen utilization in tissues. Such interventions could potentially mitigate the negative effects of hypoxia in various medical conditions.Expand Specific Solutions04 Genetic factors influencing hypoxia response
Genetic variations can affect an individual's response to hypoxic conditions and the efficiency of metabolic adaptations like the Cori cycle. Studying these genetic factors can help predict susceptibility to hypoxia-related disorders and personalize treatment approaches. This research may involve identifying specific genes or genetic markers associated with improved or impaired hypoxia tolerance.Expand Specific Solutions05 Exercise physiology and hypoxia
The Cori cycle and hypoxia metabolic responses play a significant role in exercise physiology, particularly in high-altitude or low-oxygen environments. Understanding these processes can help optimize athletic performance, develop training strategies, and prevent altitude-related illnesses. This research area focuses on how the body adapts to exercise-induced hypoxia and maintains energy balance through metabolic pathways like the Cori cycle.Expand Specific Solutions
Key Research Institutions and Scientists
The research on the Cori Cycle and hypoxia is in a mature stage, with significant contributions from established institutions and pharmaceutical companies. The market for related interventions is substantial, driven by the prevalence of metabolic disorders and hypoxia-related conditions. Key players like Harvard Medical School, AstraZeneca, and Eli Lilly are at the forefront, leveraging their extensive R&D capabilities. Emerging biotechnology firms such as FibroGen are also making notable advancements, particularly in hypoxia-inducible factor biology. The competitive landscape is characterized by a mix of academic research centers, large pharmaceutical corporations, and specialized biotech companies, indicating a robust and diverse field of study with potential for further innovations in metabolic responses and therapeutic interventions.
President & Fellows of Harvard College
Technical Solution: Harvard College has conducted extensive research on the Cori Cycle and hypoxia, focusing on metabolic responses and interventions. Their approach involves studying the interplay between glucose and lactate metabolism under hypoxic conditions. They have developed advanced imaging techniques to visualize metabolic changes in real-time, allowing for a deeper understanding of the Cori Cycle's dynamics during oxygen deprivation[1]. Additionally, they have explored the role of HIF-1α (Hypoxia-Inducible Factor 1-alpha) in regulating metabolic adaptations to hypoxia, identifying potential therapeutic targets for conditions such as ischemia and cancer[2]. Their research also extends to investigating the impact of intermittent hypoxia on metabolic health, providing insights into sleep apnea and high-altitude physiology[3].
Strengths: Access to cutting-edge research facilities and interdisciplinary collaboration. Weaknesses: Potential limitations in translating academic research to clinical applications.
AstraZeneca AB
Technical Solution: AstraZeneca AB has developed a comprehensive research program on the Cori Cycle and hypoxia, with a focus on therapeutic interventions. Their approach centers on modulating key enzymes involved in the Cori Cycle to improve metabolic efficiency under hypoxic conditions. They have made significant progress in developing small molecule inhibitors targeting lactate dehydrogenase (LDH) and pyruvate dehydrogenase kinase (PDK), which play crucial roles in the cycle[4]. AstraZeneca's research also extends to exploring the potential of these interventions in treating ischemic diseases and cancer. They have conducted preclinical studies demonstrating improved tissue oxygenation and reduced tumor growth through manipulation of the Cori Cycle[5]. Furthermore, they are investigating the use of metabolomics and artificial intelligence to predict individual responses to hypoxia and tailor treatments accordingly[6].
Strengths: Strong pharmaceutical development capabilities and global clinical trial network. Weaknesses: Potential regulatory hurdles and competition in the pharmaceutical market.
Molecular Pathways in Hypoxic Metabolism
Coumarin derivatives, processes for their preparation and uses thereof for the treatment of cancer
PatentWO2019057821A1
Innovation
- Development of specific coumarin derivatives that inhibit POLRMT, capable of inhibiting mitochondrial DNA replication and transcription, offering a novel approach for cancer treatment, including use in combination with other cancer therapies.
Compositions to Modify Intestinal Nutrient Absorption, Methods of Making and Uses Thereof
PatentPendingUS20230405042A1
Innovation
- Administering a polymer comprising L-lactate monomers that inhibit or reduce D-lactate transport across the intestinal barrier, either alone or in combination with agents promoting D-lactate degradation, to lower blood glucose, liver fat, and insulin resistance by trapping D-lactate in the intestinal lumen and preventing its absorption into the bloodstream.
Imaging Techniques for Metabolic Assessment
Imaging techniques play a crucial role in assessing metabolic responses in the context of the Cori cycle and hypoxia. These advanced methods provide valuable insights into the complex interplay between glucose metabolism, lactate production, and oxygen availability in various tissues and organs.
Positron Emission Tomography (PET) has emerged as a powerful tool for visualizing and quantifying metabolic processes in vivo. By utilizing radioactive tracers such as 18F-fluorodeoxyglucose (FDG), PET can map glucose uptake and metabolism throughout the body. This technique is particularly useful for studying the Cori cycle, as it allows researchers to track the movement of glucose between tissues and observe changes in metabolic activity under hypoxic conditions.
Magnetic Resonance Spectroscopy (MRS) offers a non-invasive approach to measure metabolite concentrations in specific tissues. This technique can detect and quantify key metabolites involved in the Cori cycle, such as glucose, lactate, and pyruvate. MRS is especially valuable for investigating metabolic shifts in the liver and skeletal muscles during hypoxia, providing detailed information on the balance between glycolysis and gluconeogenesis.
Hyperpolarized 13C Magnetic Resonance Imaging (HP 13C MRI) represents a cutting-edge method for real-time metabolic imaging. This technique enhances the sensitivity of traditional MRI by several orders of magnitude, allowing for the visualization of metabolic fluxes in vivo. HP 13C MRI is particularly useful for studying the Cori cycle, as it can track the conversion of pyruvate to lactate and back to glucose, providing dynamic information on metabolic pathways under various conditions.
Functional Near-Infrared Spectroscopy (fNIRS) offers a non-invasive approach to measure tissue oxygenation and hemodynamics. This technique is valuable for assessing the impact of hypoxia on metabolic processes, as it can detect changes in oxygen availability and utilization in real-time. fNIRS is particularly useful for studying brain metabolism during hypoxic conditions, providing insights into the relationship between oxygen availability and glucose utilization.
Bioluminescence Imaging (BLI) allows for the visualization of specific metabolic processes in living organisms. By using genetically encoded luciferase reporters, researchers can monitor the expression of key enzymes involved in the Cori cycle and hypoxia response. This technique is particularly useful for studying metabolic adaptations to hypoxia in animal models, providing longitudinal data on metabolic changes over time.
These imaging techniques, when used in combination, provide a comprehensive view of metabolic responses to hypoxia and the dynamics of the Cori cycle. By integrating data from multiple imaging modalities, researchers can gain a more complete understanding of the complex interplay between oxygen availability, glucose metabolism, and lactate production in various physiological and pathological conditions.
Positron Emission Tomography (PET) has emerged as a powerful tool for visualizing and quantifying metabolic processes in vivo. By utilizing radioactive tracers such as 18F-fluorodeoxyglucose (FDG), PET can map glucose uptake and metabolism throughout the body. This technique is particularly useful for studying the Cori cycle, as it allows researchers to track the movement of glucose between tissues and observe changes in metabolic activity under hypoxic conditions.
Magnetic Resonance Spectroscopy (MRS) offers a non-invasive approach to measure metabolite concentrations in specific tissues. This technique can detect and quantify key metabolites involved in the Cori cycle, such as glucose, lactate, and pyruvate. MRS is especially valuable for investigating metabolic shifts in the liver and skeletal muscles during hypoxia, providing detailed information on the balance between glycolysis and gluconeogenesis.
Hyperpolarized 13C Magnetic Resonance Imaging (HP 13C MRI) represents a cutting-edge method for real-time metabolic imaging. This technique enhances the sensitivity of traditional MRI by several orders of magnitude, allowing for the visualization of metabolic fluxes in vivo. HP 13C MRI is particularly useful for studying the Cori cycle, as it can track the conversion of pyruvate to lactate and back to glucose, providing dynamic information on metabolic pathways under various conditions.
Functional Near-Infrared Spectroscopy (fNIRS) offers a non-invasive approach to measure tissue oxygenation and hemodynamics. This technique is valuable for assessing the impact of hypoxia on metabolic processes, as it can detect changes in oxygen availability and utilization in real-time. fNIRS is particularly useful for studying brain metabolism during hypoxic conditions, providing insights into the relationship between oxygen availability and glucose utilization.
Bioluminescence Imaging (BLI) allows for the visualization of specific metabolic processes in living organisms. By using genetically encoded luciferase reporters, researchers can monitor the expression of key enzymes involved in the Cori cycle and hypoxia response. This technique is particularly useful for studying metabolic adaptations to hypoxia in animal models, providing longitudinal data on metabolic changes over time.
These imaging techniques, when used in combination, provide a comprehensive view of metabolic responses to hypoxia and the dynamics of the Cori cycle. By integrating data from multiple imaging modalities, researchers can gain a more complete understanding of the complex interplay between oxygen availability, glucose metabolism, and lactate production in various physiological and pathological conditions.
Ethical Considerations in Hypoxia Research
Ethical considerations in hypoxia research are paramount due to the potential risks and implications for human subjects. The study of the Cori cycle and hypoxic conditions involves complex physiological responses that require careful experimental design and participant safety protocols.
One primary ethical concern is the potential harm to research participants exposed to hypoxic conditions. Researchers must carefully weigh the scientific benefits against the risks of inducing hypoxia, even temporarily. Strict safety measures, including continuous monitoring of vital signs and immediate intervention protocols, are essential to protect participants' well-being.
Informed consent is another critical ethical aspect. Participants must be fully aware of the potential risks and discomforts associated with hypoxia experiments. Researchers have a responsibility to provide clear, comprehensive information about the study procedures, potential side effects, and long-term implications. Special consideration should be given to vulnerable populations, such as those with pre-existing cardiovascular or respiratory conditions.
The design of hypoxia studies must adhere to the principles of scientific integrity and minimize unnecessary risks. This includes using the lowest level of hypoxia necessary to achieve research objectives and limiting exposure duration. Additionally, researchers should consider alternative methods or simulations when possible to reduce the need for human subject involvement in potentially risky conditions.
Data privacy and confidentiality are also significant ethical concerns in hypoxia research. Given the sensitive nature of physiological data collected during these studies, robust measures must be in place to protect participants' personal and medical information. This includes secure data storage, anonymization procedures, and strict access controls.
Long-term follow-up and monitoring of participants in hypoxia studies are essential ethical considerations. Researchers have a responsibility to assess and address any potential long-term effects of hypoxia exposure, even after the immediate study period has concluded. This may involve periodic health check-ups and continued communication with participants.
Ethical review boards play a crucial role in ensuring the ethical conduct of hypoxia research. All studies should undergo rigorous review by institutional ethics committees to assess the balance between scientific merit and participant safety. These boards should include experts in physiology, ethics, and patient advocacy to provide comprehensive evaluation of research protocols.
Transparency in reporting research findings is another ethical imperative. Researchers must accurately and completely disclose all results, including negative outcomes or unexpected effects, to contribute to the broader scientific understanding of hypoxia and the Cori cycle. This transparency is essential for building trust in the research community and ensuring the safety of future studies.
One primary ethical concern is the potential harm to research participants exposed to hypoxic conditions. Researchers must carefully weigh the scientific benefits against the risks of inducing hypoxia, even temporarily. Strict safety measures, including continuous monitoring of vital signs and immediate intervention protocols, are essential to protect participants' well-being.
Informed consent is another critical ethical aspect. Participants must be fully aware of the potential risks and discomforts associated with hypoxia experiments. Researchers have a responsibility to provide clear, comprehensive information about the study procedures, potential side effects, and long-term implications. Special consideration should be given to vulnerable populations, such as those with pre-existing cardiovascular or respiratory conditions.
The design of hypoxia studies must adhere to the principles of scientific integrity and minimize unnecessary risks. This includes using the lowest level of hypoxia necessary to achieve research objectives and limiting exposure duration. Additionally, researchers should consider alternative methods or simulations when possible to reduce the need for human subject involvement in potentially risky conditions.
Data privacy and confidentiality are also significant ethical concerns in hypoxia research. Given the sensitive nature of physiological data collected during these studies, robust measures must be in place to protect participants' personal and medical information. This includes secure data storage, anonymization procedures, and strict access controls.
Long-term follow-up and monitoring of participants in hypoxia studies are essential ethical considerations. Researchers have a responsibility to assess and address any potential long-term effects of hypoxia exposure, even after the immediate study period has concluded. This may involve periodic health check-ups and continued communication with participants.
Ethical review boards play a crucial role in ensuring the ethical conduct of hypoxia research. All studies should undergo rigorous review by institutional ethics committees to assess the balance between scientific merit and participant safety. These boards should include experts in physiology, ethics, and patient advocacy to provide comprehensive evaluation of research protocols.
Transparency in reporting research findings is another ethical imperative. Researchers must accurately and completely disclose all results, including negative outcomes or unexpected effects, to contribute to the broader scientific understanding of hypoxia and the Cori cycle. This transparency is essential for building trust in the research community and ensuring the safety of future studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!