Cori Cycle Measurement Best Practices For Clinical Trials
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cori Cycle Overview
The Cori cycle, also known as the glucose-alanine cycle, is a metabolic pathway that plays a crucial role in glucose homeostasis and amino acid metabolism. Named after Nobel laureate Carl Cori, this cycle represents a significant interorgan cooperation between skeletal muscle and the liver. It serves as a mechanism for transporting amino groups from peripheral tissues to the liver for urea synthesis while simultaneously providing glucose for energy production in muscle tissue.
In the context of clinical trials, understanding and accurately measuring the Cori cycle is essential for evaluating metabolic disorders, assessing drug efficacy, and monitoring patient responses to various treatments. The cycle begins in skeletal muscle, where amino acids, particularly alanine, are released into the bloodstream. These amino acids are then taken up by the liver, where they undergo deamination and conversion to glucose through gluconeogenesis.
The measurement of the Cori cycle in clinical trials typically involves tracking the flux of metabolites between muscle and liver tissues. This process requires sophisticated techniques such as stable isotope tracer methodologies, which allow researchers to follow the movement of labeled molecules through the metabolic pathway. By analyzing the incorporation and distribution of these tracers, investigators can quantify the rate of glucose-alanine cycling and assess its impact on overall glucose metabolism.
One of the key challenges in measuring the Cori cycle is the need for simultaneous sampling from multiple tissue sites. This often necessitates the use of arteriovenous difference techniques, where blood samples are collected from both arterial and venous sources to determine the net uptake or release of metabolites across specific organs. Additionally, nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry have emerged as powerful tools for analyzing the metabolic flux through the Cori cycle with high precision.
The importance of the Cori cycle in clinical trials extends beyond its role in glucose metabolism. It serves as a valuable indicator of whole-body protein turnover and can provide insights into the metabolic adaptations occurring in various disease states, such as diabetes, cancer, and liver disorders. Furthermore, the cycle's activity can be influenced by factors like nutritional status, hormonal balance, and physical activity, making it a sensitive marker for assessing the efficacy of therapeutic interventions targeting metabolic pathways.
As research in metabolomics and systems biology continues to advance, new technologies and analytical approaches are being developed to enhance the accuracy and comprehensiveness of Cori cycle measurements. These advancements promise to provide a more nuanced understanding of metabolic regulation and offer new avenues for developing targeted therapies in a wide range of clinical conditions.
In the context of clinical trials, understanding and accurately measuring the Cori cycle is essential for evaluating metabolic disorders, assessing drug efficacy, and monitoring patient responses to various treatments. The cycle begins in skeletal muscle, where amino acids, particularly alanine, are released into the bloodstream. These amino acids are then taken up by the liver, where they undergo deamination and conversion to glucose through gluconeogenesis.
The measurement of the Cori cycle in clinical trials typically involves tracking the flux of metabolites between muscle and liver tissues. This process requires sophisticated techniques such as stable isotope tracer methodologies, which allow researchers to follow the movement of labeled molecules through the metabolic pathway. By analyzing the incorporation and distribution of these tracers, investigators can quantify the rate of glucose-alanine cycling and assess its impact on overall glucose metabolism.
One of the key challenges in measuring the Cori cycle is the need for simultaneous sampling from multiple tissue sites. This often necessitates the use of arteriovenous difference techniques, where blood samples are collected from both arterial and venous sources to determine the net uptake or release of metabolites across specific organs. Additionally, nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry have emerged as powerful tools for analyzing the metabolic flux through the Cori cycle with high precision.
The importance of the Cori cycle in clinical trials extends beyond its role in glucose metabolism. It serves as a valuable indicator of whole-body protein turnover and can provide insights into the metabolic adaptations occurring in various disease states, such as diabetes, cancer, and liver disorders. Furthermore, the cycle's activity can be influenced by factors like nutritional status, hormonal balance, and physical activity, making it a sensitive marker for assessing the efficacy of therapeutic interventions targeting metabolic pathways.
As research in metabolomics and systems biology continues to advance, new technologies and analytical approaches are being developed to enhance the accuracy and comprehensiveness of Cori cycle measurements. These advancements promise to provide a more nuanced understanding of metabolic regulation and offer new avenues for developing targeted therapies in a wide range of clinical conditions.
Clinical Trial Demand
The demand for clinical trials utilizing Cori Cycle measurement has been steadily increasing in recent years, driven by the growing need for more accurate and comprehensive metabolic assessments in various medical research fields. This trend is particularly evident in studies related to liver function, diabetes, and metabolic disorders, where the Cori Cycle plays a crucial role in glucose homeostasis.
Clinical researchers and pharmaceutical companies are increasingly recognizing the value of Cori Cycle measurements in evaluating the efficacy of new drugs and treatments targeting metabolic pathways. This has led to a surge in the incorporation of these measurements into clinical trial protocols, especially in Phase II and III studies focusing on metabolic diseases.
The rising prevalence of metabolic disorders worldwide has been a significant factor in driving the demand for Cori Cycle measurements in clinical trials. With the global diabetes epidemic showing no signs of slowing down, there is an urgent need for more sophisticated tools to assess glucose metabolism and insulin sensitivity. Cori Cycle measurements provide valuable insights into these processes, making them an attractive option for researchers and clinicians alike.
Furthermore, the advent of personalized medicine has created a new avenue for Cori Cycle measurements in clinical trials. As researchers strive to develop targeted therapies based on individual metabolic profiles, the ability to accurately measure and interpret Cori Cycle activity has become increasingly important. This has led to a growing demand for standardized protocols and best practices for incorporating these measurements into clinical trial designs.
The pharmaceutical industry's focus on developing novel treatments for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has also contributed to the increased demand for Cori Cycle measurements in clinical trials. These conditions, which are closely linked to metabolic dysfunction, require comprehensive assessments of liver metabolism, making Cori Cycle measurements an essential component of many studies in this field.
Additionally, the integration of advanced technologies, such as stable isotope tracers and high-resolution mass spectrometry, has enhanced the precision and reliability of Cori Cycle measurements. This technological progress has made these measurements more accessible and appealing to a broader range of clinical researchers, further driving demand in the clinical trial sector.
As the importance of metabolic health in overall well-being becomes more widely recognized, there is a growing interest in incorporating Cori Cycle measurements into clinical trials beyond traditional metabolic disease research. Fields such as oncology, cardiovascular medicine, and neurology are beginning to explore the potential applications of these measurements in their respective areas, expanding the demand for Cori Cycle assessments across various medical disciplines.
Clinical researchers and pharmaceutical companies are increasingly recognizing the value of Cori Cycle measurements in evaluating the efficacy of new drugs and treatments targeting metabolic pathways. This has led to a surge in the incorporation of these measurements into clinical trial protocols, especially in Phase II and III studies focusing on metabolic diseases.
The rising prevalence of metabolic disorders worldwide has been a significant factor in driving the demand for Cori Cycle measurements in clinical trials. With the global diabetes epidemic showing no signs of slowing down, there is an urgent need for more sophisticated tools to assess glucose metabolism and insulin sensitivity. Cori Cycle measurements provide valuable insights into these processes, making them an attractive option for researchers and clinicians alike.
Furthermore, the advent of personalized medicine has created a new avenue for Cori Cycle measurements in clinical trials. As researchers strive to develop targeted therapies based on individual metabolic profiles, the ability to accurately measure and interpret Cori Cycle activity has become increasingly important. This has led to a growing demand for standardized protocols and best practices for incorporating these measurements into clinical trial designs.
The pharmaceutical industry's focus on developing novel treatments for non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has also contributed to the increased demand for Cori Cycle measurements in clinical trials. These conditions, which are closely linked to metabolic dysfunction, require comprehensive assessments of liver metabolism, making Cori Cycle measurements an essential component of many studies in this field.
Additionally, the integration of advanced technologies, such as stable isotope tracers and high-resolution mass spectrometry, has enhanced the precision and reliability of Cori Cycle measurements. This technological progress has made these measurements more accessible and appealing to a broader range of clinical researchers, further driving demand in the clinical trial sector.
As the importance of metabolic health in overall well-being becomes more widely recognized, there is a growing interest in incorporating Cori Cycle measurements into clinical trials beyond traditional metabolic disease research. Fields such as oncology, cardiovascular medicine, and neurology are beginning to explore the potential applications of these measurements in their respective areas, expanding the demand for Cori Cycle assessments across various medical disciplines.
Measurement Challenges
The measurement of the Cori cycle in clinical trials presents several significant challenges that researchers must address to ensure accurate and reliable results. One of the primary difficulties lies in the complexity of the metabolic pathways involved, which require sophisticated techniques to quantify accurately. The Cori cycle, also known as the glucose-lactate cycle, involves the interconversion of glucose and lactate between the liver and peripheral tissues, making it challenging to isolate and measure specific components of the cycle.
A major challenge in Cori cycle measurement is the need for simultaneous assessment of multiple metabolic processes. This requires the use of advanced imaging techniques, such as positron emission tomography (PET) or magnetic resonance spectroscopy (MRS), combined with isotope tracers. These methods, while powerful, can be costly and may not be readily available in all clinical trial settings, potentially limiting the scope and feasibility of studies.
The dynamic nature of the Cori cycle poses another significant challenge. The cycle's activity can fluctuate rapidly in response to various physiological and environmental factors, including exercise, stress, and nutritional status. This variability makes it difficult to obtain consistent measurements and necessitates careful control of experimental conditions. Researchers must develop standardized protocols that account for these fluctuations and ensure that measurements are taken under comparable conditions across all subjects and time points.
Interindividual variability in metabolic rates and responses to interventions further complicates Cori cycle measurements in clinical trials. Factors such as age, sex, body composition, and genetic background can significantly influence the cycle's activity, requiring researchers to carefully consider subject selection criteria and potentially increase sample sizes to account for this variability.
The invasive nature of some measurement techniques presents both ethical and practical challenges. While less invasive methods are preferable, they may not provide the same level of detail or accuracy as more invasive approaches. Striking a balance between measurement precision and subject comfort is crucial for maintaining ethical standards and ensuring participant compliance throughout the trial.
Lastly, the interpretation of Cori cycle measurements in the context of clinical outcomes presents a significant challenge. Establishing clear correlations between changes in cycle activity and clinically relevant endpoints requires careful study design and statistical analysis. Researchers must develop robust methods for translating complex metabolic data into meaningful insights that can inform clinical decision-making and drug development processes.
A major challenge in Cori cycle measurement is the need for simultaneous assessment of multiple metabolic processes. This requires the use of advanced imaging techniques, such as positron emission tomography (PET) or magnetic resonance spectroscopy (MRS), combined with isotope tracers. These methods, while powerful, can be costly and may not be readily available in all clinical trial settings, potentially limiting the scope and feasibility of studies.
The dynamic nature of the Cori cycle poses another significant challenge. The cycle's activity can fluctuate rapidly in response to various physiological and environmental factors, including exercise, stress, and nutritional status. This variability makes it difficult to obtain consistent measurements and necessitates careful control of experimental conditions. Researchers must develop standardized protocols that account for these fluctuations and ensure that measurements are taken under comparable conditions across all subjects and time points.
Interindividual variability in metabolic rates and responses to interventions further complicates Cori cycle measurements in clinical trials. Factors such as age, sex, body composition, and genetic background can significantly influence the cycle's activity, requiring researchers to carefully consider subject selection criteria and potentially increase sample sizes to account for this variability.
The invasive nature of some measurement techniques presents both ethical and practical challenges. While less invasive methods are preferable, they may not provide the same level of detail or accuracy as more invasive approaches. Striking a balance between measurement precision and subject comfort is crucial for maintaining ethical standards and ensuring participant compliance throughout the trial.
Lastly, the interpretation of Cori cycle measurements in the context of clinical outcomes presents a significant challenge. Establishing clear correlations between changes in cycle activity and clinically relevant endpoints requires careful study design and statistical analysis. Researchers must develop robust methods for translating complex metabolic data into meaningful insights that can inform clinical decision-making and drug development processes.
Current Measurement
01 Metabolic pathway analysis techniques
Advanced techniques for analyzing metabolic pathways, including the Cori cycle, involve using computational models and data integration methods. These approaches allow for more accurate measurement and understanding of the cycle's dynamics, incorporating factors such as enzyme kinetics and substrate concentrations.- Metabolic pathway analysis techniques: Advanced techniques for analyzing metabolic pathways, including the Cori cycle, involve using computational models and data integration methods. These approaches help in understanding the flux of metabolites and energy in the cycle, providing insights into glucose homeostasis and liver-muscle interactions.
- Isotope labeling and tracing methods: Isotope labeling and tracing methods are crucial for measuring Cori cycle activity. These techniques involve using stable isotopes to track the movement of glucose and lactate between tissues, allowing for precise quantification of cycle flux and turnover rates.
- Real-time monitoring systems: Development of real-time monitoring systems for Cori cycle measurements involves integrating biosensors and continuous glucose monitoring technologies. These systems enable dynamic assessment of glucose-lactate interconversion and provide valuable data on cycle kinetics under various physiological conditions.
- Data analysis and machine learning algorithms: Advanced data analysis techniques and machine learning algorithms are employed to process and interpret complex datasets generated from Cori cycle measurements. These computational approaches help in identifying patterns, predicting cycle behavior, and optimizing measurement protocols.
- Standardization and quality control protocols: Establishing standardized protocols and quality control measures for Cori cycle measurements is essential for ensuring reproducibility and reliability of results. This includes developing best practices for sample collection, processing, and data reporting across different research settings.
02 Real-time monitoring systems
Implementing real-time monitoring systems for the Cori cycle involves using biosensors and continuous data collection methods. These systems can track glucose and lactate levels, providing instant feedback on the cycle's activity and allowing for more precise measurements and interventions.Expand Specific Solutions03 Machine learning algorithms for data analysis
Applying machine learning algorithms to analyze Cori cycle data can improve the accuracy of measurements and predictions. These algorithms can identify patterns and correlations in large datasets, helping to refine measurement practices and interpret complex metabolic interactions.Expand Specific Solutions04 Standardization of measurement protocols
Developing standardized protocols for Cori cycle measurements ensures consistency and comparability across different studies and laboratories. This includes specifying sample collection methods, timing of measurements, and data reporting formats to minimize variability and improve reproducibility.Expand Specific Solutions05 Integration with other metabolic assessments
Integrating Cori cycle measurements with other metabolic assessments provides a more comprehensive understanding of overall metabolic health. This holistic approach combines data from various pathways and biomarkers to create a more accurate picture of metabolic function and potential dysfunctions.Expand Specific Solutions
Key Industry Players
The Cori Cycle measurement for clinical trials is in a developing stage, with the market showing potential for growth as the importance of metabolic assessments in clinical research increases. The technology's maturity varies among key players, with established companies like Bio-Rad Laboratories and Cerner Innovation leading in innovation. Emerging firms such as Evelo Biosciences and POCARED Diagnostics are also contributing to advancements. Academic institutions like MIT and Baylor College of Medicine are driving research, while healthcare providers like Cedars-Sinai Medical Center are implementing these technologies in clinical settings. The competitive landscape is diverse, with both specialized biotech firms and larger healthcare conglomerates vying for market share in this niche but growing field.
The Board of Regents of The University of Texas System
Technical Solution: The University of Texas System has developed a comprehensive approach to Cori Cycle measurement in clinical trials. Their method involves using stable isotope tracers to accurately measure glucose production and gluconeogenesis rates[1]. They employ a combination of gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectroscopy for precise quantification of metabolites[2]. The protocol includes standardized fasting periods and controlled infusion rates of labeled glucose to ensure consistency across subjects. Additionally, they have implemented a novel algorithm for data analysis that accounts for individual variations in metabolic rates, improving the accuracy of results in diverse patient populations[3].
Strengths: High precision in metabolite quantification, standardized protocol for consistency, advanced data analysis. Weaknesses: Requires specialized equipment and expertise, potentially higher cost and complexity compared to simpler methods.
The Regents of the University of California
Technical Solution: The University of California has pioneered a multi-omics approach to Cori Cycle measurement in clinical trials. Their method integrates metabolomics, transcriptomics, and proteomics data to provide a comprehensive view of gluconeogenesis and glycolysis pathways[4]. They use high-resolution liquid chromatography-mass spectrometry (LC-MS) for metabolite profiling, coupled with RNA sequencing and targeted proteomics to assess enzyme expression levels[5]. This integrated approach allows for the identification of novel biomarkers and regulatory mechanisms affecting the Cori Cycle. The UC system has also developed a machine learning algorithm that predicts Cori Cycle flux based on these multi-omics datasets, potentially reducing the need for invasive measurements in some clinical scenarios[6].
Strengths: Comprehensive pathway analysis, identification of novel biomarkers, potential for non-invasive measurements. Weaknesses: Complex data integration and interpretation, requires extensive bioinformatics support.
Innovative Techniques
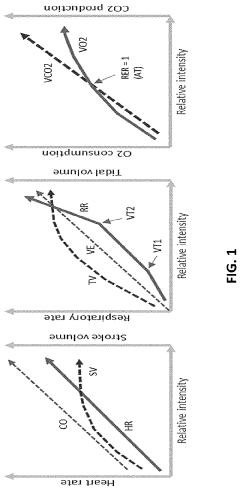
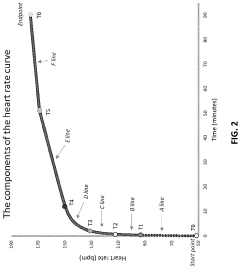
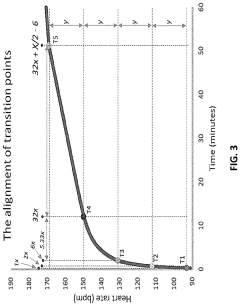
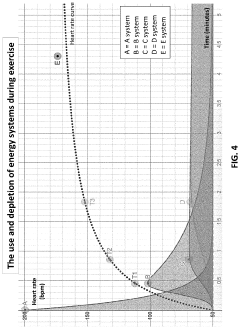
A method and system for determining exercise parameters including aerobic endurance based on heart rate curve analysis
PatentPendingUS20230138921A1
Innovation
- A computer-implemented method and system that analyzes heart rate data from submaximal exercises to determine exercise parameters like endurance, maximum speed, and lactate threshold, using a curve-fitting approach to identify transition points and calculate exercise parameters, compatible with various wearable devices like Apple Watch, Garmin, and Fitbit, providing a universal fitness metric and predicting race performance.
Prevention and/or treatment of chronic fatigue syndrome
PatentInactiveEP3544603A2
Innovation
- A composition comprising oxalic acid or its derivatives, administered as a pharmaceutical or nutritional supplement, along with a diagnostic method using abnormal lactate levels in the blood to indicate CFS/ME/SEID, involving lactate patterns and loads measured over time.
Regulatory Compliance
Regulatory compliance is a critical aspect of conducting Cori Cycle measurements in clinical trials. The Cori Cycle, which describes the metabolic pathway between the liver and skeletal muscles, requires careful monitoring and adherence to established guidelines to ensure the validity and reliability of results in clinical research settings.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating clinical trials, including those involving Cori Cycle measurements. Researchers must comply with Good Clinical Practice (GCP) guidelines, which are international ethical and scientific quality standards for designing, conducting, recording, and reporting trials involving human subjects.
The European Medicines Agency (EMA) also provides regulatory oversight for clinical trials conducted in the European Union. Their guidelines emphasize the importance of standardized protocols and quality control measures in metabolic studies, including those related to the Cori Cycle.
Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, is essential when handling patient information in Cori Cycle studies. These regulations mandate strict protocols for data collection, storage, and sharing.
Institutional Review Boards (IRBs) or Ethics Committees play a crucial role in ensuring regulatory compliance. They review and approve study protocols, informed consent forms, and other relevant documents to protect the rights and welfare of human subjects participating in Cori Cycle measurement studies.
Researchers must also adhere to specific regulations regarding the use of radioisotopes or other tracers often employed in Cori Cycle measurements. This includes obtaining necessary licenses and following safety protocols as mandated by regulatory bodies such as the Nuclear Regulatory Commission in the US.
Documentation and record-keeping are paramount in maintaining regulatory compliance. Detailed logs of equipment calibration, sample handling procedures, and raw data must be maintained and made available for regulatory inspections.
Adherence to international standards, such as those set by the International Conference on Harmonisation (ICH), ensures that Cori Cycle measurement practices in clinical trials meet global regulatory requirements. This is particularly important for multi-center or international studies.
Continuous monitoring and auditing of clinical trial processes related to Cori Cycle measurements are necessary to maintain compliance. This includes regular training of staff on updated regulations and best practices to ensure ongoing adherence to regulatory standards.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating clinical trials, including those involving Cori Cycle measurements. Researchers must comply with Good Clinical Practice (GCP) guidelines, which are international ethical and scientific quality standards for designing, conducting, recording, and reporting trials involving human subjects.
The European Medicines Agency (EMA) also provides regulatory oversight for clinical trials conducted in the European Union. Their guidelines emphasize the importance of standardized protocols and quality control measures in metabolic studies, including those related to the Cori Cycle.
Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US, is essential when handling patient information in Cori Cycle studies. These regulations mandate strict protocols for data collection, storage, and sharing.
Institutional Review Boards (IRBs) or Ethics Committees play a crucial role in ensuring regulatory compliance. They review and approve study protocols, informed consent forms, and other relevant documents to protect the rights and welfare of human subjects participating in Cori Cycle measurement studies.
Researchers must also adhere to specific regulations regarding the use of radioisotopes or other tracers often employed in Cori Cycle measurements. This includes obtaining necessary licenses and following safety protocols as mandated by regulatory bodies such as the Nuclear Regulatory Commission in the US.
Documentation and record-keeping are paramount in maintaining regulatory compliance. Detailed logs of equipment calibration, sample handling procedures, and raw data must be maintained and made available for regulatory inspections.
Adherence to international standards, such as those set by the International Conference on Harmonisation (ICH), ensures that Cori Cycle measurement practices in clinical trials meet global regulatory requirements. This is particularly important for multi-center or international studies.
Continuous monitoring and auditing of clinical trial processes related to Cori Cycle measurements are necessary to maintain compliance. This includes regular training of staff on updated regulations and best practices to ensure ongoing adherence to regulatory standards.
Data Management
Data management is a critical component in ensuring the accuracy and reliability of Cori cycle measurements in clinical trials. Effective data management practices are essential for maintaining data integrity, facilitating analysis, and supporting regulatory compliance.
One key aspect of data management for Cori cycle measurements is the implementation of standardized data collection protocols. These protocols should clearly define the methods for measuring and recording glucose and insulin levels, as well as other relevant parameters. Consistency in data collection across different sites and time points is crucial for the validity of the study results.
Data validation processes should be established to identify and address potential errors or inconsistencies in the collected data. This may include automated checks for out-of-range values, logical inconsistencies, and missing data points. Regular quality control reviews should be conducted to ensure adherence to the established protocols and to identify any systematic issues in data collection or recording.
Secure data storage and backup systems are essential to protect the integrity and confidentiality of the collected information. Clinical trial data should be stored in validated electronic data capture (EDC) systems that comply with regulatory requirements, such as 21 CFR Part 11. These systems should have robust access controls, audit trails, and data encryption measures in place.
Data integration from multiple sources, such as laboratory results, patient-reported outcomes, and electronic health records, may be necessary for comprehensive analysis of Cori cycle measurements. Establishing clear data integration procedures and ensuring data compatibility across different systems is crucial for maintaining data quality and facilitating efficient analysis.
Regular data monitoring and interim analyses should be conducted to assess the quality and completeness of the collected data. This allows for early identification of potential issues and enables timely corrective actions to be taken. It also provides an opportunity to assess the progress of the study and make informed decisions about potential protocol modifications if necessary.
Data cleaning and preparation procedures should be well-documented and consistently applied to ensure the accuracy and reliability of the final dataset. This may include handling missing data, addressing outliers, and applying appropriate transformations to the data as needed for analysis.
Finally, comprehensive documentation of all data management processes, including standard operating procedures (SOPs), data management plans, and data dictionaries, is essential for regulatory compliance and to facilitate reproducibility of the study results. This documentation should be maintained throughout the clinical trial and be readily available for audits or regulatory inspections.
One key aspect of data management for Cori cycle measurements is the implementation of standardized data collection protocols. These protocols should clearly define the methods for measuring and recording glucose and insulin levels, as well as other relevant parameters. Consistency in data collection across different sites and time points is crucial for the validity of the study results.
Data validation processes should be established to identify and address potential errors or inconsistencies in the collected data. This may include automated checks for out-of-range values, logical inconsistencies, and missing data points. Regular quality control reviews should be conducted to ensure adherence to the established protocols and to identify any systematic issues in data collection or recording.
Secure data storage and backup systems are essential to protect the integrity and confidentiality of the collected information. Clinical trial data should be stored in validated electronic data capture (EDC) systems that comply with regulatory requirements, such as 21 CFR Part 11. These systems should have robust access controls, audit trails, and data encryption measures in place.
Data integration from multiple sources, such as laboratory results, patient-reported outcomes, and electronic health records, may be necessary for comprehensive analysis of Cori cycle measurements. Establishing clear data integration procedures and ensuring data compatibility across different systems is crucial for maintaining data quality and facilitating efficient analysis.
Regular data monitoring and interim analyses should be conducted to assess the quality and completeness of the collected data. This allows for early identification of potential issues and enables timely corrective actions to be taken. It also provides an opportunity to assess the progress of the study and make informed decisions about potential protocol modifications if necessary.
Data cleaning and preparation procedures should be well-documented and consistently applied to ensure the accuracy and reliability of the final dataset. This may include handling missing data, addressing outliers, and applying appropriate transformations to the data as needed for analysis.
Finally, comprehensive documentation of all data management processes, including standard operating procedures (SOPs), data management plans, and data dictionaries, is essential for regulatory compliance and to facilitate reproducibility of the study results. This documentation should be maintained throughout the clinical trial and be readily available for audits or regulatory inspections.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!