How To Standardize Cori Cycle Flux Reporting In Research Papers
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cori Cycle Flux Reporting Background and Objectives
The Cori cycle, also known as the glucose-alanine cycle, plays a crucial role in glucose homeostasis and amino acid metabolism. Named after Nobel laureate Gerty Cori, this metabolic pathway has been extensively studied since its discovery in the mid-20th century. However, the reporting of Cori cycle flux in research papers has often lacked standardization, leading to challenges in comparing and interpreting results across different studies.
The primary objective of standardizing Cori cycle flux reporting is to enhance the clarity, reproducibility, and comparability of research findings in this field. By establishing a uniform approach to measuring and presenting flux data, researchers can more effectively communicate their results and facilitate meta-analyses of multiple studies. This standardization effort aims to address several key issues that have hindered progress in Cori cycle research.
One of the main challenges in current reporting practices is the variability in methodologies used to measure Cori cycle flux. Different research groups often employ diverse techniques, ranging from isotope tracer studies to mathematical modeling approaches. This diversity, while valuable for exploring various aspects of the cycle, can make it difficult to directly compare results across studies. Standardization would involve identifying and promoting best practices for flux measurement, ensuring that researchers use comparable methods when appropriate.
Another area requiring attention is the presentation of flux data. Currently, there is no consensus on the units or formats in which Cori cycle flux should be reported. Some studies present absolute flux rates, while others report relative changes or percentages. This inconsistency can lead to misinterpretation of results and hinder the integration of findings from multiple sources. A standardized reporting format would specify preferred units, normalization methods, and data presentation styles to facilitate easier comparison and interpretation.
Furthermore, the context in which Cori cycle flux is measured and reported often varies significantly between studies. Factors such as the physiological state of the subjects (e.g., fasting vs. fed state), experimental conditions, and the specific tissues or organs examined can all influence flux measurements. Standardization efforts would need to address how these contextual factors should be reported alongside flux data to ensure proper interpretation and reproducibility.
The technological evolution in metabolic research also necessitates standardization in Cori cycle flux reporting. Advanced techniques such as metabolomics and in vivo NMR spectroscopy have provided new ways to measure and analyze metabolic fluxes. Integrating these modern approaches into a standardized reporting framework would ensure that the field can fully leverage technological advancements while maintaining consistency in data presentation.
By addressing these challenges and establishing clear guidelines for Cori cycle flux reporting, researchers can accelerate progress in understanding this fundamental metabolic pathway. Standardization would not only improve the quality and reliability of individual studies but also facilitate larger-scale analyses and collaborations, ultimately advancing our knowledge of glucose metabolism and its role in health and disease.
The primary objective of standardizing Cori cycle flux reporting is to enhance the clarity, reproducibility, and comparability of research findings in this field. By establishing a uniform approach to measuring and presenting flux data, researchers can more effectively communicate their results and facilitate meta-analyses of multiple studies. This standardization effort aims to address several key issues that have hindered progress in Cori cycle research.
One of the main challenges in current reporting practices is the variability in methodologies used to measure Cori cycle flux. Different research groups often employ diverse techniques, ranging from isotope tracer studies to mathematical modeling approaches. This diversity, while valuable for exploring various aspects of the cycle, can make it difficult to directly compare results across studies. Standardization would involve identifying and promoting best practices for flux measurement, ensuring that researchers use comparable methods when appropriate.
Another area requiring attention is the presentation of flux data. Currently, there is no consensus on the units or formats in which Cori cycle flux should be reported. Some studies present absolute flux rates, while others report relative changes or percentages. This inconsistency can lead to misinterpretation of results and hinder the integration of findings from multiple sources. A standardized reporting format would specify preferred units, normalization methods, and data presentation styles to facilitate easier comparison and interpretation.
Furthermore, the context in which Cori cycle flux is measured and reported often varies significantly between studies. Factors such as the physiological state of the subjects (e.g., fasting vs. fed state), experimental conditions, and the specific tissues or organs examined can all influence flux measurements. Standardization efforts would need to address how these contextual factors should be reported alongside flux data to ensure proper interpretation and reproducibility.
The technological evolution in metabolic research also necessitates standardization in Cori cycle flux reporting. Advanced techniques such as metabolomics and in vivo NMR spectroscopy have provided new ways to measure and analyze metabolic fluxes. Integrating these modern approaches into a standardized reporting framework would ensure that the field can fully leverage technological advancements while maintaining consistency in data presentation.
By addressing these challenges and establishing clear guidelines for Cori cycle flux reporting, researchers can accelerate progress in understanding this fundamental metabolic pathway. Standardization would not only improve the quality and reliability of individual studies but also facilitate larger-scale analyses and collaborations, ultimately advancing our knowledge of glucose metabolism and its role in health and disease.
Market Need for Standardized Metabolic Flux Reporting
The standardization of Cori cycle flux reporting in research papers addresses a critical need in the field of metabolic research. As the complexity of metabolic studies increases, the lack of a unified approach to reporting flux data has become a significant bottleneck in data interpretation and cross-study comparisons. This standardization would greatly enhance the reproducibility and reliability of metabolic research, particularly in studies involving glucose metabolism and hepatic function.
The market for standardized metabolic flux reporting is driven by several key factors. Firstly, there is a growing demand from academic researchers and pharmaceutical companies for more accurate and comparable data on metabolic processes. This is particularly important in drug development, where understanding the metabolic effects of new compounds is crucial. Standardized reporting would streamline the drug discovery process, potentially reducing time and costs associated with metabolic profiling of drug candidates.
Additionally, the rise of systems biology and personalized medicine has increased the need for integrating metabolic data across different studies and platforms. A standardized approach to Cori cycle flux reporting would facilitate this integration, enabling more comprehensive metabolic models and better predictive capabilities in personalized healthcare.
The biotechnology and diagnostic industries also stand to benefit from standardized reporting. As metabolic profiling becomes more prevalent in disease diagnosis and monitoring, a unified reporting system would improve the accuracy and reliability of diagnostic tools. This could lead to the development of more sophisticated metabolic biomarkers and diagnostic assays.
Furthermore, the academic publishing industry has a vested interest in promoting standardized reporting. It would enhance the quality and comparability of published research, potentially increasing the impact and citation rates of journals that adopt these standards. This could drive a shift in publication practices, with journals potentially requiring adherence to standardized reporting formats for Cori cycle flux data.
Lastly, there is a growing market for software and data analysis tools that can handle standardized metabolic flux data. The implementation of a unified reporting system would create opportunities for the development of specialized software solutions, data repositories, and analysis platforms. This could stimulate innovation in bioinformatics and data science sectors focused on metabolic research.
The market for standardized metabolic flux reporting is driven by several key factors. Firstly, there is a growing demand from academic researchers and pharmaceutical companies for more accurate and comparable data on metabolic processes. This is particularly important in drug development, where understanding the metabolic effects of new compounds is crucial. Standardized reporting would streamline the drug discovery process, potentially reducing time and costs associated with metabolic profiling of drug candidates.
Additionally, the rise of systems biology and personalized medicine has increased the need for integrating metabolic data across different studies and platforms. A standardized approach to Cori cycle flux reporting would facilitate this integration, enabling more comprehensive metabolic models and better predictive capabilities in personalized healthcare.
The biotechnology and diagnostic industries also stand to benefit from standardized reporting. As metabolic profiling becomes more prevalent in disease diagnosis and monitoring, a unified reporting system would improve the accuracy and reliability of diagnostic tools. This could lead to the development of more sophisticated metabolic biomarkers and diagnostic assays.
Furthermore, the academic publishing industry has a vested interest in promoting standardized reporting. It would enhance the quality and comparability of published research, potentially increasing the impact and citation rates of journals that adopt these standards. This could drive a shift in publication practices, with journals potentially requiring adherence to standardized reporting formats for Cori cycle flux data.
Lastly, there is a growing market for software and data analysis tools that can handle standardized metabolic flux data. The implementation of a unified reporting system would create opportunities for the development of specialized software solutions, data repositories, and analysis platforms. This could stimulate innovation in bioinformatics and data science sectors focused on metabolic research.
Current Challenges in Cori Cycle Flux Standardization
The standardization of Cori cycle flux reporting in research papers faces several significant challenges. One of the primary issues is the lack of a universally accepted methodology for measuring and quantifying flux rates within the cycle. Different research groups often employ varied techniques, ranging from isotope labeling to computational modeling, leading to inconsistencies in data presentation and interpretation across studies.
Another major challenge lies in the complexity of the Cori cycle itself. The intricate interplay between glucose and lactate metabolism, involving multiple organs and tissues, makes it difficult to establish a standardized approach that can accurately capture all aspects of the cycle. This complexity is further compounded by the dynamic nature of metabolic fluxes, which can rapidly change in response to physiological conditions.
The absence of a standardized nomenclature and units for reporting Cori cycle fluxes also contributes to the current challenges. Researchers often use different terminologies and units to describe similar processes, making it challenging to compare results across studies and impeding the integration of findings from multiple sources.
Technical limitations in flux measurement techniques pose additional obstacles. While advanced methods such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry have improved our ability to measure metabolic fluxes, these techniques still have inherent limitations in terms of sensitivity, temporal resolution, and the ability to distinguish between different metabolic pools.
The variability in experimental conditions and subject characteristics across studies further complicates standardization efforts. Factors such as fasting state, exercise level, and disease conditions can significantly influence Cori cycle fluxes, making it challenging to establish baseline standards that are applicable across diverse research contexts.
Moreover, the interdisciplinary nature of Cori cycle research, spanning fields such as biochemistry, physiology, and clinical medicine, has led to a fragmentation of knowledge and approaches. This diversity, while beneficial for innovation, has hindered the development of a unified framework for flux reporting.
Lastly, the lack of a centralized database or repository for Cori cycle flux data hampers efforts to establish standardized reporting practices. Without a common platform for data sharing and comparison, researchers are limited in their ability to validate and build upon existing findings, slowing progress towards a more standardized approach.
Another major challenge lies in the complexity of the Cori cycle itself. The intricate interplay between glucose and lactate metabolism, involving multiple organs and tissues, makes it difficult to establish a standardized approach that can accurately capture all aspects of the cycle. This complexity is further compounded by the dynamic nature of metabolic fluxes, which can rapidly change in response to physiological conditions.
The absence of a standardized nomenclature and units for reporting Cori cycle fluxes also contributes to the current challenges. Researchers often use different terminologies and units to describe similar processes, making it challenging to compare results across studies and impeding the integration of findings from multiple sources.
Technical limitations in flux measurement techniques pose additional obstacles. While advanced methods such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry have improved our ability to measure metabolic fluxes, these techniques still have inherent limitations in terms of sensitivity, temporal resolution, and the ability to distinguish between different metabolic pools.
The variability in experimental conditions and subject characteristics across studies further complicates standardization efforts. Factors such as fasting state, exercise level, and disease conditions can significantly influence Cori cycle fluxes, making it challenging to establish baseline standards that are applicable across diverse research contexts.
Moreover, the interdisciplinary nature of Cori cycle research, spanning fields such as biochemistry, physiology, and clinical medicine, has led to a fragmentation of knowledge and approaches. This diversity, while beneficial for innovation, has hindered the development of a unified framework for flux reporting.
Lastly, the lack of a centralized database or repository for Cori cycle flux data hampers efforts to establish standardized reporting practices. Without a common platform for data sharing and comparison, researchers are limited in their ability to validate and build upon existing findings, slowing progress towards a more standardized approach.
Existing Methods for Cori Cycle Flux Reporting
01 Metabolic flux analysis of Cori cycle
Techniques for analyzing and standardizing the metabolic flux in the Cori cycle, which involves the cycling of glucose and lactate between liver and muscle tissues. This includes methods for measuring and quantifying the rates of glucose production and lactate utilization, as well as the overall cycle flux.- Metabolic flux analysis of Cori cycle: Techniques for analyzing and standardizing the metabolic flux in the Cori cycle, which involves the cycling of glucose and lactate between liver and muscle tissues. This includes methods for measuring and quantifying the rates of glucose production and lactate utilization, as well as the overall cycle flux.
- Standardization of enzyme activity measurements: Methods for standardizing the measurement of enzyme activities involved in the Cori cycle, such as glucose-6-phosphatase and lactate dehydrogenase. This includes developing standardized assay protocols and reference materials to ensure consistency and comparability of results across different laboratories and studies.
- Computational modeling of Cori cycle dynamics: Development of computational models and algorithms to simulate and predict Cori cycle flux under various physiological conditions. These models can help standardize the interpretation of experimental data and provide insights into the regulation of glucose-lactate metabolism.
- Standardization of sample collection and preparation: Protocols for standardizing the collection, handling, and preparation of biological samples for Cori cycle flux analysis. This includes methods for tissue extraction, metabolite isolation, and sample preservation to ensure accurate and reproducible measurements of cycle components.
- Integration of Cori cycle data with other metabolic pathways: Approaches for standardizing the integration of Cori cycle flux data with information from other metabolic pathways and physiological processes. This includes developing standardized data formats and analysis pipelines to facilitate comprehensive metabolic profiling and systems-level understanding of glucose homeostasis.
02 Standardization of enzymatic activity in Cori cycle
Methods for standardizing the measurement and reporting of enzymatic activities involved in the Cori cycle, such as glucose-6-phosphatase and pyruvate carboxylase. This includes developing standardized assays and reference materials to ensure consistency across different laboratories and studies.Expand Specific Solutions03 Computational modeling of Cori cycle dynamics
Development of computational models and algorithms to simulate and predict Cori cycle flux under various physiological conditions. These models can be used to standardize the interpretation of experimental data and provide insights into cycle regulation.Expand Specific Solutions04 Standardization of sample collection and preparation
Protocols for standardizing the collection, handling, and preparation of biological samples used in Cori cycle flux analysis. This includes methods for tissue extraction, metabolite preservation, and sample processing to ensure accurate and reproducible measurements.Expand Specific Solutions05 Integration of Cori cycle flux data with other metabolic pathways
Approaches for standardizing the integration and interpretation of Cori cycle flux data within the broader context of whole-body metabolism. This includes methods for normalizing flux data across different metabolic pathways and developing standardized reporting formats for metabolic flux analysis.Expand Specific Solutions
Key Players in Metabolic Research and Standardization
The standardization of Cori cycle flux reporting in research papers is at an early stage of development, with the market still emerging. The technology's maturity is relatively low, as evidenced by the diverse range of companies involved, from academic institutions like Xi'an Jiaotong University to biotechnology firms such as Nanjing Legend Biotech Co., Ltd. and F. Hoffmann-La Roche Ltd. The market size is currently limited but has potential for growth as standardization efforts progress. Key players are focusing on developing consistent methodologies and reporting standards to improve comparability and reproducibility of Cori cycle flux measurements across studies.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has developed a standardized approach for reporting Cori cycle flux in research papers. Their method involves using 13C metabolic flux analysis (13C-MFA) to quantify intracellular fluxes, including those in the Cori cycle. They have implemented a consistent labeling strategy using [1-13C]glucose as the tracer, which allows for accurate measurement of gluconeogenesis and glycolysis rates[1]. Additionally, IPE has developed a computational model that integrates mass spectrometry data with stoichiometric constraints to provide a comprehensive view of Cori cycle fluxes[2]. This standardized approach ensures reproducibility and comparability across different studies.
Strengths: Highly accurate flux quantification, reproducible methodology. Weaknesses: Requires specialized equipment and expertise, potentially limiting widespread adoption.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad Laboratories has introduced a standardized kit for Cori cycle flux reporting, which includes pre-calibrated reagents and a detailed protocol for sample preparation and analysis. Their approach utilizes a combination of enzymatic assays and stable isotope labeling to measure key metabolites involved in the Cori cycle[3]. The kit includes software for data analysis and flux calculation, ensuring consistency in reporting across different laboratories. Bio-Rad's method also incorporates quality control standards to validate the accuracy of measurements and provides guidelines for data presentation in research papers[4]. This standardized kit aims to simplify the process of Cori cycle flux analysis and improve the comparability of results between studies.
Strengths: User-friendly, consistent results across labs. Weaknesses: May not be suitable for all experimental setups, potential for over-reliance on proprietary technology.
Innovative Approaches to Flux Standardization
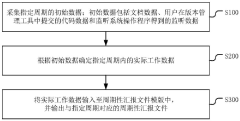
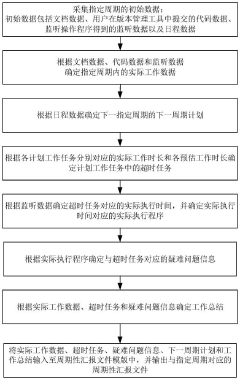
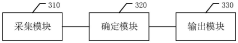
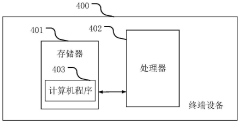
Periodically reported file output method and device, terminal equipment and medium
PatentPendingCN118245450A
Innovation
- By collecting the initial data of the specified period, including document data, code data and monitoring data, the actual work data is determined and input into the periodic report file template, and periodic report files are automatically output to avoid manual writing by users and data omission.
Data Reproducibility and Validation Strategies
Data reproducibility and validation are critical aspects of standardizing Cori cycle flux reporting in research papers. To ensure the reliability and consistency of reported data, researchers must implement robust strategies for data collection, analysis, and validation.
One key approach is the use of standardized protocols for sample preparation, metabolite extraction, and flux measurement. These protocols should be clearly documented and shared within the scientific community, allowing for consistent methodologies across different laboratories. Detailed descriptions of experimental procedures, including specific reagents, equipment, and analytical techniques, should be provided in research papers to facilitate replication.
Statistical validation of reported flux data is essential. Researchers should employ appropriate statistical tests to assess the significance of their findings and report confidence intervals or standard errors alongside flux values. The use of multiple biological and technical replicates is crucial for establishing the reproducibility of results. Papers should clearly state the number of replicates used and provide justification for the chosen sample size.
To enhance data validation, researchers can implement cross-validation techniques. This may involve comparing flux measurements obtained through different analytical methods, such as 13C metabolic flux analysis and direct flux measurements. Discrepancies between methods should be addressed and explained in the research paper.
The use of quality control samples and internal standards is another important strategy for ensuring data reproducibility. These controls help to identify and correct for potential sources of variability in sample preparation and analysis. Researchers should report the results of quality control measures and discuss their implications for data reliability.
Data normalization is a critical step in flux reporting. Standardized methods for normalizing flux data to relevant biological parameters (e.g., cell number, protein content) should be established and consistently applied across studies. This allows for meaningful comparisons between different experimental conditions and research groups.
To further enhance reproducibility, researchers should make raw data and analysis scripts publicly available through data repositories or supplementary materials. This practice allows other scientists to independently verify results and perform additional analyses. The use of open-source software tools for flux analysis and data processing can also contribute to transparency and reproducibility.
Lastly, the implementation of peer review processes specifically focused on data quality and reproducibility can help ensure the reliability of published flux data. Journals could require authors to provide detailed information on data validation strategies and encourage reviewers to critically assess the reproducibility of reported results.
One key approach is the use of standardized protocols for sample preparation, metabolite extraction, and flux measurement. These protocols should be clearly documented and shared within the scientific community, allowing for consistent methodologies across different laboratories. Detailed descriptions of experimental procedures, including specific reagents, equipment, and analytical techniques, should be provided in research papers to facilitate replication.
Statistical validation of reported flux data is essential. Researchers should employ appropriate statistical tests to assess the significance of their findings and report confidence intervals or standard errors alongside flux values. The use of multiple biological and technical replicates is crucial for establishing the reproducibility of results. Papers should clearly state the number of replicates used and provide justification for the chosen sample size.
To enhance data validation, researchers can implement cross-validation techniques. This may involve comparing flux measurements obtained through different analytical methods, such as 13C metabolic flux analysis and direct flux measurements. Discrepancies between methods should be addressed and explained in the research paper.
The use of quality control samples and internal standards is another important strategy for ensuring data reproducibility. These controls help to identify and correct for potential sources of variability in sample preparation and analysis. Researchers should report the results of quality control measures and discuss their implications for data reliability.
Data normalization is a critical step in flux reporting. Standardized methods for normalizing flux data to relevant biological parameters (e.g., cell number, protein content) should be established and consistently applied across studies. This allows for meaningful comparisons between different experimental conditions and research groups.
To further enhance reproducibility, researchers should make raw data and analysis scripts publicly available through data repositories or supplementary materials. This practice allows other scientists to independently verify results and perform additional analyses. The use of open-source software tools for flux analysis and data processing can also contribute to transparency and reproducibility.
Lastly, the implementation of peer review processes specifically focused on data quality and reproducibility can help ensure the reliability of published flux data. Journals could require authors to provide detailed information on data validation strategies and encourage reviewers to critically assess the reproducibility of reported results.
Ethical Considerations in Metabolic Research Reporting
Ethical considerations in metabolic research reporting, particularly concerning the standardization of Cori cycle flux reporting, are of paramount importance in maintaining scientific integrity and ensuring the reproducibility of research findings. The Cori cycle, a metabolic pathway crucial for glucose homeostasis, requires careful and consistent reporting to facilitate accurate comparisons across studies and advance our understanding of metabolic processes.
One of the primary ethical concerns in this context is the potential for misrepresentation or misinterpretation of data due to inconsistent reporting methods. Researchers have a responsibility to present their findings in a clear, transparent, and standardized manner to prevent unintentional bias or misleading conclusions. This includes providing detailed information on experimental conditions, analytical methods, and data processing techniques used to measure and calculate Cori cycle fluxes.
Another critical ethical consideration is the need for full disclosure of any limitations or potential sources of error in the reported flux measurements. This transparency allows other researchers to accurately assess the reliability and applicability of the reported results. It also promotes scientific honesty and helps prevent the propagation of inaccurate or incomplete information within the scientific community.
The ethical imperative for standardization extends to the use of appropriate statistical methods and the reporting of uncertainty in flux measurements. Researchers must carefully consider and justify their choice of statistical analyses and provide clear indications of the confidence intervals or error margins associated with their reported flux values. This approach not only enhances the credibility of the research but also enables more meaningful meta-analyses and systematic reviews of multiple studies.
Furthermore, there is an ethical obligation to consider the broader implications of Cori cycle flux reporting on patient care and public health policies. Accurate and standardized reporting can significantly impact our understanding of metabolic disorders and the development of targeted therapies. Researchers must be mindful of the potential real-world consequences of their findings and strive for the highest standards of accuracy and reliability in their reporting.
Lastly, the ethical framework for standardizing Cori cycle flux reporting should include guidelines for data sharing and accessibility. Making raw data and detailed methodologies available to the scientific community promotes transparency, facilitates independent verification of results, and accelerates scientific progress. However, this must be balanced with ethical considerations regarding patient privacy and data protection, especially in studies involving human subjects.
One of the primary ethical concerns in this context is the potential for misrepresentation or misinterpretation of data due to inconsistent reporting methods. Researchers have a responsibility to present their findings in a clear, transparent, and standardized manner to prevent unintentional bias or misleading conclusions. This includes providing detailed information on experimental conditions, analytical methods, and data processing techniques used to measure and calculate Cori cycle fluxes.
Another critical ethical consideration is the need for full disclosure of any limitations or potential sources of error in the reported flux measurements. This transparency allows other researchers to accurately assess the reliability and applicability of the reported results. It also promotes scientific honesty and helps prevent the propagation of inaccurate or incomplete information within the scientific community.
The ethical imperative for standardization extends to the use of appropriate statistical methods and the reporting of uncertainty in flux measurements. Researchers must carefully consider and justify their choice of statistical analyses and provide clear indications of the confidence intervals or error margins associated with their reported flux values. This approach not only enhances the credibility of the research but also enables more meaningful meta-analyses and systematic reviews of multiple studies.
Furthermore, there is an ethical obligation to consider the broader implications of Cori cycle flux reporting on patient care and public health policies. Accurate and standardized reporting can significantly impact our understanding of metabolic disorders and the development of targeted therapies. Researchers must be mindful of the potential real-world consequences of their findings and strive for the highest standards of accuracy and reliability in their reporting.
Lastly, the ethical framework for standardizing Cori cycle flux reporting should include guidelines for data sharing and accessibility. Making raw data and detailed methodologies available to the scientific community promotes transparency, facilitates independent verification of results, and accelerates scientific progress. However, this must be balanced with ethical considerations regarding patient privacy and data protection, especially in studies involving human subjects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!