How To Incorporate Cori Cycle Metrics Into Metabolic Models
AUG 21, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cori Cycle Integration Goals
The integration of Cori cycle metrics into metabolic models represents a significant advancement in our understanding of glucose homeostasis and energy metabolism. The primary goal of this integration is to enhance the accuracy and predictive power of existing metabolic models by incorporating the dynamic interplay between glucose and lactate metabolism.
One key objective is to capture the bidirectional flux of glucose and lactate between the liver and peripheral tissues, which is central to the Cori cycle. This involves developing mathematical equations that describe the rates of glucose production in the liver, glucose uptake by muscles and other tissues, lactate production in muscles, and lactate uptake by the liver for gluconeogenesis.
Another important aim is to incorporate the regulatory mechanisms that control the Cori cycle, including hormonal influences such as insulin and glucagon. These hormones play crucial roles in modulating the cycle's activity in response to varying metabolic states, such as fasting or exercise. By including these regulatory elements, the models can better simulate the body's adaptive responses to different physiological conditions.
Quantifying the energy costs associated with the Cori cycle is another critical goal. This includes accounting for the ATP consumption during gluconeogenesis in the liver and the energy expenditure in muscle tissues during glycolysis. Accurate representation of these energy balances will provide a more comprehensive view of whole-body energy metabolism.
Furthermore, the integration aims to elucidate the contribution of the Cori cycle to overall glucose homeostasis under various pathological conditions, such as diabetes or liver diseases. This could involve modeling how alterations in enzyme activities or substrate availabilities affect the cycle's efficiency and its impact on blood glucose levels.
Temporal dynamics are also a key consideration in this integration. The goal is to create models that can simulate the Cori cycle's behavior over different time scales, from rapid responses to exercise to longer-term adaptations to dietary changes. This temporal aspect is crucial for understanding how the cycle contributes to both acute and chronic metabolic regulation.
Lastly, the integration seeks to establish a framework for linking the Cori cycle to other metabolic pathways, such as lipid metabolism and amino acid metabolism. This holistic approach will provide a more complete picture of cellular and systemic metabolism, allowing for better predictions of metabolic outcomes in complex scenarios.
One key objective is to capture the bidirectional flux of glucose and lactate between the liver and peripheral tissues, which is central to the Cori cycle. This involves developing mathematical equations that describe the rates of glucose production in the liver, glucose uptake by muscles and other tissues, lactate production in muscles, and lactate uptake by the liver for gluconeogenesis.
Another important aim is to incorporate the regulatory mechanisms that control the Cori cycle, including hormonal influences such as insulin and glucagon. These hormones play crucial roles in modulating the cycle's activity in response to varying metabolic states, such as fasting or exercise. By including these regulatory elements, the models can better simulate the body's adaptive responses to different physiological conditions.
Quantifying the energy costs associated with the Cori cycle is another critical goal. This includes accounting for the ATP consumption during gluconeogenesis in the liver and the energy expenditure in muscle tissues during glycolysis. Accurate representation of these energy balances will provide a more comprehensive view of whole-body energy metabolism.
Furthermore, the integration aims to elucidate the contribution of the Cori cycle to overall glucose homeostasis under various pathological conditions, such as diabetes or liver diseases. This could involve modeling how alterations in enzyme activities or substrate availabilities affect the cycle's efficiency and its impact on blood glucose levels.
Temporal dynamics are also a key consideration in this integration. The goal is to create models that can simulate the Cori cycle's behavior over different time scales, from rapid responses to exercise to longer-term adaptations to dietary changes. This temporal aspect is crucial for understanding how the cycle contributes to both acute and chronic metabolic regulation.
Lastly, the integration seeks to establish a framework for linking the Cori cycle to other metabolic pathways, such as lipid metabolism and amino acid metabolism. This holistic approach will provide a more complete picture of cellular and systemic metabolism, allowing for better predictions of metabolic outcomes in complex scenarios.
Metabolic Modeling Market Trends
The metabolic modeling market is experiencing significant growth and transformation, driven by advancements in systems biology, computational capabilities, and the increasing demand for personalized medicine. This market segment is closely tied to the broader bioinformatics and computational biology sectors, which are projected to expand rapidly in the coming years.
One of the key trends in metabolic modeling is the integration of multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics. This holistic approach allows for more comprehensive and accurate models of cellular metabolism, enabling researchers to better understand complex biological processes and their regulation. The incorporation of Cori cycle metrics into these models represents an important step in this direction, as it provides crucial insights into glucose homeostasis and energy metabolism.
Another notable trend is the increasing adoption of machine learning and artificial intelligence techniques in metabolic modeling. These advanced computational methods are being used to improve model predictions, identify novel metabolic pathways, and optimize experimental design. As a result, the accuracy and predictive power of metabolic models are continuously improving, making them more valuable tools for both research and industrial applications.
The pharmaceutical industry has emerged as a major driver of growth in the metabolic modeling market. Drug discovery and development processes are increasingly relying on in silico models to predict drug efficacy, toxicity, and metabolic interactions. This trend is expected to accelerate as the industry seeks to reduce the time and cost associated with bringing new drugs to market.
Personalized medicine is another area where metabolic modeling is gaining traction. By incorporating individual genetic and metabolic profiles into models, researchers and clinicians can develop tailored treatment strategies for various metabolic disorders, including diabetes, obesity, and certain types of cancer. This personalized approach is expected to significantly improve patient outcomes and drive further investment in metabolic modeling technologies.
The biotechnology sector is also contributing to market growth, with an increasing focus on metabolic engineering for the production of biofuels, biochemicals, and other high-value compounds. Metabolic models are being used to optimize microbial strains and bioprocesses, leading to improved yields and reduced production costs.
Geographically, North America and Europe currently dominate the metabolic modeling market, due to their strong research infrastructure and significant investments in life sciences. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by increasing research funding, growing biotechnology sectors, and rising healthcare expenditures in countries like China and India.
One of the key trends in metabolic modeling is the integration of multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics. This holistic approach allows for more comprehensive and accurate models of cellular metabolism, enabling researchers to better understand complex biological processes and their regulation. The incorporation of Cori cycle metrics into these models represents an important step in this direction, as it provides crucial insights into glucose homeostasis and energy metabolism.
Another notable trend is the increasing adoption of machine learning and artificial intelligence techniques in metabolic modeling. These advanced computational methods are being used to improve model predictions, identify novel metabolic pathways, and optimize experimental design. As a result, the accuracy and predictive power of metabolic models are continuously improving, making them more valuable tools for both research and industrial applications.
The pharmaceutical industry has emerged as a major driver of growth in the metabolic modeling market. Drug discovery and development processes are increasingly relying on in silico models to predict drug efficacy, toxicity, and metabolic interactions. This trend is expected to accelerate as the industry seeks to reduce the time and cost associated with bringing new drugs to market.
Personalized medicine is another area where metabolic modeling is gaining traction. By incorporating individual genetic and metabolic profiles into models, researchers and clinicians can develop tailored treatment strategies for various metabolic disorders, including diabetes, obesity, and certain types of cancer. This personalized approach is expected to significantly improve patient outcomes and drive further investment in metabolic modeling technologies.
The biotechnology sector is also contributing to market growth, with an increasing focus on metabolic engineering for the production of biofuels, biochemicals, and other high-value compounds. Metabolic models are being used to optimize microbial strains and bioprocesses, leading to improved yields and reduced production costs.
Geographically, North America and Europe currently dominate the metabolic modeling market, due to their strong research infrastructure and significant investments in life sciences. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by increasing research funding, growing biotechnology sectors, and rising healthcare expenditures in countries like China and India.
Cori Cycle Modeling Challenges
Incorporating Cori cycle metrics into metabolic models presents several significant challenges. The Cori cycle, also known as the glucose-lactate cycle, is a complex metabolic pathway that plays a crucial role in glucose homeostasis. Integrating this cycle into existing metabolic models requires addressing multiple technical hurdles.
One of the primary challenges is accurately representing the bidirectional nature of the Cori cycle. The cycle involves the conversion of glucose to lactate in peripheral tissues and the subsequent reconversion of lactate back to glucose in the liver. This bidirectional flow of metabolites is difficult to capture in traditional metabolic models, which often assume unidirectional reactions.
Another significant obstacle is the need to account for the spatial separation of the cycle's components. The Cori cycle operates across different tissues and organs, primarily involving the liver and skeletal muscles. Metabolic models typically focus on cellular-level processes, making it challenging to incorporate the inter-organ dynamics inherent in the Cori cycle.
The temporal aspects of the Cori cycle also pose modeling difficulties. The cycle's activity fluctuates based on various factors, including exercise, fasting, and feeding states. Capturing these dynamic changes in metabolic flux over time requires sophisticated modeling techniques that can handle time-dependent variables and regulatory mechanisms.
Furthermore, the integration of hormonal regulation into the model presents a substantial challenge. The Cori cycle is heavily influenced by hormones such as insulin and glucagon, which regulate the direction and intensity of metabolic flux. Incorporating these regulatory elements into metabolic models requires a deep understanding of endocrine signaling pathways and their effects on metabolic processes.
The quantification of metabolic fluxes within the Cori cycle is another significant hurdle. Accurate measurement of in vivo fluxes, especially in human subjects, is technically challenging and often relies on indirect methods or tracer studies. This limitation in data availability and accuracy can lead to uncertainties in model parameterization and validation.
Additionally, the Cori cycle interacts with numerous other metabolic pathways, including glycolysis, gluconeogenesis, and the citric acid cycle. Modeling these complex interactions and feedback loops requires a systems-level approach that can capture the intricate interdependencies between different metabolic processes.
Lastly, the computational complexity of incorporating Cori cycle metrics into large-scale metabolic models is a significant challenge. As the level of detail and the number of variables increase, so does the computational power required to simulate and analyze the model. Balancing model complexity with computational feasibility remains an ongoing challenge in metabolic modeling.
One of the primary challenges is accurately representing the bidirectional nature of the Cori cycle. The cycle involves the conversion of glucose to lactate in peripheral tissues and the subsequent reconversion of lactate back to glucose in the liver. This bidirectional flow of metabolites is difficult to capture in traditional metabolic models, which often assume unidirectional reactions.
Another significant obstacle is the need to account for the spatial separation of the cycle's components. The Cori cycle operates across different tissues and organs, primarily involving the liver and skeletal muscles. Metabolic models typically focus on cellular-level processes, making it challenging to incorporate the inter-organ dynamics inherent in the Cori cycle.
The temporal aspects of the Cori cycle also pose modeling difficulties. The cycle's activity fluctuates based on various factors, including exercise, fasting, and feeding states. Capturing these dynamic changes in metabolic flux over time requires sophisticated modeling techniques that can handle time-dependent variables and regulatory mechanisms.
Furthermore, the integration of hormonal regulation into the model presents a substantial challenge. The Cori cycle is heavily influenced by hormones such as insulin and glucagon, which regulate the direction and intensity of metabolic flux. Incorporating these regulatory elements into metabolic models requires a deep understanding of endocrine signaling pathways and their effects on metabolic processes.
The quantification of metabolic fluxes within the Cori cycle is another significant hurdle. Accurate measurement of in vivo fluxes, especially in human subjects, is technically challenging and often relies on indirect methods or tracer studies. This limitation in data availability and accuracy can lead to uncertainties in model parameterization and validation.
Additionally, the Cori cycle interacts with numerous other metabolic pathways, including glycolysis, gluconeogenesis, and the citric acid cycle. Modeling these complex interactions and feedback loops requires a systems-level approach that can capture the intricate interdependencies between different metabolic processes.
Lastly, the computational complexity of incorporating Cori cycle metrics into large-scale metabolic models is a significant challenge. As the level of detail and the number of variables increase, so does the computational power required to simulate and analyze the model. Balancing model complexity with computational feasibility remains an ongoing challenge in metabolic modeling.
Current Cori Cycle Modeling Approaches
01 Metabolic modeling of the Cori cycle
Computational models are developed to simulate and analyze the Cori cycle, which is a metabolic pathway involving glucose-lactate metabolism between the liver and muscles. These models help in understanding the dynamics of glucose and lactate exchange, providing insights into energy metabolism and homeostasis.- Metabolic modeling of the Cori cycle: Computational models are developed to simulate and analyze the Cori cycle, which is a metabolic pathway involving glucose-lactate metabolism between the liver and muscles. These models help in understanding the dynamics of glucose and lactate exchange, providing insights into energy metabolism and homeostasis.
- Metrics for assessing Cori cycle efficiency: Various metrics are developed to quantify and evaluate the efficiency of the Cori cycle. These may include measures of glucose-lactate conversion rates, energy expenditure, and overall cycle throughput. Such metrics aid in assessing metabolic health and identifying potential disruptions in the cycle.
- Integration of Cori cycle models in broader metabolic networks: The Cori cycle models are incorporated into larger-scale metabolic network simulations. This integration allows for a more comprehensive understanding of how the cycle interacts with other metabolic pathways and its role in overall energy metabolism and substrate utilization.
- Application of machine learning to Cori cycle analysis: Machine learning techniques are applied to analyze large datasets related to the Cori cycle. These methods help in identifying patterns, predicting cycle behavior, and optimizing metabolic models for improved accuracy in representing the glucose-lactate shuttle.
- Cori cycle metrics in disease diagnosis and treatment: Metrics derived from Cori cycle models are utilized in the diagnosis and treatment of metabolic disorders. These metrics serve as biomarkers for conditions such as diabetes, helping in early detection and monitoring of treatment efficacy by quantifying alterations in glucose-lactate metabolism.
02 Metrics for assessing Cori cycle efficiency
Various metrics are developed to quantify and evaluate the efficiency of the Cori cycle. These may include measures of glucose-lactate conversion rates, energy expenditure, and overall cycle throughput. Such metrics aid in assessing metabolic health and identifying potential disruptions in the cycle.Expand Specific Solutions03 Integration of Cori cycle models in broader metabolic networks
The Cori cycle models are incorporated into larger-scale metabolic network simulations. This integration allows for a more comprehensive understanding of how the cycle interacts with other metabolic pathways and its role in overall energy metabolism and substrate utilization.Expand Specific Solutions04 Application of machine learning to Cori cycle analysis
Machine learning techniques are applied to analyze large datasets related to the Cori cycle. These methods help in identifying patterns, predicting cycle behavior, and optimizing metabolic models for improved accuracy in representing the glucose-lactate shuttle.Expand Specific Solutions05 Cori cycle metrics in disease diagnosis and treatment
Metrics derived from Cori cycle models are utilized in the diagnosis and treatment of metabolic disorders. By analyzing deviations from normal cycle parameters, healthcare professionals can identify potential metabolic issues and develop targeted interventions to restore balance.Expand Specific Solutions
Key Players in Metabolomics
The incorporation of Cori cycle metrics into metabolic models represents an evolving field at the intersection of systems biology and metabolic engineering. The competitive landscape is characterized by a mix of academic institutions, research centers, and biotechnology companies, indicating a growing but not yet fully mature market. Key players like Massachusetts Institute of Technology, Arizona State University, and Sorbonne Université are driving academic research, while companies such as Sartorius Stedim Data Analytics AB and SimBioSys, Inc. are developing commercial applications. The technology's maturity varies, with some established modeling techniques and emerging AI-driven approaches, suggesting a dynamic and innovative environment with significant potential for growth and refinement.
Hoffmann-La Roche, Inc.
Technical Solution: Hoffmann-La Roche has developed a systems pharmacology approach to incorporate Cori cycle metrics into metabolic models for drug discovery and development. Their method combines physiologically-based pharmacokinetic (PBPK) modeling with detailed representations of the Cori cycle and related metabolic pathways[12]. The model accounts for organ-specific glucose metabolism, including hepatic glucose production and peripheral glucose utilization. Roche's approach also integrates the effects of hormones such as insulin and glucagon on Cori cycle fluxes, allowing for simulation of various metabolic disorders[14]. The platform enables in silico testing of potential drug candidates and their impact on glucose homeostasis, facilitating the development of novel therapies for metabolic diseases[16].
Strengths: Integration with PBPK modeling, focus on drug discovery applications, and simulation of metabolic disorders. Weaknesses: Potential overemphasis on pharmaceutical applications and limited consideration of non-drug interventions.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced computational models that incorporate Cori cycle metrics into metabolic simulations. Their approach utilizes flux balance analysis (FBA) and dynamic flux balance analysis (dFBA) to integrate Cori cycle parameters with genome-scale metabolic models[1]. This allows for more accurate predictions of cellular metabolism under various conditions, including exercise and fasting states. The model accounts for glucose-lactate cycling between tissues, incorporating tissue-specific enzyme kinetics and regulatory mechanisms[3]. MIT's method also includes a multi-tissue framework that captures the interplay between liver, muscle, and adipose tissue in glucose homeostasis[5].
Strengths: Comprehensive multi-tissue modeling, integration of regulatory mechanisms, and improved prediction accuracy. Weaknesses: Computational complexity and potential limitations in real-time applications.
Innovative Cori Cycle Metrics
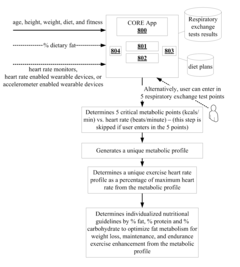
Calorie optimization respiratory exchange (CORE) metabolic profile system and method
PatentActiveUS20160379521A1
Innovation
- A calorie optimization respiratory exchange (CORE) metabolic profile system and method that uses biometric data from wearable devices and previous respiratory exchange tests to generate individualized metabolic profiles, exercise heart rate zones, and nutritional guidelines, accessible through a website or mobile app, providing a cost-effective alternative for nutrition and diet planning.
Metabolic Model Validation Methods
Metabolic model validation is a critical step in ensuring the accuracy and reliability of computational representations of cellular metabolism. Various methods have been developed to assess the quality and predictive power of these models. One key approach is the comparison of model predictions with experimental data, particularly flux measurements obtained through techniques such as 13C metabolic flux analysis. This method allows researchers to evaluate the model's ability to accurately represent the distribution of metabolic fluxes within the cell.
Another important validation technique is the use of growth phenotype data. By comparing the model's predictions of growth rates under different environmental conditions with experimental observations, researchers can assess the model's ability to capture the overall metabolic capabilities of the organism. This approach is particularly useful for identifying gaps in the model or inaccuracies in the representation of specific metabolic pathways.
Gene essentiality predictions provide another valuable means of model validation. By comparing the model's predictions of essential genes with experimental gene knockout data, researchers can evaluate the model's ability to capture the interdependencies between different metabolic pathways and the overall robustness of the metabolic network.
Metabolomics data can also be leveraged for model validation. By comparing predicted metabolite concentrations or changes in metabolite levels with experimental measurements, researchers can assess the model's ability to capture the dynamic behavior of the metabolic network. This approach is particularly useful for validating kinetic models of metabolism.
Transcriptomics and proteomics data can be integrated into the validation process to assess the model's ability to capture regulatory effects on metabolism. By comparing predicted changes in metabolic fluxes or gene expression levels with experimental data, researchers can evaluate the model's ability to represent the complex interplay between gene regulation and metabolic activity.
Finally, sensitivity analysis and uncertainty quantification techniques can be employed to assess the robustness of the model's predictions and identify key parameters or reactions that have a significant impact on model behavior. These methods help researchers understand the limitations of the model and guide further refinement and improvement efforts.
Another important validation technique is the use of growth phenotype data. By comparing the model's predictions of growth rates under different environmental conditions with experimental observations, researchers can assess the model's ability to capture the overall metabolic capabilities of the organism. This approach is particularly useful for identifying gaps in the model or inaccuracies in the representation of specific metabolic pathways.
Gene essentiality predictions provide another valuable means of model validation. By comparing the model's predictions of essential genes with experimental gene knockout data, researchers can evaluate the model's ability to capture the interdependencies between different metabolic pathways and the overall robustness of the metabolic network.
Metabolomics data can also be leveraged for model validation. By comparing predicted metabolite concentrations or changes in metabolite levels with experimental measurements, researchers can assess the model's ability to capture the dynamic behavior of the metabolic network. This approach is particularly useful for validating kinetic models of metabolism.
Transcriptomics and proteomics data can be integrated into the validation process to assess the model's ability to capture regulatory effects on metabolism. By comparing predicted changes in metabolic fluxes or gene expression levels with experimental data, researchers can evaluate the model's ability to represent the complex interplay between gene regulation and metabolic activity.
Finally, sensitivity analysis and uncertainty quantification techniques can be employed to assess the robustness of the model's predictions and identify key parameters or reactions that have a significant impact on model behavior. These methods help researchers understand the limitations of the model and guide further refinement and improvement efforts.
Computational Resources for Modeling
Incorporating Cori cycle metrics into metabolic models requires significant computational resources due to the complexity of the metabolic pathways involved. High-performance computing (HPC) clusters are essential for handling the large-scale simulations and data processing required. These clusters typically consist of multiple interconnected nodes, each with multi-core processors and substantial memory capacity, allowing for parallel processing of complex metabolic calculations.
Software tools specifically designed for metabolic modeling are crucial for integrating Cori cycle metrics effectively. Platforms such as COBRA (COnstraint-Based Reconstruction and Analysis) Toolbox, which runs on MATLAB, provide a comprehensive set of methods for genome-scale metabolic modeling. These tools offer functionalities for flux balance analysis, metabolic flux analysis, and dynamic simulations, which are vital for accurately representing the Cori cycle within larger metabolic networks.
Cloud computing services have become increasingly important for metabolic modeling. Platforms like Amazon Web Services (AWS) or Google Cloud Platform offer scalable resources that can be tailored to the specific computational needs of incorporating Cori cycle metrics. These services provide on-demand access to powerful computing resources without the need for significant upfront investment in hardware.
Specialized databases and repositories play a crucial role in supporting metabolic modeling efforts. Resources such as BiGG Models (Biochemically, Genetically, and Genomically structured) provide curated, genome-scale metabolic network reconstructions that can serve as a foundation for incorporating Cori cycle metrics. These databases offer standardized formats and annotations, facilitating the integration of new metabolic data and models.
Machine learning and artificial intelligence algorithms are emerging as valuable tools for enhancing metabolic modeling capabilities. These techniques can help in predicting metabolic fluxes, identifying key regulatory points in the Cori cycle, and optimizing model parameters. GPU acceleration is often employed to speed up these machine learning processes, particularly for deep learning applications in metabolic network analysis.
Version control systems and collaborative platforms, such as Git and GitHub, are essential for managing the development and iteration of metabolic models. These tools allow researchers to track changes, collaborate effectively, and maintain reproducibility when incorporating complex elements like Cori cycle metrics into existing models.
Software tools specifically designed for metabolic modeling are crucial for integrating Cori cycle metrics effectively. Platforms such as COBRA (COnstraint-Based Reconstruction and Analysis) Toolbox, which runs on MATLAB, provide a comprehensive set of methods for genome-scale metabolic modeling. These tools offer functionalities for flux balance analysis, metabolic flux analysis, and dynamic simulations, which are vital for accurately representing the Cori cycle within larger metabolic networks.
Cloud computing services have become increasingly important for metabolic modeling. Platforms like Amazon Web Services (AWS) or Google Cloud Platform offer scalable resources that can be tailored to the specific computational needs of incorporating Cori cycle metrics. These services provide on-demand access to powerful computing resources without the need for significant upfront investment in hardware.
Specialized databases and repositories play a crucial role in supporting metabolic modeling efforts. Resources such as BiGG Models (Biochemically, Genetically, and Genomically structured) provide curated, genome-scale metabolic network reconstructions that can serve as a foundation for incorporating Cori cycle metrics. These databases offer standardized formats and annotations, facilitating the integration of new metabolic data and models.
Machine learning and artificial intelligence algorithms are emerging as valuable tools for enhancing metabolic modeling capabilities. These techniques can help in predicting metabolic fluxes, identifying key regulatory points in the Cori cycle, and optimizing model parameters. GPU acceleration is often employed to speed up these machine learning processes, particularly for deep learning applications in metabolic network analysis.
Version control systems and collaborative platforms, such as Git and GitHub, are essential for managing the development and iteration of metabolic models. These tools allow researchers to track changes, collaborate effectively, and maintain reproducibility when incorporating complex elements like Cori cycle metrics into existing models.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!