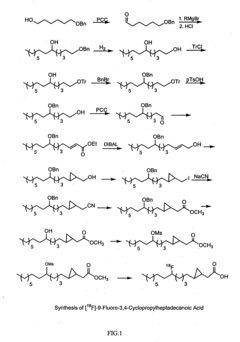
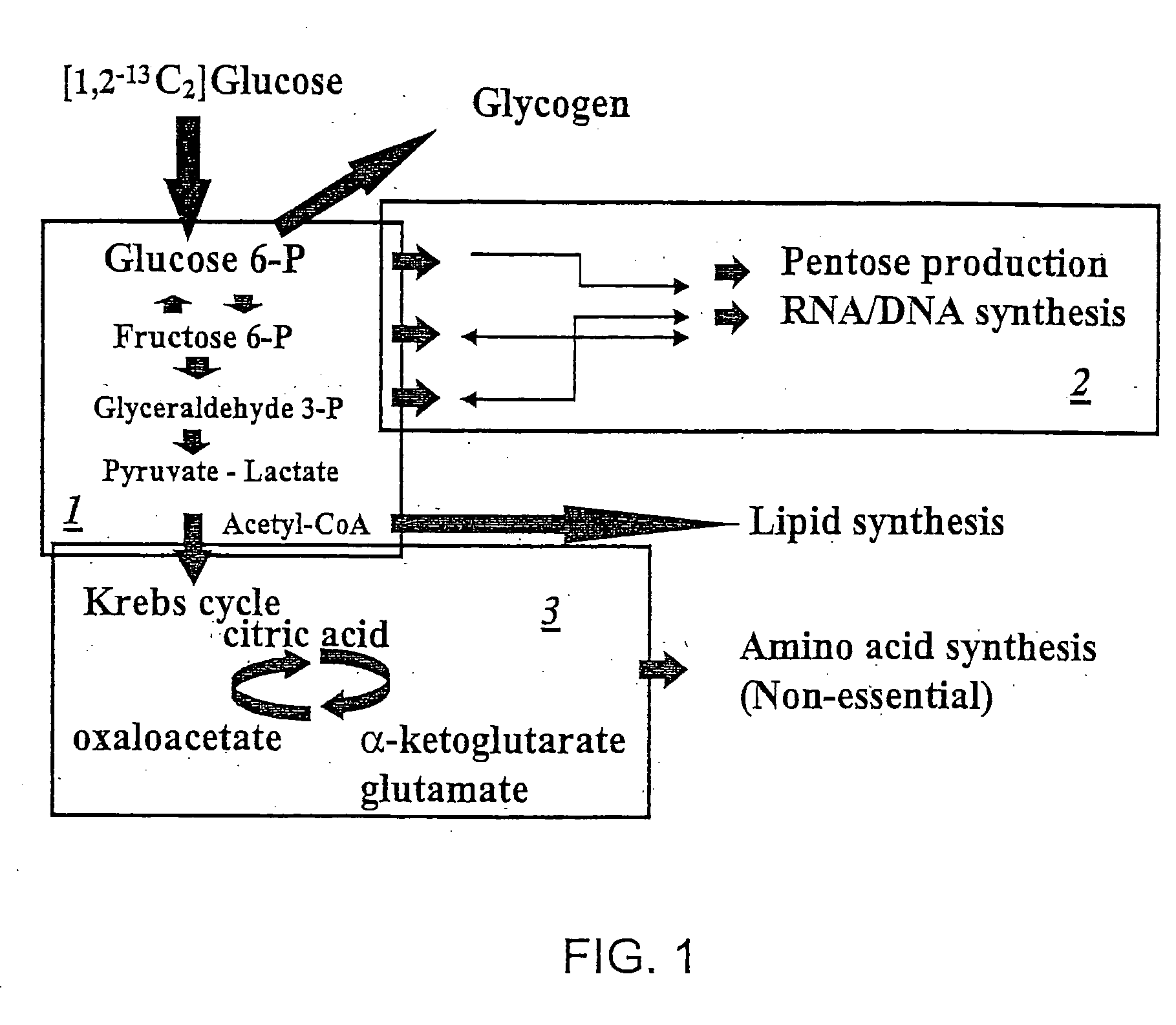
How To Differentiate Cori Cycle From Alanine Cycle In Tracer Studies
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tracer Study Objectives
Tracer studies are essential tools in metabolic research, allowing researchers to track the movement and transformation of specific molecules through biochemical pathways. In the context of differentiating the Cori cycle from the alanine cycle, the primary objective is to elucidate the distinct roles these cycles play in glucose homeostasis and amino acid metabolism. This differentiation is crucial for understanding the body's response to various physiological states, particularly during fasting or exercise.
The main goal of such tracer studies is to quantify the flux of metabolites through these cycles, providing insights into the relative contributions of each pathway to overall glucose production and utilization. By using isotopically labeled tracers, researchers aim to track the movement of carbon atoms from glucose to lactate and back to glucose (Cori cycle), as well as from alanine to glucose (alanine cycle).
Another key objective is to determine the tissue-specific involvement in these cycles. The Cori cycle primarily involves the liver and skeletal muscle, while the alanine cycle includes skeletal muscle and the liver but also incorporates amino acid metabolism. Tracer studies seek to delineate the precise contributions of each tissue to the overall metabolic flux through these pathways.
Researchers also aim to understand the regulatory mechanisms governing these cycles under different physiological conditions. This includes investigating how hormones, such as insulin and glucagon, influence the activity of key enzymes in each cycle. By manipulating these factors and observing the resulting changes in tracer distribution, scientists can gain valuable insights into the control of glucose homeostasis.
Furthermore, tracer studies are designed to elucidate the temporal dynamics of these cycles. This involves measuring the rates of substrate conversion and transport between tissues, providing a comprehensive view of how quickly the body can respond to changes in glucose demand or supply through these pathways.
An additional objective is to assess the energy costs associated with each cycle. The Cori cycle is known to be energy-consuming, while the alanine cycle plays a role in nitrogen transport. Quantifying the ATP requirements and overall energetic efficiency of these processes is crucial for understanding their relative importance in different metabolic states.
Finally, tracer studies aim to explore potential therapeutic interventions targeting these cycles. By identifying rate-limiting steps or key regulatory points, researchers can develop strategies to modulate these pathways in metabolic disorders such as diabetes or liver disease. This translational aspect of tracer studies bridges the gap between basic metabolic research and clinical applications, potentially leading to new treatment approaches for metabolic disorders.
The main goal of such tracer studies is to quantify the flux of metabolites through these cycles, providing insights into the relative contributions of each pathway to overall glucose production and utilization. By using isotopically labeled tracers, researchers aim to track the movement of carbon atoms from glucose to lactate and back to glucose (Cori cycle), as well as from alanine to glucose (alanine cycle).
Another key objective is to determine the tissue-specific involvement in these cycles. The Cori cycle primarily involves the liver and skeletal muscle, while the alanine cycle includes skeletal muscle and the liver but also incorporates amino acid metabolism. Tracer studies seek to delineate the precise contributions of each tissue to the overall metabolic flux through these pathways.
Researchers also aim to understand the regulatory mechanisms governing these cycles under different physiological conditions. This includes investigating how hormones, such as insulin and glucagon, influence the activity of key enzymes in each cycle. By manipulating these factors and observing the resulting changes in tracer distribution, scientists can gain valuable insights into the control of glucose homeostasis.
Furthermore, tracer studies are designed to elucidate the temporal dynamics of these cycles. This involves measuring the rates of substrate conversion and transport between tissues, providing a comprehensive view of how quickly the body can respond to changes in glucose demand or supply through these pathways.
An additional objective is to assess the energy costs associated with each cycle. The Cori cycle is known to be energy-consuming, while the alanine cycle plays a role in nitrogen transport. Quantifying the ATP requirements and overall energetic efficiency of these processes is crucial for understanding their relative importance in different metabolic states.
Finally, tracer studies aim to explore potential therapeutic interventions targeting these cycles. By identifying rate-limiting steps or key regulatory points, researchers can develop strategies to modulate these pathways in metabolic disorders such as diabetes or liver disease. This translational aspect of tracer studies bridges the gap between basic metabolic research and clinical applications, potentially leading to new treatment approaches for metabolic disorders.
Clinical Relevance
The differentiation between the Cori cycle and the alanine cycle in tracer studies holds significant clinical relevance in understanding metabolic disorders and optimizing patient care. These cycles play crucial roles in glucose homeostasis and amino acid metabolism, making their distinction essential for accurate diagnosis and treatment of various metabolic conditions.
In clinical settings, tracer studies utilizing these cycles provide valuable insights into liver function, muscle metabolism, and overall energy balance. The Cori cycle, also known as the glucose-lactate cycle, is particularly important in assessing liver glycogen metabolism and gluconeogenesis. Abnormalities in this cycle can indicate liver dysfunction, such as in cirrhosis or hepatic steatosis. By accurately differentiating the Cori cycle in tracer studies, clinicians can better evaluate liver health and metabolic efficiency in patients with suspected liver disorders.
The alanine cycle, on the other hand, is critical for understanding protein catabolism and nitrogen balance. Its differentiation in tracer studies allows for the assessment of muscle protein breakdown and amino acid utilization. This information is particularly valuable in managing conditions such as sarcopenia, cachexia, and protein-energy malnutrition. In critically ill patients or those with chronic wasting diseases, monitoring the alanine cycle can guide nutritional interventions and improve overall patient outcomes.
Furthermore, the ability to distinguish between these cycles in tracer studies has implications for diabetes management. Both cycles contribute to glucose production, but through different mechanisms. In type 2 diabetes, where insulin resistance affects glucose uptake and utilization, understanding the relative contributions of the Cori and alanine cycles to glucose production can inform targeted therapeutic approaches. This differentiation can help in tailoring medications and lifestyle interventions to address specific metabolic imbalances.
In oncology, differentiating these cycles can provide insights into tumor metabolism. Cancer cells often exhibit altered metabolic profiles, including changes in glucose and amino acid utilization. By distinguishing between the Cori and alanine cycles, oncologists can better understand tumor energy metabolism and potentially identify new targets for cancer therapy or markers for treatment response.
The clinical relevance of differentiating these cycles extends to the field of exercise physiology and sports medicine. During intense physical activity, both cycles are activated to maintain energy supply and manage metabolic byproducts. Accurate differentiation in tracer studies can help in optimizing athletic performance, designing effective training regimens, and preventing exercise-induced metabolic disturbances.
In conclusion, the ability to differentiate between the Cori cycle and the alanine cycle in tracer studies has far-reaching clinical implications. It enhances our understanding of metabolic disorders, improves diagnostic accuracy, and enables more targeted therapeutic interventions across a wide range of medical specialties. As metabolic research continues to advance, refining these differentiation techniques will undoubtedly lead to improved patient care and more personalized treatment strategies.
In clinical settings, tracer studies utilizing these cycles provide valuable insights into liver function, muscle metabolism, and overall energy balance. The Cori cycle, also known as the glucose-lactate cycle, is particularly important in assessing liver glycogen metabolism and gluconeogenesis. Abnormalities in this cycle can indicate liver dysfunction, such as in cirrhosis or hepatic steatosis. By accurately differentiating the Cori cycle in tracer studies, clinicians can better evaluate liver health and metabolic efficiency in patients with suspected liver disorders.
The alanine cycle, on the other hand, is critical for understanding protein catabolism and nitrogen balance. Its differentiation in tracer studies allows for the assessment of muscle protein breakdown and amino acid utilization. This information is particularly valuable in managing conditions such as sarcopenia, cachexia, and protein-energy malnutrition. In critically ill patients or those with chronic wasting diseases, monitoring the alanine cycle can guide nutritional interventions and improve overall patient outcomes.
Furthermore, the ability to distinguish between these cycles in tracer studies has implications for diabetes management. Both cycles contribute to glucose production, but through different mechanisms. In type 2 diabetes, where insulin resistance affects glucose uptake and utilization, understanding the relative contributions of the Cori and alanine cycles to glucose production can inform targeted therapeutic approaches. This differentiation can help in tailoring medications and lifestyle interventions to address specific metabolic imbalances.
In oncology, differentiating these cycles can provide insights into tumor metabolism. Cancer cells often exhibit altered metabolic profiles, including changes in glucose and amino acid utilization. By distinguishing between the Cori and alanine cycles, oncologists can better understand tumor energy metabolism and potentially identify new targets for cancer therapy or markers for treatment response.
The clinical relevance of differentiating these cycles extends to the field of exercise physiology and sports medicine. During intense physical activity, both cycles are activated to maintain energy supply and manage metabolic byproducts. Accurate differentiation in tracer studies can help in optimizing athletic performance, designing effective training regimens, and preventing exercise-induced metabolic disturbances.
In conclusion, the ability to differentiate between the Cori cycle and the alanine cycle in tracer studies has far-reaching clinical implications. It enhances our understanding of metabolic disorders, improves diagnostic accuracy, and enables more targeted therapeutic interventions across a wide range of medical specialties. As metabolic research continues to advance, refining these differentiation techniques will undoubtedly lead to improved patient care and more personalized treatment strategies.
Metabolic Pathway Comparison
The Cori cycle and alanine cycle are both crucial metabolic pathways involved in glucose homeostasis and energy metabolism. While they share some similarities, there are distinct differences that can be identified through tracer studies. These differences are primarily related to the organs involved, the specific metabolites transported, and the overall impact on energy balance.
The Cori cycle, also known as the glucose-lactate cycle, primarily involves the liver and skeletal muscles. In this pathway, lactate produced by anaerobic glycolysis in the muscles is transported to the liver, where it is converted back to glucose through gluconeogenesis. This newly formed glucose is then released into the bloodstream and can be utilized by the muscles again. In tracer studies, the Cori cycle can be identified by following the fate of labeled lactate or glucose molecules.
In contrast, the alanine cycle, also called the glucose-alanine cycle, involves the skeletal muscles and the liver as well, but with alanine as the key metabolite instead of lactate. In this cycle, amino acids in the muscles are transaminated to form alanine, which is then transported to the liver. In the liver, alanine is converted to glucose through gluconeogenesis, and the resulting glucose is released into the bloodstream. Tracer studies can differentiate this cycle by tracking labeled alanine or glucose molecules.
One key difference between these cycles lies in their energy efficiency. The Cori cycle is essentially energy-neutral, as the energy expended in gluconeogenesis in the liver is roughly equal to the energy gained from glycolysis in the muscles. However, the alanine cycle results in a net loss of energy, as the conversion of amino acids to glucose is an energy-consuming process.
Another distinguishing factor is the source of the carbon skeletons used for glucose production. In the Cori cycle, the carbon comes from lactate, which is derived from glucose through glycolysis. In the alanine cycle, the carbon skeletons originate from amino acids, specifically alanine, which is formed from the breakdown of other amino acids or from pyruvate.
Tracer studies can effectively differentiate these cycles by using isotope-labeled molecules. For the Cori cycle, researchers can use 13C-labeled lactate or glucose to track the conversion between these molecules. For the alanine cycle, 15N-labeled alanine or 13C-labeled alanine can be employed to follow the fate of alanine and its conversion to glucose.
Furthermore, the timing and location of labeled molecule appearance can help distinguish between these cycles. In the Cori cycle, labeled glucose derived from lactate would appear more rapidly in the bloodstream compared to glucose derived from alanine in the alanine cycle, due to the additional steps involved in amino acid metabolism.
The Cori cycle, also known as the glucose-lactate cycle, primarily involves the liver and skeletal muscles. In this pathway, lactate produced by anaerobic glycolysis in the muscles is transported to the liver, where it is converted back to glucose through gluconeogenesis. This newly formed glucose is then released into the bloodstream and can be utilized by the muscles again. In tracer studies, the Cori cycle can be identified by following the fate of labeled lactate or glucose molecules.
In contrast, the alanine cycle, also called the glucose-alanine cycle, involves the skeletal muscles and the liver as well, but with alanine as the key metabolite instead of lactate. In this cycle, amino acids in the muscles are transaminated to form alanine, which is then transported to the liver. In the liver, alanine is converted to glucose through gluconeogenesis, and the resulting glucose is released into the bloodstream. Tracer studies can differentiate this cycle by tracking labeled alanine or glucose molecules.
One key difference between these cycles lies in their energy efficiency. The Cori cycle is essentially energy-neutral, as the energy expended in gluconeogenesis in the liver is roughly equal to the energy gained from glycolysis in the muscles. However, the alanine cycle results in a net loss of energy, as the conversion of amino acids to glucose is an energy-consuming process.
Another distinguishing factor is the source of the carbon skeletons used for glucose production. In the Cori cycle, the carbon comes from lactate, which is derived from glucose through glycolysis. In the alanine cycle, the carbon skeletons originate from amino acids, specifically alanine, which is formed from the breakdown of other amino acids or from pyruvate.
Tracer studies can effectively differentiate these cycles by using isotope-labeled molecules. For the Cori cycle, researchers can use 13C-labeled lactate or glucose to track the conversion between these molecules. For the alanine cycle, 15N-labeled alanine or 13C-labeled alanine can be employed to follow the fate of alanine and its conversion to glucose.
Furthermore, the timing and location of labeled molecule appearance can help distinguish between these cycles. In the Cori cycle, labeled glucose derived from lactate would appear more rapidly in the bloodstream compared to glucose derived from alanine in the alanine cycle, due to the additional steps involved in amino acid metabolism.
Current Differentiation Methods
01 Substrate differences
The Cori cycle primarily involves glucose and lactate, while the alanine cycle mainly uses alanine as a substrate. This fundamental difference in substrates leads to distinct metabolic pathways and end products in each cycle.- Metabolic pathways and glucose homeostasis: The Cori cycle and alanine cycle are both important metabolic pathways involved in glucose homeostasis. The Cori cycle primarily involves the liver and muscle tissues, while the alanine cycle involves the liver and muscle tissues as well as other organs. Both cycles play crucial roles in maintaining blood glucose levels during periods of fasting or exercise.
- Substrate differences: The main difference between the Cori cycle and alanine cycle lies in their substrates. The Cori cycle uses lactate as its primary substrate, which is produced by anaerobic glycolysis in muscle cells. In contrast, the alanine cycle uses alanine as its primary substrate, which is derived from the transamination of pyruvate in muscle cells.
- Energy efficiency: The Cori cycle and alanine cycle differ in their energy efficiency. The Cori cycle is less energy-efficient as it requires more ATP to convert lactate back to glucose in the liver. The alanine cycle is more energy-efficient as it allows for the transfer of both carbon and nitrogen from muscle to liver, providing substrates for both gluconeogenesis and urea synthesis.
- Nitrogen balance: The alanine cycle plays a significant role in nitrogen balance, while the Cori cycle does not. In the alanine cycle, alanine serves as a carrier of both carbon and nitrogen from muscle to liver, contributing to amino acid metabolism and urea synthesis. The Cori cycle, on the other hand, primarily focuses on glucose metabolism without directly impacting nitrogen balance.
- Regulation and hormonal control: Both the Cori cycle and alanine cycle are regulated by hormones, but their responses to hormonal signals may differ. The Cori cycle is primarily influenced by insulin and glucagon, which regulate glucose metabolism. The alanine cycle is also affected by these hormones, but additionally responds to cortisol and other stress hormones that influence protein metabolism and amino acid utilization.
02 Organ involvement
The Cori cycle mainly occurs between the liver and skeletal muscles, whereas the alanine cycle involves the liver and various tissues, particularly muscle tissue. This difference in organ involvement affects the overall metabolic impact of each cycle.Expand Specific Solutions03 Nitrogen handling
The alanine cycle plays a crucial role in nitrogen transport from peripheral tissues to the liver for urea synthesis, while the Cori cycle does not directly involve nitrogen metabolism. This distinction is important for understanding the broader physiological roles of each cycle.Expand Specific Solutions04 Energy efficiency
The Cori cycle is less energy-efficient compared to the alanine cycle, as it requires more ATP for glucose regeneration. This difference in energy efficiency impacts the overall metabolic cost and utility of each cycle in various physiological states.Expand Specific Solutions05 Metabolic regulation
Both cycles are regulated differently by hormones and metabolic factors. The Cori cycle is more sensitive to insulin and glucagon, while the alanine cycle is influenced by cortisol and other stress hormones. Understanding these regulatory differences is crucial for comprehending their roles in various metabolic states.Expand Specific Solutions
Key Research Institutions
The differentiation of Cori cycle from alanine cycle in tracer studies represents a complex technical challenge in metabolic research. The field is currently in a mature stage, with established methodologies and a significant market size due to its importance in understanding metabolic disorders. Companies like F. Hoffmann-La Roche Ltd. and Illumina, Inc. are at the forefront, leveraging advanced technologies such as mass spectrometry and next-generation sequencing to enhance the precision and efficiency of these studies. The technological maturity is evident in the sophisticated analytical tools and biomarker detection methods developed by firms like Astute Medical, Inc. and Meso Scale Technologies LLC, enabling more accurate differentiation between these closely related metabolic cycles.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed advanced metabolomics platforms for tracer studies, utilizing high-resolution mass spectrometry to differentiate between Cori and alanine cycles. Their approach involves isotope-labeled glucose and alanine tracers, coupled with sophisticated data analysis algorithms. The technique measures the incorporation of labeled carbons into specific metabolites, allowing precise quantification of flux through each cycle. Roche's method employs 13C-NMR spectroscopy to track the fate of labeled carbons, providing detailed pathway analysis[1][3]. This technology enables researchers to simultaneously monitor multiple metabolic pathways, offering a comprehensive view of cellular metabolism in various physiological and pathological states.
Strengths: High sensitivity and specificity in pathway differentiation, comprehensive metabolic profiling. Weaknesses: Requires expensive equipment and specialized expertise, potentially limiting widespread adoption.
Life Technologies Corp.
Technical Solution: Life Technologies has pioneered a multi-omics approach to differentiate between Cori and alanine cycles in tracer studies. Their method integrates transcriptomics, proteomics, and metabolomics data to provide a holistic view of metabolic flux. By using stable isotope-labeled precursors and advanced mass spectrometry techniques, they can track the fate of labeled molecules through both cycles with high precision. The company's proprietary software algorithms analyze the complex data sets, identifying unique metabolic signatures for each cycle. This approach allows for the simultaneous quantification of flux through multiple pathways, including the Cori and alanine cycles, in various biological systems[2][4]. The technology has been successfully applied in studies of liver metabolism, diabetes research, and cancer metabolism.
Strengths: Comprehensive multi-omics approach, high-throughput capability, and robust data analysis. Weaknesses: Complex data interpretation, requiring significant computational resources and expertise.
Analytical Instrumentation
Method for monitoring blood flow and metabolic uptake in tissue with radiolabeled alkanoic acid
PatentInactiveUS20080214851A1
Innovation
- Development of novel radiolabeled fatty acid analogs with cyclic organic substituents that block the catabolic pathway, allowing them to be retained in cardiac tissue for extended periods without significant metabolism or migration to other tissues, using positron or gamma-emitting labels placed on the fatty acid backbone.
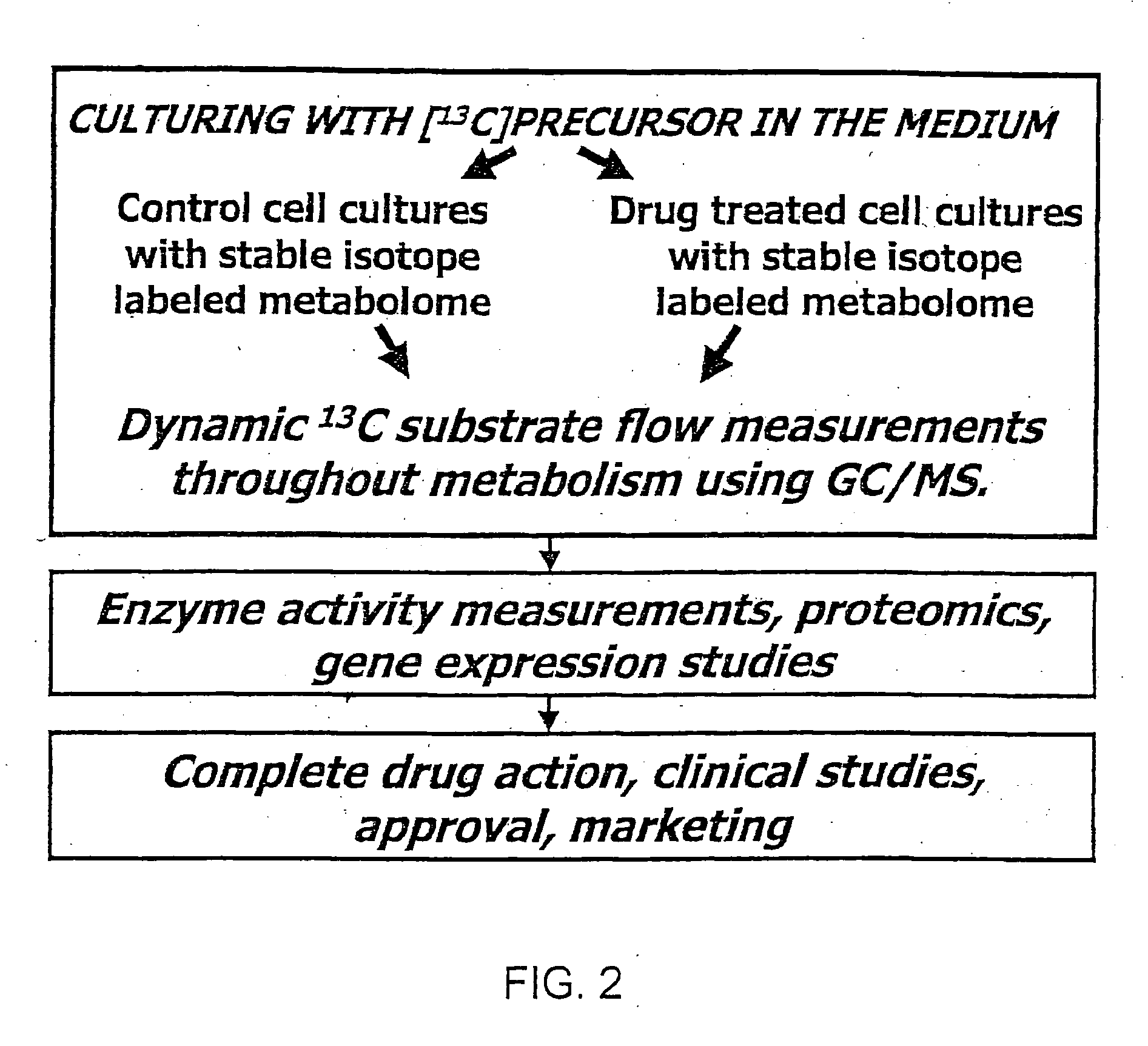
Stable isotope based dynamic metabolic profiling of living organisms for characterization of metabolic diseases, drug testing and drug development
PatentInactiveUS20050281745A1
Innovation
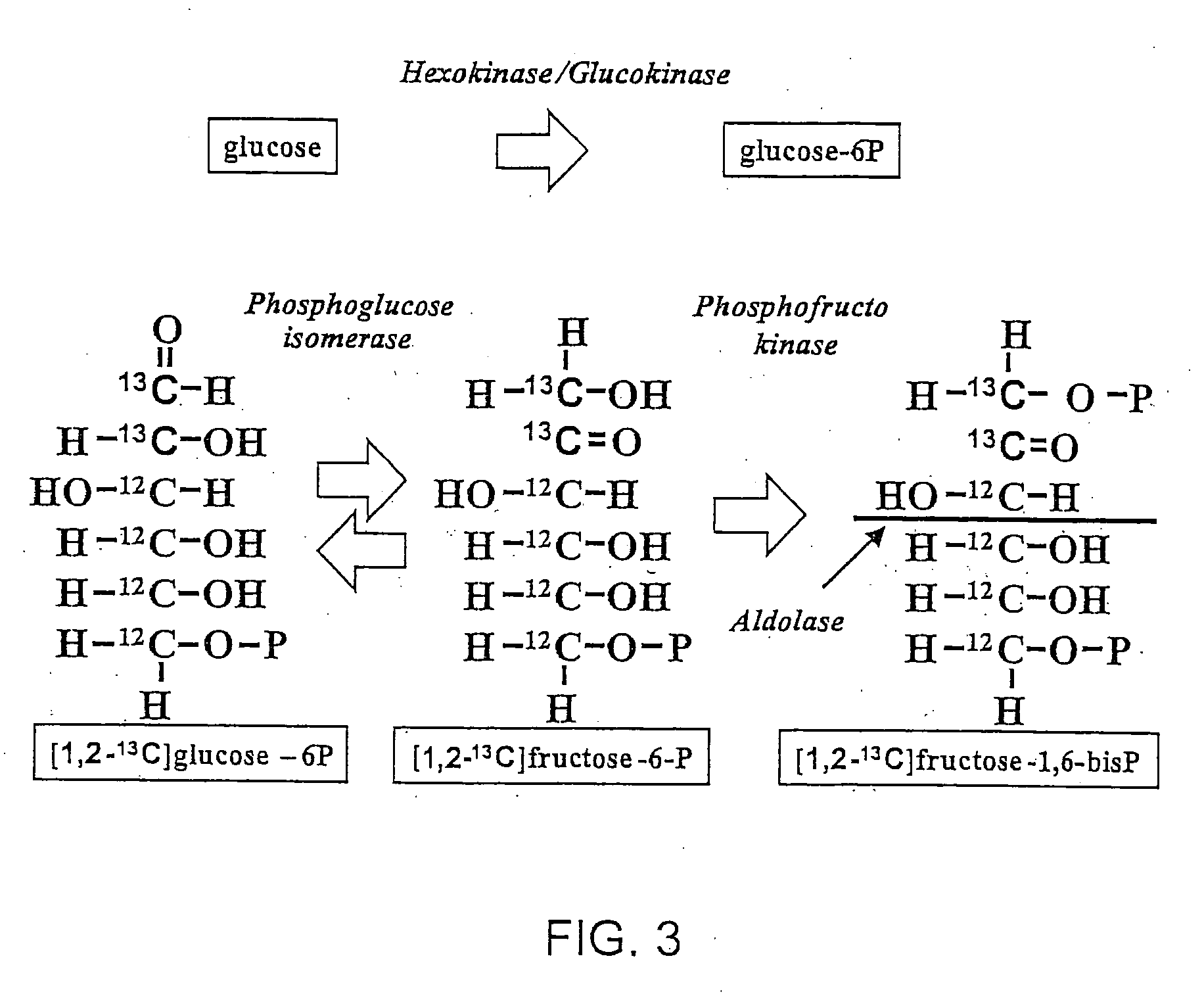
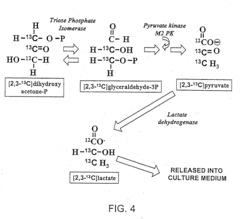
- The use of 13C labeled glucose substrate to dynamically label intracellular metabolic pathways, allowing for the tracking of substrate flow and distribution through multiple metabolic reactions, thereby providing a comprehensive and dynamic metabolic profiling system that reveals metabolic pathway flux changes and enzyme activity changes.
Regulatory Considerations
In the context of differentiating the Cori cycle from the alanine cycle in tracer studies, regulatory considerations play a crucial role in ensuring the safety, efficacy, and ethical conduct of research. These studies often involve the use of isotopically labeled compounds, which may require specific approvals and oversight from regulatory bodies.
Researchers must adhere to guidelines set forth by national and international regulatory agencies when conducting tracer studies. In the United States, the Food and Drug Administration (FDA) oversees the use of radioisotopes in clinical research, while the Nuclear Regulatory Commission (NRC) regulates the handling and disposal of radioactive materials. Similar regulatory bodies exist in other countries, such as the European Medicines Agency (EMA) in the European Union.
When designing tracer studies to differentiate between the Cori and alanine cycles, investigators must consider the type and amount of isotopes used. The choice of isotope (e.g., 13C, 14C, or 15N) may impact regulatory requirements, as some isotopes are subject to stricter controls due to their potential health risks. Researchers must justify the use of specific tracers and demonstrate that the benefits of the study outweigh any potential risks to participants.
Institutional Review Board (IRB) approval is essential for any tracer study involving human subjects. The IRB will assess the study protocol, including the dosage and administration of labeled compounds, to ensure participant safety and informed consent. Additionally, researchers must comply with Good Clinical Practice (GCP) guidelines, which provide an international ethical and scientific quality standard for designing, conducting, and reporting clinical trials.
Data protection and privacy regulations, such as the General Data Protection Regulation (GDPR) in the European Union, must be considered when collecting and storing participant information in tracer studies. Researchers must implement appropriate safeguards to protect sensitive medical data and ensure compliance with data protection laws.
Environmental regulations may also apply to tracer studies, particularly those involving the disposal of radioactive materials. Proper handling, storage, and disposal procedures must be in place to minimize environmental impact and comply with local and national regulations.
Lastly, researchers should be aware of any specific regulatory requirements related to the interpretation and reporting of tracer study results. Accurate differentiation between the Cori and alanine cycles may have implications for drug development or metabolic disorder diagnoses, necessitating adherence to regulatory standards for data analysis and reporting.
Researchers must adhere to guidelines set forth by national and international regulatory agencies when conducting tracer studies. In the United States, the Food and Drug Administration (FDA) oversees the use of radioisotopes in clinical research, while the Nuclear Regulatory Commission (NRC) regulates the handling and disposal of radioactive materials. Similar regulatory bodies exist in other countries, such as the European Medicines Agency (EMA) in the European Union.
When designing tracer studies to differentiate between the Cori and alanine cycles, investigators must consider the type and amount of isotopes used. The choice of isotope (e.g., 13C, 14C, or 15N) may impact regulatory requirements, as some isotopes are subject to stricter controls due to their potential health risks. Researchers must justify the use of specific tracers and demonstrate that the benefits of the study outweigh any potential risks to participants.
Institutional Review Board (IRB) approval is essential for any tracer study involving human subjects. The IRB will assess the study protocol, including the dosage and administration of labeled compounds, to ensure participant safety and informed consent. Additionally, researchers must comply with Good Clinical Practice (GCP) guidelines, which provide an international ethical and scientific quality standard for designing, conducting, and reporting clinical trials.
Data protection and privacy regulations, such as the General Data Protection Regulation (GDPR) in the European Union, must be considered when collecting and storing participant information in tracer studies. Researchers must implement appropriate safeguards to protect sensitive medical data and ensure compliance with data protection laws.
Environmental regulations may also apply to tracer studies, particularly those involving the disposal of radioactive materials. Proper handling, storage, and disposal procedures must be in place to minimize environmental impact and comply with local and national regulations.
Lastly, researchers should be aware of any specific regulatory requirements related to the interpretation and reporting of tracer study results. Accurate differentiation between the Cori and alanine cycles may have implications for drug development or metabolic disorder diagnoses, necessitating adherence to regulatory standards for data analysis and reporting.
Data Interpretation Challenges
Differentiating between the Cori cycle and the alanine cycle in tracer studies presents several data interpretation challenges. These challenges stem from the complex nature of metabolic pathways and the limitations of current tracer methodologies.
One primary challenge is the overlap in metabolic intermediates between the two cycles. Both the Cori and alanine cycles involve glucose and lactate, making it difficult to distinguish their individual contributions to overall metabolism. This overlap can lead to ambiguous data interpretation, especially when using single-tracer techniques.
The dynamic nature of these cycles further complicates data analysis. The rates of substrate flux through each cycle can vary significantly depending on physiological conditions, such as exercise intensity or nutritional status. This variability makes it challenging to establish baseline measurements and interpret changes in tracer distribution accurately.
Another significant challenge lies in the compartmentalization of metabolic processes. The Cori cycle primarily occurs between the liver and skeletal muscle, while the alanine cycle involves the liver and various tissues, including muscle. Tracer studies often struggle to capture these tissue-specific differences, leading to potential misinterpretation of whole-body metabolism.
The choice of tracer compound and labeling position can also impact data interpretation. Different tracers may have varying degrees of specificity for each cycle, and the position of isotopic labels can affect how the tracer is metabolized. This variability in tracer behavior necessitates careful consideration when designing experiments and analyzing results.
Time-dependent changes in tracer distribution pose another challenge. The Cori and alanine cycles operate at different rates, and the turnover of labeled compounds can vary between the two. Capturing these temporal dynamics requires sophisticated sampling protocols and mathematical modeling, which can introduce additional complexities in data interpretation.
Lastly, individual variations in metabolism can significantly impact tracer studies. Factors such as genetic differences, hormonal status, and overall health can influence the relative activities of the Cori and alanine cycles. Accounting for these individual variations while drawing generalizable conclusions remains a persistent challenge in metabolic research.
One primary challenge is the overlap in metabolic intermediates between the two cycles. Both the Cori and alanine cycles involve glucose and lactate, making it difficult to distinguish their individual contributions to overall metabolism. This overlap can lead to ambiguous data interpretation, especially when using single-tracer techniques.
The dynamic nature of these cycles further complicates data analysis. The rates of substrate flux through each cycle can vary significantly depending on physiological conditions, such as exercise intensity or nutritional status. This variability makes it challenging to establish baseline measurements and interpret changes in tracer distribution accurately.
Another significant challenge lies in the compartmentalization of metabolic processes. The Cori cycle primarily occurs between the liver and skeletal muscle, while the alanine cycle involves the liver and various tissues, including muscle. Tracer studies often struggle to capture these tissue-specific differences, leading to potential misinterpretation of whole-body metabolism.
The choice of tracer compound and labeling position can also impact data interpretation. Different tracers may have varying degrees of specificity for each cycle, and the position of isotopic labels can affect how the tracer is metabolized. This variability in tracer behavior necessitates careful consideration when designing experiments and analyzing results.
Time-dependent changes in tracer distribution pose another challenge. The Cori and alanine cycles operate at different rates, and the turnover of labeled compounds can vary between the two. Capturing these temporal dynamics requires sophisticated sampling protocols and mathematical modeling, which can introduce additional complexities in data interpretation.
Lastly, individual variations in metabolism can significantly impact tracer studies. Factors such as genetic differences, hormonal status, and overall health can influence the relative activities of the Cori and alanine cycles. Accounting for these individual variations while drawing generalizable conclusions remains a persistent challenge in metabolic research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!