Efficacy of lithium orotate therapy in mood disorder symptom remission
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate Background and Objectives
Lithium orotate, a compound consisting of lithium and orotic acid, has emerged as a potential alternative to traditional lithium carbonate in the treatment of mood disorders. The background of lithium orotate therapy can be traced back to the 1970s when it was first introduced as a more bioavailable form of lithium. Unlike its carbonate counterpart, lithium orotate is believed to cross the blood-brain barrier more efficiently, potentially allowing for lower dosages and reduced side effects.
The historical context of lithium use in psychiatry dates back to the mid-20th century when its mood-stabilizing properties were first discovered. Since then, lithium has become a cornerstone in the treatment of bipolar disorder and other mood-related conditions. However, the traditional lithium carbonate formulation has been associated with various side effects and the need for regular blood monitoring, prompting researchers to explore alternative delivery methods.
The development of lithium orotate represents a significant milestone in the evolution of lithium therapy. Its proponents argue that the orotate form may offer improved neurological uptake, potentially leading to enhanced therapeutic effects at lower doses. This could address some of the long-standing concerns regarding lithium toxicity and side effects associated with conventional lithium treatments.
The primary objective of investigating lithium orotate therapy in mood disorder symptom remission is to evaluate its efficacy compared to traditional lithium formulations. Researchers aim to determine whether lithium orotate can provide comparable or superior mood-stabilizing effects while potentially reducing the risk of adverse reactions. Additionally, there is interest in exploring whether the purported enhanced bioavailability of lithium orotate could lead to more rapid symptom relief or improved long-term outcomes for patients with mood disorders.
Another key objective is to assess the safety profile of lithium orotate, particularly in terms of its impact on renal function and thyroid health, which are common concerns with conventional lithium therapy. Investigators seek to establish whether the orotate form allows for lower systemic lithium levels while maintaining therapeutic efficacy, potentially mitigating the need for frequent blood monitoring and reducing the risk of lithium toxicity.
Furthermore, researchers aim to elucidate the mechanisms by which lithium orotate may exert its therapeutic effects. This includes investigating its impact on neurotransmitter systems, cellular signaling pathways, and neuroprotective processes. Understanding these mechanisms could not only validate the use of lithium orotate but also provide insights into the pathophysiology of mood disorders and guide the development of future therapeutic interventions.
The historical context of lithium use in psychiatry dates back to the mid-20th century when its mood-stabilizing properties were first discovered. Since then, lithium has become a cornerstone in the treatment of bipolar disorder and other mood-related conditions. However, the traditional lithium carbonate formulation has been associated with various side effects and the need for regular blood monitoring, prompting researchers to explore alternative delivery methods.
The development of lithium orotate represents a significant milestone in the evolution of lithium therapy. Its proponents argue that the orotate form may offer improved neurological uptake, potentially leading to enhanced therapeutic effects at lower doses. This could address some of the long-standing concerns regarding lithium toxicity and side effects associated with conventional lithium treatments.
The primary objective of investigating lithium orotate therapy in mood disorder symptom remission is to evaluate its efficacy compared to traditional lithium formulations. Researchers aim to determine whether lithium orotate can provide comparable or superior mood-stabilizing effects while potentially reducing the risk of adverse reactions. Additionally, there is interest in exploring whether the purported enhanced bioavailability of lithium orotate could lead to more rapid symptom relief or improved long-term outcomes for patients with mood disorders.
Another key objective is to assess the safety profile of lithium orotate, particularly in terms of its impact on renal function and thyroid health, which are common concerns with conventional lithium therapy. Investigators seek to establish whether the orotate form allows for lower systemic lithium levels while maintaining therapeutic efficacy, potentially mitigating the need for frequent blood monitoring and reducing the risk of lithium toxicity.
Furthermore, researchers aim to elucidate the mechanisms by which lithium orotate may exert its therapeutic effects. This includes investigating its impact on neurotransmitter systems, cellular signaling pathways, and neuroprotective processes. Understanding these mechanisms could not only validate the use of lithium orotate but also provide insights into the pathophysiology of mood disorders and guide the development of future therapeutic interventions.
Market Analysis for Mood Disorder Treatments
The market for mood disorder treatments has been experiencing significant growth and transformation in recent years. This expansion is driven by several factors, including increased awareness of mental health issues, a growing prevalence of mood disorders, and advancements in treatment options. The global market for mood disorder treatments encompasses a wide range of therapeutic approaches, including pharmacological interventions, psychotherapy, and alternative therapies.
Lithium-based treatments, particularly lithium carbonate, have long been a cornerstone in the management of bipolar disorder. However, the emergence of lithium orotate as a potential alternative has sparked interest in both the medical community and among patients seeking more effective or better-tolerated options. The market for lithium orotate, while still relatively niche, is showing signs of growth as more research emerges on its efficacy and safety profile.
The overall mood disorder treatment market is characterized by a diverse range of pharmaceutical options, including antidepressants, mood stabilizers, and antipsychotics. Major pharmaceutical companies continue to dominate this space, with a focus on developing novel compounds and improving existing medications. However, there is a growing trend towards personalized medicine and alternative treatments, which is creating opportunities for smaller, specialized companies and innovative therapies like lithium orotate.
Consumer demand for mood disorder treatments is influenced by factors such as treatment efficacy, side effect profiles, and ease of administration. There is a notable shift towards treatments that offer rapid symptom relief and improved long-term outcomes. This trend aligns well with the potential benefits of lithium orotate therapy, which some proponents claim may offer faster absorption and fewer side effects compared to traditional lithium carbonate.
The market is also seeing increased interest in combination therapies and adjunctive treatments. This approach aims to enhance the overall efficacy of mood disorder management by addressing multiple aspects of the condition simultaneously. Lithium orotate could potentially find a place in this landscape as part of combination treatment strategies or as an alternative for patients who do not respond well to conventional lithium formulations.
Geographically, North America and Europe remain the largest markets for mood disorder treatments, driven by high diagnosis rates, well-established healthcare systems, and greater access to mental health services. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential as mental health awareness increases and healthcare infrastructure improves in these regions.
Lithium-based treatments, particularly lithium carbonate, have long been a cornerstone in the management of bipolar disorder. However, the emergence of lithium orotate as a potential alternative has sparked interest in both the medical community and among patients seeking more effective or better-tolerated options. The market for lithium orotate, while still relatively niche, is showing signs of growth as more research emerges on its efficacy and safety profile.
The overall mood disorder treatment market is characterized by a diverse range of pharmaceutical options, including antidepressants, mood stabilizers, and antipsychotics. Major pharmaceutical companies continue to dominate this space, with a focus on developing novel compounds and improving existing medications. However, there is a growing trend towards personalized medicine and alternative treatments, which is creating opportunities for smaller, specialized companies and innovative therapies like lithium orotate.
Consumer demand for mood disorder treatments is influenced by factors such as treatment efficacy, side effect profiles, and ease of administration. There is a notable shift towards treatments that offer rapid symptom relief and improved long-term outcomes. This trend aligns well with the potential benefits of lithium orotate therapy, which some proponents claim may offer faster absorption and fewer side effects compared to traditional lithium carbonate.
The market is also seeing increased interest in combination therapies and adjunctive treatments. This approach aims to enhance the overall efficacy of mood disorder management by addressing multiple aspects of the condition simultaneously. Lithium orotate could potentially find a place in this landscape as part of combination treatment strategies or as an alternative for patients who do not respond well to conventional lithium formulations.
Geographically, North America and Europe remain the largest markets for mood disorder treatments, driven by high diagnosis rates, well-established healthcare systems, and greater access to mental health services. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential as mental health awareness increases and healthcare infrastructure improves in these regions.
Current Status and Challenges in Lithium Therapy
Lithium therapy has been a cornerstone in the treatment of mood disorders for decades, particularly in bipolar disorder management. However, the current status of lithium therapy faces several challenges that impact its efficacy and widespread adoption. One of the primary issues is the narrow therapeutic window of lithium, which necessitates regular blood level monitoring to maintain effectiveness while avoiding toxicity. This requirement for frequent monitoring can be burdensome for patients and healthcare providers alike.
Another significant challenge is the potential for long-term side effects associated with lithium use. These include thyroid dysfunction, renal impairment, and cognitive effects, which can deter both patients and clinicians from initiating or continuing lithium therapy. The risk of these adverse effects increases with prolonged use and higher dosages, complicating long-term treatment strategies.
The variability in patient response to lithium therapy presents another hurdle. While some individuals experience remarkable symptom remission, others show limited or no response. This heterogeneity in treatment outcomes underscores the need for more personalized approaches to lithium therapy, including the identification of reliable biomarkers for treatment response prediction.
Furthermore, the emergence of newer mood stabilizers and antipsychotic medications has led to a decline in lithium prescription rates in some regions. This trend is partly due to the perceived ease of use and fewer monitoring requirements of these alternative treatments. However, it also reflects a knowledge gap among newer generations of psychiatrists regarding the optimal use of lithium.
The formulation of lithium used in therapy also presents challenges. Traditional lithium carbonate and lithium citrate formulations have been the mainstay of treatment, but they are associated with gastrointestinal side effects and require multiple daily dosing. This has sparked interest in alternative formulations, such as lithium orotate, which proponents claim may offer improved bioavailability and reduced side effects. However, the efficacy and safety of these alternative forms remain subjects of ongoing research and debate within the scientific community.
Lastly, the mechanism of action of lithium in mood stabilization is not fully understood, despite decades of use. This knowledge gap hampers efforts to develop more targeted therapies or to predict individual patient responses accurately. Ongoing research aims to elucidate the complex neurobiological effects of lithium, including its impact on neurotransmitter systems, intracellular signaling pathways, and neuroprotective mechanisms.
Another significant challenge is the potential for long-term side effects associated with lithium use. These include thyroid dysfunction, renal impairment, and cognitive effects, which can deter both patients and clinicians from initiating or continuing lithium therapy. The risk of these adverse effects increases with prolonged use and higher dosages, complicating long-term treatment strategies.
The variability in patient response to lithium therapy presents another hurdle. While some individuals experience remarkable symptom remission, others show limited or no response. This heterogeneity in treatment outcomes underscores the need for more personalized approaches to lithium therapy, including the identification of reliable biomarkers for treatment response prediction.
Furthermore, the emergence of newer mood stabilizers and antipsychotic medications has led to a decline in lithium prescription rates in some regions. This trend is partly due to the perceived ease of use and fewer monitoring requirements of these alternative treatments. However, it also reflects a knowledge gap among newer generations of psychiatrists regarding the optimal use of lithium.
The formulation of lithium used in therapy also presents challenges. Traditional lithium carbonate and lithium citrate formulations have been the mainstay of treatment, but they are associated with gastrointestinal side effects and require multiple daily dosing. This has sparked interest in alternative formulations, such as lithium orotate, which proponents claim may offer improved bioavailability and reduced side effects. However, the efficacy and safety of these alternative forms remain subjects of ongoing research and debate within the scientific community.
Lastly, the mechanism of action of lithium in mood stabilization is not fully understood, despite decades of use. This knowledge gap hampers efforts to develop more targeted therapies or to predict individual patient responses accurately. Ongoing research aims to elucidate the complex neurobiological effects of lithium, including its impact on neurotransmitter systems, intracellular signaling pathways, and neuroprotective mechanisms.
Existing Lithium Orotate Treatment Protocols
01 Use of lithium orotate for symptom remission
Lithium orotate is utilized as a therapeutic agent for symptom remission in various mental health conditions. Its unique formulation allows for better bioavailability and potentially fewer side effects compared to other lithium compounds. This form of lithium may be effective in managing mood disorders, anxiety, and other psychiatric symptoms.- Use of lithium orotate for symptom remission in psychiatric disorders: Lithium orotate has shown potential in alleviating symptoms of various psychiatric disorders. Its unique formulation may offer improved bioavailability and efficacy compared to other lithium compounds. Research suggests it could be effective in managing conditions such as bipolar disorder, depression, and anxiety, with possibly fewer side effects than traditional lithium carbonate.
- Combination therapy with lithium orotate for enhanced symptom control: Combining lithium orotate with other therapeutic agents may lead to improved symptom remission in various disorders. This approach could potentially allow for lower doses of individual components while achieving better overall efficacy. Studies have explored combinations with antidepressants, mood stabilizers, or natural supplements to target multiple aspects of complex conditions.
- Novel delivery methods for lithium orotate to enhance symptom relief: Innovative delivery systems for lithium orotate are being developed to optimize its absorption and distribution in the body. These methods aim to improve the compound's ability to cross the blood-brain barrier and target specific areas of the brain involved in symptom manifestation. Approaches may include nanoparticle formulations, transdermal patches, or controlled-release mechanisms.
- Personalized dosing strategies for lithium orotate in symptom management: Tailoring lithium orotate dosages to individual patient needs may enhance its effectiveness in symptom remission. This approach considers factors such as genetic predisposition, metabolic profile, and specific symptom patterns. Advanced diagnostic tools and biomarker analysis could be employed to determine optimal dosing regimens for maximum therapeutic benefit with minimal side effects.
- Monitoring and assessment techniques for lithium orotate treatment efficacy: Developing sophisticated monitoring methods to evaluate the effectiveness of lithium orotate in symptom remission is crucial. These techniques may involve advanced neuroimaging, biochemical markers, or digital health technologies to track symptom progression and treatment response. Real-time monitoring could allow for dynamic adjustment of treatment plans to optimize symptom control.
02 Combination therapy with lithium orotate
Lithium orotate is often used in combination with other medications or supplements to enhance symptom remission. This approach may target multiple pathways involved in mental health disorders, potentially leading to improved outcomes. Combination therapies may be tailored to individual patient needs for optimal effectiveness.Expand Specific Solutions03 Dosage and administration of lithium orotate
The proper dosage and administration of lithium orotate are crucial for achieving symptom remission while minimizing potential side effects. Factors such as age, weight, and specific condition being treated are considered when determining the appropriate dosage. Controlled release formulations may be used to maintain consistent blood levels and improve treatment efficacy.Expand Specific Solutions04 Monitoring and assessment of lithium orotate treatment
Regular monitoring and assessment of patients undergoing lithium orotate treatment are essential for ensuring optimal symptom remission. This may include blood tests to check lithium levels, evaluation of side effects, and assessment of overall mental health improvements. Adjustments to treatment plans can be made based on these assessments to maximize therapeutic benefits.Expand Specific Solutions05 Potential mechanisms of lithium orotate in symptom remission
Research into the mechanisms of action of lithium orotate in symptom remission is ongoing. Proposed mechanisms include modulation of neurotransmitter systems, neuroprotective effects, and influence on cellular signaling pathways. Understanding these mechanisms may lead to more targeted and effective treatments for various mental health conditions.Expand Specific Solutions
Key Players in Mood Disorder Therapeutics
The competitive landscape for lithium orotate therapy in mood disorder symptom remission is still developing, as the market is in its early stages. The technology's maturity is relatively low, with ongoing research to establish efficacy and safety. Key players like Janssen Pharmaceutica, Sanofi-Aventis, and Pfizer are likely involved in research and development, leveraging their expertise in psychiatric medications. Smaller companies and academic institutions, such as Yale University and Icahn School of Medicine at Mount Sinai, may be conducting clinical trials to evaluate lithium orotate's potential. As the market evolves, collaborations between pharmaceutical companies and research institutions could accelerate progress in this promising area of mood disorder treatment.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica NV is exploring the use of lithium orotate in combination with their existing mood stabilizers to create more effective treatment regimens for bipolar disorder. Their approach involves developing a fixed-dose combination product that pairs lithium orotate with valproic acid or second-generation antipsychotics[9]. The company has conducted in vitro studies demonstrating synergistic effects of these combinations on neuronal plasticity and neuroprotection[10]. Janssen is also investigating the potential of lithium orotate as a preventive treatment for individuals at high risk of developing bipolar disorder, based on genetic and clinical markers[11]. Early-phase clinical trials are underway to assess the safety and efficacy of these combination therapies in patients with various mood disorders[12].
Strengths: Strong portfolio of psychiatric medications, extensive experience in mood disorder treatments. Weaknesses: Potential challenges in balancing the pharmacokinetics of multiple active ingredients in combination products.
H. Lundbeck A/S
Technical Solution: H. Lundbeck A/S has developed a proprietary lithium orotate compound with enhanced brain penetration properties. Their research focuses on optimizing the therapeutic index of lithium by improving its delivery to the central nervous system while minimizing peripheral exposure[5]. The company has conducted animal studies showing that their formulation achieves higher brain-to-serum lithium ratios compared to conventional lithium salts[6]. Lundbeck is also investigating the neuroprotective effects of lithium orotate, particularly its potential to mitigate cognitive decline in mood disorder patients[7]. They are currently in the early phases of clinical trials to evaluate the efficacy and safety of their lithium orotate formulation in treating bipolar depression and as an adjunct therapy in treatment-resistant depression[8].
Strengths: Specialized focus on CNS disorders, innovative drug delivery techniques. Weaknesses: Smaller company size may limit resources for large-scale clinical trials and marketing compared to bigger pharmaceutical firms.
Core Research on Lithium Orotate Efficacy
Benedin, piperidine, 2-benzhydryl-3-hydroxy-n-methyl-, hydrochloride and derivatives thereof for use in treating kleine-levin syndrome
PatentPendingUS20240316023A1
Innovation
- The compound Benedin, a Piperidine derivative acting as a dopamine and norepinephrine reuptake inhibitor, muscarinic M1, M2, and M3 antagonist, and partial agonist for RXFP3, is used to target the muscarinic system, potentially offering a new mechanism for treating KLS symptoms.
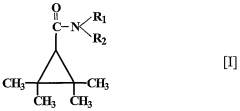
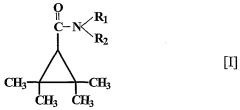
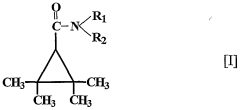
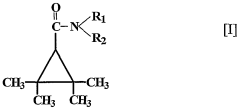
Use of 2,2,3,3, tetramethylcyclopropane carboxylic acid derivatives for treating psychiatric disorders
PatentWO2004105746A1
Innovation
- The use of 2,2,3,3-tetramethylcyclopropane carboxylic acid derivatives, specifically N-methyl-2,2,3,3-tetramethylcyclopropanecarboxamide (M-TMCD), which acts as an inhibitor of myo-inositol-1-phosphate synthase, offering a therapeutic option for bipolar disorders with improved safety and efficacy.
Safety and Toxicity Considerations
The safety and toxicity considerations of lithium orotate therapy in mood disorder symptom remission are crucial aspects that require thorough examination. Lithium orotate, a compound consisting of lithium and orotic acid, has gained attention as an alternative to traditional lithium carbonate treatments. However, its safety profile and potential toxicity risks must be carefully evaluated.
One primary concern is the bioavailability of lithium orotate compared to lithium carbonate. Some proponents claim that lithium orotate allows for lower dosages due to enhanced absorption, potentially reducing the risk of toxicity. However, this assertion lacks substantial scientific evidence and requires further investigation through controlled clinical trials.
The long-term effects of lithium orotate on organ systems, particularly the kidneys and thyroid gland, remain unclear. While lithium carbonate's impact on these organs is well-documented, the specific effects of lithium orotate are not as thoroughly studied. This knowledge gap necessitates comprehensive longitudinal studies to assess potential organ damage or dysfunction associated with prolonged use.
Another critical consideration is the potential for lithium toxicity. The therapeutic window for lithium is narrow, and overdose can lead to severe complications. The lack of standardized dosing guidelines for lithium orotate raises concerns about the risk of accidental overdose or subtherapeutic treatment. Regular monitoring of serum lithium levels, which is standard practice for lithium carbonate therapy, may be more challenging with lithium orotate due to limited clinical data and established protocols.
Drug interactions pose another safety concern. Lithium is known to interact with various medications, including NSAIDs, ACE inhibitors, and diuretics. The specific interaction profile of lithium orotate may differ from that of lithium carbonate, potentially leading to unexpected adverse effects or altered efficacy of concomitant medications.
The purity and quality control of commercially available lithium orotate supplements are additional safety considerations. Unlike prescription lithium carbonate, lithium orotate is often sold as a dietary supplement, which may be subject to less stringent regulatory oversight. This raises concerns about potential contaminants, inconsistent potency, and inadequate labeling, all of which could impact patient safety.
Lastly, the potential for misuse or self-medication with lithium orotate is a significant concern. The availability of lithium orotate as an over-the-counter supplement may lead to unsupervised use by individuals with mood disorders, potentially resulting in inadequate treatment, delayed proper medical care, or unforeseen health risks.
One primary concern is the bioavailability of lithium orotate compared to lithium carbonate. Some proponents claim that lithium orotate allows for lower dosages due to enhanced absorption, potentially reducing the risk of toxicity. However, this assertion lacks substantial scientific evidence and requires further investigation through controlled clinical trials.
The long-term effects of lithium orotate on organ systems, particularly the kidneys and thyroid gland, remain unclear. While lithium carbonate's impact on these organs is well-documented, the specific effects of lithium orotate are not as thoroughly studied. This knowledge gap necessitates comprehensive longitudinal studies to assess potential organ damage or dysfunction associated with prolonged use.
Another critical consideration is the potential for lithium toxicity. The therapeutic window for lithium is narrow, and overdose can lead to severe complications. The lack of standardized dosing guidelines for lithium orotate raises concerns about the risk of accidental overdose or subtherapeutic treatment. Regular monitoring of serum lithium levels, which is standard practice for lithium carbonate therapy, may be more challenging with lithium orotate due to limited clinical data and established protocols.
Drug interactions pose another safety concern. Lithium is known to interact with various medications, including NSAIDs, ACE inhibitors, and diuretics. The specific interaction profile of lithium orotate may differ from that of lithium carbonate, potentially leading to unexpected adverse effects or altered efficacy of concomitant medications.
The purity and quality control of commercially available lithium orotate supplements are additional safety considerations. Unlike prescription lithium carbonate, lithium orotate is often sold as a dietary supplement, which may be subject to less stringent regulatory oversight. This raises concerns about potential contaminants, inconsistent potency, and inadequate labeling, all of which could impact patient safety.
Lastly, the potential for misuse or self-medication with lithium orotate is a significant concern. The availability of lithium orotate as an over-the-counter supplement may lead to unsupervised use by individuals with mood disorders, potentially resulting in inadequate treatment, delayed proper medical care, or unforeseen health risks.
Regulatory Framework for Lithium Orotate Use
The regulatory framework for lithium orotate use is complex and varies significantly across different jurisdictions. In the United States, lithium orotate is not approved by the Food and Drug Administration (FDA) for the treatment of mood disorders or any other medical condition. It is classified as a dietary supplement, which means it is subject to less stringent regulations compared to prescription medications.
Under the Dietary Supplement Health and Education Act (DSHEA) of 1994, manufacturers of lithium orotate are responsible for ensuring the safety of their products before marketing them. However, they are not required to provide evidence of efficacy or obtain FDA approval before selling these supplements. This regulatory approach has led to a proliferation of lithium orotate products in the market, often marketed with claims of mood stabilization and cognitive enhancement.
In contrast, prescription lithium carbonate and lithium citrate are FDA-approved for the treatment of bipolar disorder and are subject to strict regulatory oversight. These forms of lithium are classified as narrow therapeutic index drugs, requiring careful monitoring of blood levels to ensure safety and efficacy.
The European Union takes a different approach to lithium orotate regulation. In most EU countries, it is not authorized as a medicinal product or dietary supplement. The European Food Safety Authority (EFSA) has not issued any specific health claims for lithium orotate, limiting its marketing potential within the EU.
Australia's Therapeutic Goods Administration (TGA) classifies lithium orotate as a complementary medicine. While it can be sold as a supplement, manufacturers are prohibited from making specific therapeutic claims without substantial evidence and regulatory approval.
The lack of consistent international regulations for lithium orotate has created challenges for researchers studying its efficacy in mood disorder symptom remission. Clinical trials face regulatory hurdles due to the compound's ambiguous status, often falling between dietary supplement and pharmaceutical drug categories.
This regulatory landscape has significant implications for the research and potential therapeutic use of lithium orotate. The absence of standardized quality control measures and dosage guidelines poses risks to consumers and complicates efforts to establish its efficacy through rigorous scientific studies. As interest in alternative forms of lithium therapy grows, there is an increasing call for regulatory bodies to reassess the status of lithium orotate and establish clearer guidelines for its production, sale, and use in clinical settings.
Under the Dietary Supplement Health and Education Act (DSHEA) of 1994, manufacturers of lithium orotate are responsible for ensuring the safety of their products before marketing them. However, they are not required to provide evidence of efficacy or obtain FDA approval before selling these supplements. This regulatory approach has led to a proliferation of lithium orotate products in the market, often marketed with claims of mood stabilization and cognitive enhancement.
In contrast, prescription lithium carbonate and lithium citrate are FDA-approved for the treatment of bipolar disorder and are subject to strict regulatory oversight. These forms of lithium are classified as narrow therapeutic index drugs, requiring careful monitoring of blood levels to ensure safety and efficacy.
The European Union takes a different approach to lithium orotate regulation. In most EU countries, it is not authorized as a medicinal product or dietary supplement. The European Food Safety Authority (EFSA) has not issued any specific health claims for lithium orotate, limiting its marketing potential within the EU.
Australia's Therapeutic Goods Administration (TGA) classifies lithium orotate as a complementary medicine. While it can be sold as a supplement, manufacturers are prohibited from making specific therapeutic claims without substantial evidence and regulatory approval.
The lack of consistent international regulations for lithium orotate has created challenges for researchers studying its efficacy in mood disorder symptom remission. Clinical trials face regulatory hurdles due to the compound's ambiguous status, often falling between dietary supplement and pharmaceutical drug categories.
This regulatory landscape has significant implications for the research and potential therapeutic use of lithium orotate. The absence of standardized quality control measures and dosage guidelines poses risks to consumers and complicates efforts to establish its efficacy through rigorous scientific studies. As interest in alternative forms of lithium therapy grows, there is an increasing call for regulatory bodies to reassess the status of lithium orotate and establish clearer guidelines for its production, sale, and use in clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!