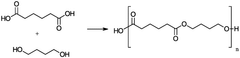Exploring biodegradable polymers in single-use laryngoscopes.
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biodegradable Polymers in Medical Devices: Background and Objectives
Biodegradable polymers have emerged as a revolutionary material in the medical device industry, particularly in the development of single-use laryngoscopes. These innovative materials offer a sustainable solution to the growing environmental concerns associated with traditional plastic medical devices. The evolution of biodegradable polymers in medical applications can be traced back to the late 20th century, with significant advancements occurring in the past two decades.
The primary objective of incorporating biodegradable polymers in single-use laryngoscopes is to reduce the environmental impact of medical waste while maintaining the high standards of safety and efficacy required in medical procedures. This technology aims to address the critical balance between single-use device benefits, such as reduced risk of cross-contamination, and the need for environmentally responsible healthcare practices.
Biodegradable polymers used in medical devices are designed to break down into non-toxic components over time, typically through hydrolysis or enzymatic degradation. This characteristic makes them ideal for applications where temporary presence is required, such as in single-use laryngoscopes. The most commonly used biodegradable polymers in this field include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers.
The development of biodegradable polymers for medical devices has been driven by several factors, including increasing environmental awareness, stricter regulations on medical waste disposal, and the healthcare industry's push towards more sustainable practices. These materials offer a promising alternative to conventional plastics, potentially reducing the carbon footprint of medical procedures and alleviating the burden on waste management systems.
In the context of single-use laryngoscopes, the application of biodegradable polymers presents unique challenges and opportunities. The material must meet stringent requirements for biocompatibility, mechanical strength, and sterilization compatibility while also demonstrating controlled degradation properties. Researchers and manufacturers are focusing on optimizing polymer formulations to achieve the ideal balance between functionality during use and rapid degradation after disposal.
The ongoing research in this field aims to expand the range of biodegradable polymers suitable for medical devices, improve their performance characteristics, and develop cost-effective manufacturing processes. As the technology continues to evolve, it is expected to play a crucial role in shaping the future of sustainable healthcare practices, particularly in the realm of single-use medical devices like laryngoscopes.
The primary objective of incorporating biodegradable polymers in single-use laryngoscopes is to reduce the environmental impact of medical waste while maintaining the high standards of safety and efficacy required in medical procedures. This technology aims to address the critical balance between single-use device benefits, such as reduced risk of cross-contamination, and the need for environmentally responsible healthcare practices.
Biodegradable polymers used in medical devices are designed to break down into non-toxic components over time, typically through hydrolysis or enzymatic degradation. This characteristic makes them ideal for applications where temporary presence is required, such as in single-use laryngoscopes. The most commonly used biodegradable polymers in this field include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers.
The development of biodegradable polymers for medical devices has been driven by several factors, including increasing environmental awareness, stricter regulations on medical waste disposal, and the healthcare industry's push towards more sustainable practices. These materials offer a promising alternative to conventional plastics, potentially reducing the carbon footprint of medical procedures and alleviating the burden on waste management systems.
In the context of single-use laryngoscopes, the application of biodegradable polymers presents unique challenges and opportunities. The material must meet stringent requirements for biocompatibility, mechanical strength, and sterilization compatibility while also demonstrating controlled degradation properties. Researchers and manufacturers are focusing on optimizing polymer formulations to achieve the ideal balance between functionality during use and rapid degradation after disposal.
The ongoing research in this field aims to expand the range of biodegradable polymers suitable for medical devices, improve their performance characteristics, and develop cost-effective manufacturing processes. As the technology continues to evolve, it is expected to play a crucial role in shaping the future of sustainable healthcare practices, particularly in the realm of single-use medical devices like laryngoscopes.
Market Analysis for Eco-Friendly Laryngoscopes
The market for eco-friendly laryngoscopes, particularly those utilizing biodegradable polymers, is experiencing significant growth driven by increasing environmental awareness and stringent healthcare regulations. This segment is part of the broader single-use medical devices market, which is projected to expand substantially in the coming years.
Healthcare facilities worldwide are showing a growing preference for disposable laryngoscopes due to their reduced risk of cross-contamination and lower maintenance costs. However, the environmental impact of traditional plastic-based disposable devices has become a major concern, creating a strong demand for sustainable alternatives.
The adoption of biodegradable polymers in single-use laryngoscopes addresses both clinical and environmental needs. These eco-friendly devices offer the same benefits as conventional disposables in terms of infection control and convenience, while significantly reducing plastic waste and environmental footprint.
Market research indicates that hospitals and ambulatory surgical centers are the primary end-users of eco-friendly laryngoscopes. The increasing number of surgical procedures, coupled with the rising prevalence of respiratory diseases, is driving the demand for these devices. Additionally, the COVID-19 pandemic has further accelerated the shift towards single-use medical equipment, including laryngoscopes, to minimize infection risks.
Geographically, North America and Europe currently lead the market for eco-friendly laryngoscopes, owing to stringent regulations on medical waste management and higher environmental consciousness. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by improving healthcare infrastructure and increasing awareness of sustainable healthcare practices.
Key market drivers include the growing focus on reducing healthcare-associated infections, increasing government initiatives to promote environmentally friendly medical devices, and the rising demand for cost-effective healthcare solutions. However, challenges such as higher initial costs compared to traditional disposables and the need for extensive clinical validation of new biodegradable materials may impact market growth.
The competitive landscape of the eco-friendly laryngoscope market is characterized by a mix of established medical device manufacturers and innovative start-ups. These companies are investing heavily in research and development to improve the performance and cost-effectiveness of biodegradable polymers in medical applications.
In conclusion, the market for eco-friendly laryngoscopes made from biodegradable polymers presents significant opportunities for growth and innovation. As healthcare providers increasingly prioritize sustainability alongside patient safety and cost-effectiveness, the demand for these devices is expected to surge, reshaping the medical device industry's approach to single-use products.
Healthcare facilities worldwide are showing a growing preference for disposable laryngoscopes due to their reduced risk of cross-contamination and lower maintenance costs. However, the environmental impact of traditional plastic-based disposable devices has become a major concern, creating a strong demand for sustainable alternatives.
The adoption of biodegradable polymers in single-use laryngoscopes addresses both clinical and environmental needs. These eco-friendly devices offer the same benefits as conventional disposables in terms of infection control and convenience, while significantly reducing plastic waste and environmental footprint.
Market research indicates that hospitals and ambulatory surgical centers are the primary end-users of eco-friendly laryngoscopes. The increasing number of surgical procedures, coupled with the rising prevalence of respiratory diseases, is driving the demand for these devices. Additionally, the COVID-19 pandemic has further accelerated the shift towards single-use medical equipment, including laryngoscopes, to minimize infection risks.
Geographically, North America and Europe currently lead the market for eco-friendly laryngoscopes, owing to stringent regulations on medical waste management and higher environmental consciousness. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by improving healthcare infrastructure and increasing awareness of sustainable healthcare practices.
Key market drivers include the growing focus on reducing healthcare-associated infections, increasing government initiatives to promote environmentally friendly medical devices, and the rising demand for cost-effective healthcare solutions. However, challenges such as higher initial costs compared to traditional disposables and the need for extensive clinical validation of new biodegradable materials may impact market growth.
The competitive landscape of the eco-friendly laryngoscope market is characterized by a mix of established medical device manufacturers and innovative start-ups. These companies are investing heavily in research and development to improve the performance and cost-effectiveness of biodegradable polymers in medical applications.
In conclusion, the market for eco-friendly laryngoscopes made from biodegradable polymers presents significant opportunities for growth and innovation. As healthcare providers increasingly prioritize sustainability alongside patient safety and cost-effectiveness, the demand for these devices is expected to surge, reshaping the medical device industry's approach to single-use products.
Current Challenges in Biodegradable Medical Instruments
The development of biodegradable medical instruments, particularly single-use laryngoscopes, faces several significant challenges. One of the primary obstacles is achieving the delicate balance between biodegradability and mechanical performance. Biodegradable polymers must maintain their structural integrity and functionality during use while also being capable of decomposing within a reasonable timeframe after disposal.
Material selection poses another major challenge. Researchers are exploring various biodegradable polymers, such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers. However, each material has its limitations in terms of strength, flexibility, and degradation rate. Finding the optimal polymer or polymer blend that meets all the requirements for laryngoscope manufacturing remains a complex task.
Sterilization compatibility is a critical issue in medical device development. Traditional sterilization methods, such as ethylene oxide treatment or gamma irradiation, can potentially degrade or alter the properties of biodegradable polymers. This necessitates the development of new sterilization techniques or the modification of existing polymers to withstand sterilization processes without compromising their biodegradability or mechanical properties.
Cost-effectiveness presents another hurdle in the widespread adoption of biodegradable laryngoscopes. Currently, the production of biodegradable polymers and their processing into medical-grade instruments is more expensive than conventional plastic manufacturing. This cost differential can be a significant barrier to market entry and widespread adoption, especially in healthcare systems with tight budget constraints.
Regulatory compliance and standardization pose additional challenges. As biodegradable medical instruments are relatively new, regulatory frameworks and testing standards are still evolving. Manufacturers must navigate complex approval processes and demonstrate the safety, efficacy, and environmental benefits of their products to gain market acceptance.
Lastly, the environmental impact of biodegradable laryngoscopes throughout their lifecycle requires careful consideration. While biodegradability offers clear end-of-life benefits, the production and disposal processes must be optimized to ensure a net positive environmental impact. This includes addressing concerns about the potential release of microplastics during degradation and the overall carbon footprint of manufacturing biodegradable polymers.
Material selection poses another major challenge. Researchers are exploring various biodegradable polymers, such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers. However, each material has its limitations in terms of strength, flexibility, and degradation rate. Finding the optimal polymer or polymer blend that meets all the requirements for laryngoscope manufacturing remains a complex task.
Sterilization compatibility is a critical issue in medical device development. Traditional sterilization methods, such as ethylene oxide treatment or gamma irradiation, can potentially degrade or alter the properties of biodegradable polymers. This necessitates the development of new sterilization techniques or the modification of existing polymers to withstand sterilization processes without compromising their biodegradability or mechanical properties.
Cost-effectiveness presents another hurdle in the widespread adoption of biodegradable laryngoscopes. Currently, the production of biodegradable polymers and their processing into medical-grade instruments is more expensive than conventional plastic manufacturing. This cost differential can be a significant barrier to market entry and widespread adoption, especially in healthcare systems with tight budget constraints.
Regulatory compliance and standardization pose additional challenges. As biodegradable medical instruments are relatively new, regulatory frameworks and testing standards are still evolving. Manufacturers must navigate complex approval processes and demonstrate the safety, efficacy, and environmental benefits of their products to gain market acceptance.
Lastly, the environmental impact of biodegradable laryngoscopes throughout their lifecycle requires careful consideration. While biodegradability offers clear end-of-life benefits, the production and disposal processes must be optimized to ensure a net positive environmental impact. This includes addressing concerns about the potential release of microplastics during degradation and the overall carbon footprint of manufacturing biodegradable polymers.
Existing Biodegradable Polymer Solutions for Laryngoscopes
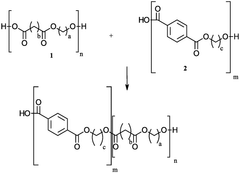
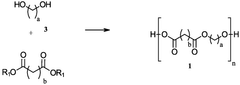
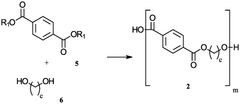
01 Synthesis and composition of biodegradable polymers
Various methods and compositions for synthesizing biodegradable polymers are described. These include techniques for creating polymers with specific properties, such as controlled degradation rates or enhanced mechanical strength. The polymers may be derived from natural or synthetic sources and can be tailored for different applications.- Biodegradable polymer compositions: Various compositions of biodegradable polymers are developed for different applications. These compositions may include blends of different biodegradable polymers or combinations with other materials to enhance properties such as strength, flexibility, or degradation rate. The compositions are designed to meet specific requirements for applications in areas such as packaging, medical devices, and agricultural products.
- Synthesis methods for biodegradable polymers: Different methods are employed to synthesize biodegradable polymers, including polymerization techniques and modification of existing polymers. These methods aim to control the molecular weight, structure, and properties of the resulting polymers. Innovations in synthesis methods focus on improving efficiency, reducing environmental impact, and enhancing the scalability of production processes.
- Applications of biodegradable polymers: Biodegradable polymers find applications in various fields, including medicine, agriculture, and consumer goods. In medicine, they are used for drug delivery systems, tissue engineering scaffolds, and biodegradable implants. Agricultural applications include controlled-release fertilizers and mulch films. Consumer goods applications range from packaging materials to disposable items, aiming to reduce environmental impact.
- Degradation mechanisms and control: Research focuses on understanding and controlling the degradation mechanisms of biodegradable polymers. This includes studying factors that influence degradation rates, such as environmental conditions, polymer structure, and additives. Efforts are made to develop polymers with predictable and controllable degradation profiles to suit specific applications and end-of-life scenarios.
- Biodegradable polymer blends and composites: Blending biodegradable polymers or creating composites with natural fibers or inorganic materials is explored to enhance properties and expand applications. These blends and composites aim to combine the benefits of different materials, such as improved mechanical properties, processability, or degradation characteristics. Research in this area focuses on optimizing compatibility between components and achieving desired performance.
02 Applications of biodegradable polymers in medical field
Biodegradable polymers find extensive use in medical applications, including drug delivery systems, tissue engineering scaffolds, and implantable devices. These materials offer the advantage of being absorbed by the body over time, eliminating the need for removal procedures and reducing long-term complications.Expand Specific Solutions03 Biodegradable polymers for packaging and environmental applications
The use of biodegradable polymers in packaging and other environmental applications is explored. These materials offer sustainable alternatives to traditional plastics, reducing environmental impact and waste accumulation. Various formulations and processing techniques are developed to enhance the performance and degradability of these polymers.Expand Specific Solutions04 Modification and blending of biodegradable polymers
Techniques for modifying and blending biodegradable polymers to enhance their properties are discussed. This includes the creation of composite materials, copolymers, and polymer blends to achieve desired characteristics such as improved mechanical strength, flexibility, or degradation rates.Expand Specific Solutions05 Biodegradable polymer processing and manufacturing
Various methods for processing and manufacturing biodegradable polymers are explored. This includes techniques for molding, extrusion, and other fabrication processes to create products with specific shapes and properties. Considerations for scalability and industrial production are also addressed.Expand Specific Solutions
Key Players in Biodegradable Medical Device Industry
The exploration of biodegradable polymers in single-use laryngoscopes is in its early stages, with the market showing significant growth potential. This emerging field combines the increasing demand for sustainable medical devices with the need for high-performance disposable equipment. Companies like Coloplast A/S, Medtronic Vascular, Inc., and Alcon AG are likely to play key roles in advancing this technology. The market size is expected to expand rapidly as healthcare facilities seek eco-friendly alternatives. While the technology is still developing, collaborations between academic institutions like Rutgers University and California Institute of Technology with industry leaders are accelerating progress towards commercially viable biodegradable laryngoscopes.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular, Inc. has developed a biodegradable polymer technology for medical devices, including potential applications in single-use laryngoscopes. Their approach focuses on using a proprietary blend of polyesters and polycarbonates that offer controlled degradation rates[7]. The company has engineered these polymers to maintain their mechanical properties during use while gradually breaking down into biocompatible monomers over time. Medtronic's biodegradable polymers for laryngoscopes are designed to degrade within 12-18 months after disposal, balancing the need for durability during use with environmental considerations[8]. The company has also incorporated antimicrobial agents into the polymer matrix to reduce the risk of infection during use[9].
Strengths: Controlled degradation rates, incorporation of antimicrobial properties, and extensive experience in medical device manufacturing. Weaknesses: Longer degradation time compared to some competitors and potential regulatory hurdles for novel materials.
3M Innovative Properties Co.
Technical Solution: 3M Innovative Properties Co. has developed a range of biodegradable polymers suitable for medical devices, including single-use laryngoscopes. Their approach involves using a combination of aliphatic polyesters and natural polymers to create materials that balance performance with environmental sustainability[10]. 3M's biodegradable polymers for laryngoscopes are engineered to maintain their structural integrity and optical clarity during use while breaking down into non-toxic components within 6-9 months after disposal[11]. The company has also developed specialized coatings that can be applied to these biodegradable polymers to enhance their barrier properties and resistance to moisture, extending their shelf life and ensuring reliability in medical settings[12].
Strengths: Advanced polymer engineering capabilities, integration of specialized coatings, and global manufacturing infrastructure. Weaknesses: Potential trade-offs between biodegradability and performance in extreme conditions.
Innovative Biodegradable Polymer Compositions for Medical Use
Biodegradable hospital curtain and curtain system, method of making and composition
PatentWO2025024163A2
Innovation
- A biodegradable disposable curtain system with a lockable quick-release mechanism, made from nonwoven biodegradable materials, which can be easily cleaned and disposed of, reducing environmental impact.
Biodegradable healthcare disposables and method of making
PatentPendingUS20250034382A1
Innovation
- Development of a biodegradable disposable material made from a blend or copolymer of poly-lactic acid (PLA) or polyhydroxyalkanoates (PHA) with a poly-terephthalate, which can be mixed with non-biodegradable polymers like polypropylene to create a biodegradable composition suitable for healthcare disposables.
Environmental Impact Assessment of Biodegradable Medical Devices
The environmental impact assessment of biodegradable medical devices, particularly single-use laryngoscopes made from biodegradable polymers, is a critical aspect of their development and implementation. These devices offer potential benefits in reducing medical waste and its associated environmental footprint, but their full lifecycle impact must be carefully evaluated.
Biodegradable polymers used in medical devices can significantly reduce the accumulation of plastic waste in landfills and oceans. Traditional single-use laryngoscopes, often made from non-biodegradable plastics, contribute to the growing problem of medical waste. By transitioning to biodegradable alternatives, healthcare facilities can minimize their long-term environmental impact.
The production process of biodegradable polymers for medical devices generally requires less energy and produces fewer greenhouse gas emissions compared to conventional plastics. This reduced carbon footprint during manufacturing is a key environmental advantage. However, the specific environmental impact varies depending on the type of biodegradable polymer used and the manufacturing techniques employed.
When assessing the end-of-life impact, biodegradable laryngoscopes offer clear advantages. Unlike their non-biodegradable counterparts, these devices can decompose naturally, reducing the burden on waste management systems. The rate and completeness of biodegradation depend on the specific polymer used and the disposal environment, factors that must be carefully considered in the device design.
Water consumption and pollution are also important considerations in the environmental assessment. The production of biodegradable polymers often requires less water compared to traditional plastics. Additionally, the potential for water pollution from decomposing medical devices is generally lower with biodegradable materials, although this aspect requires thorough testing and monitoring.
The use of renewable resources in the production of biodegradable polymers for laryngoscopes can further enhance their environmental profile. Many biodegradable polymers are derived from plant-based sources, reducing reliance on fossil fuels and promoting a more sustainable supply chain.
However, it's crucial to consider the potential trade-offs. For instance, if biodegradable laryngoscopes require more frequent replacement due to shorter shelf life or reduced durability, the increased production and transportation could offset some environmental benefits. A comprehensive lifecycle analysis is necessary to fully understand these dynamics.
In conclusion, while biodegradable single-use laryngoscopes show promise in reducing the environmental impact of medical waste, a thorough assessment considering all stages of the product lifecycle is essential. This evaluation should include raw material sourcing, manufacturing processes, usage patterns, and end-of-life disposal to ensure that the transition to biodegradable alternatives truly results in a net positive environmental impact.
Biodegradable polymers used in medical devices can significantly reduce the accumulation of plastic waste in landfills and oceans. Traditional single-use laryngoscopes, often made from non-biodegradable plastics, contribute to the growing problem of medical waste. By transitioning to biodegradable alternatives, healthcare facilities can minimize their long-term environmental impact.
The production process of biodegradable polymers for medical devices generally requires less energy and produces fewer greenhouse gas emissions compared to conventional plastics. This reduced carbon footprint during manufacturing is a key environmental advantage. However, the specific environmental impact varies depending on the type of biodegradable polymer used and the manufacturing techniques employed.
When assessing the end-of-life impact, biodegradable laryngoscopes offer clear advantages. Unlike their non-biodegradable counterparts, these devices can decompose naturally, reducing the burden on waste management systems. The rate and completeness of biodegradation depend on the specific polymer used and the disposal environment, factors that must be carefully considered in the device design.
Water consumption and pollution are also important considerations in the environmental assessment. The production of biodegradable polymers often requires less water compared to traditional plastics. Additionally, the potential for water pollution from decomposing medical devices is generally lower with biodegradable materials, although this aspect requires thorough testing and monitoring.
The use of renewable resources in the production of biodegradable polymers for laryngoscopes can further enhance their environmental profile. Many biodegradable polymers are derived from plant-based sources, reducing reliance on fossil fuels and promoting a more sustainable supply chain.
However, it's crucial to consider the potential trade-offs. For instance, if biodegradable laryngoscopes require more frequent replacement due to shorter shelf life or reduced durability, the increased production and transportation could offset some environmental benefits. A comprehensive lifecycle analysis is necessary to fully understand these dynamics.
In conclusion, while biodegradable single-use laryngoscopes show promise in reducing the environmental impact of medical waste, a thorough assessment considering all stages of the product lifecycle is essential. This evaluation should include raw material sourcing, manufacturing processes, usage patterns, and end-of-life disposal to ensure that the transition to biodegradable alternatives truly results in a net positive environmental impact.
Regulatory Framework for Biodegradable Medical Instruments
The regulatory framework for biodegradable medical instruments, particularly in the context of single-use laryngoscopes, is a complex and evolving landscape. As the healthcare industry increasingly focuses on sustainability and environmental responsibility, regulatory bodies worldwide are adapting their guidelines to accommodate innovative materials and technologies.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating medical devices, including biodegradable laryngoscopes. The FDA's Center for Devices and Radiological Health (CDRH) oversees the approval process for such devices, considering factors such as safety, efficacy, and environmental impact. Manufacturers seeking to introduce biodegradable laryngoscopes must navigate the premarket notification (510(k)) or premarket approval (PMA) pathways, depending on the device's classification and risk level.
The European Union's regulatory framework is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation places a strong emphasis on the entire lifecycle of medical devices, including their disposal. For biodegradable laryngoscopes, manufacturers must demonstrate compliance with essential requirements related to safety and performance, as well as provide evidence of the device's biodegradability and environmental impact.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of medical devices. The Japanese regulatory framework has been evolving to align more closely with international standards, including considerations for innovative materials like biodegradable polymers. Manufacturers must provide comprehensive data on the device's safety, efficacy, and environmental impact to obtain approval.
Globally, the International Organization for Standardization (ISO) has developed standards that are relevant to biodegradable medical instruments. ISO 10993, which addresses the biological evaluation of medical devices, includes considerations for biodegradable materials. Additionally, ISO 14001, which focuses on environmental management systems, may be applicable to the manufacturing processes of biodegradable laryngoscopes.
As the field of biodegradable medical instruments continues to advance, regulatory bodies are likely to refine their guidelines further. This may include the development of specific standards for biodegradable polymers in medical devices, more stringent requirements for demonstrating biodegradability, and increased focus on the environmental impact throughout the product lifecycle.
Manufacturers and researchers working on biodegradable laryngoscopes must stay abreast of these evolving regulations and engage proactively with regulatory bodies. Early consultation and collaboration with regulatory agencies can help streamline the approval process and ensure that innovative, environmentally friendly medical devices reach the market efficiently while maintaining the highest standards of patient safety and environmental responsibility.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating medical devices, including biodegradable laryngoscopes. The FDA's Center for Devices and Radiological Health (CDRH) oversees the approval process for such devices, considering factors such as safety, efficacy, and environmental impact. Manufacturers seeking to introduce biodegradable laryngoscopes must navigate the premarket notification (510(k)) or premarket approval (PMA) pathways, depending on the device's classification and risk level.
The European Union's regulatory framework is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation places a strong emphasis on the entire lifecycle of medical devices, including their disposal. For biodegradable laryngoscopes, manufacturers must demonstrate compliance with essential requirements related to safety and performance, as well as provide evidence of the device's biodegradability and environmental impact.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of medical devices. The Japanese regulatory framework has been evolving to align more closely with international standards, including considerations for innovative materials like biodegradable polymers. Manufacturers must provide comprehensive data on the device's safety, efficacy, and environmental impact to obtain approval.
Globally, the International Organization for Standardization (ISO) has developed standards that are relevant to biodegradable medical instruments. ISO 10993, which addresses the biological evaluation of medical devices, includes considerations for biodegradable materials. Additionally, ISO 14001, which focuses on environmental management systems, may be applicable to the manufacturing processes of biodegradable laryngoscopes.
As the field of biodegradable medical instruments continues to advance, regulatory bodies are likely to refine their guidelines further. This may include the development of specific standards for biodegradable polymers in medical devices, more stringent requirements for demonstrating biodegradability, and increased focus on the environmental impact throughout the product lifecycle.
Manufacturers and researchers working on biodegradable laryngoscopes must stay abreast of these evolving regulations and engage proactively with regulatory bodies. Early consultation and collaboration with regulatory agencies can help streamline the approval process and ensure that innovative, environmentally friendly medical devices reach the market efficiently while maintaining the highest standards of patient safety and environmental responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!