Laryngoscope operative interfaces: Trends and developments.
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Laryngoscope Interface Evolution and Objectives
Laryngoscopes have undergone significant evolution since their inception, driven by the need for improved visualization and ergonomics in airway management. The primary objective of laryngoscope interface development has been to enhance the operator's ability to visualize the larynx and facilitate intubation while minimizing patient trauma and operator fatigue.
The early laryngoscopes featured simple, rigid designs with direct line-of-sight visualization. These traditional instruments, while effective, often required significant skill and experience to use effectively, particularly in challenging airway scenarios. The advent of fiber optic technology in the 1960s marked a significant milestone in laryngoscope interface evolution, allowing for improved illumination and the potential for indirect visualization.
The late 20th and early 21st centuries saw the introduction of video laryngoscopes, representing a paradigm shift in laryngoscope interfaces. These devices incorporate miniature cameras and high-resolution displays, providing a magnified, indirect view of the larynx. This technological leap aimed to reduce the learning curve for intubation and improve success rates, especially in difficult airway cases.
Recent trends in laryngoscope interface development have focused on ergonomic design, aiming to reduce operator strain during prolonged procedures. Adjustable handles, lightweight materials, and optimized blade shapes have been introduced to enhance user comfort and control. Additionally, the integration of advanced imaging technologies, such as augmented reality overlays and 3D visualization, is being explored to further assist operators in navigating complex airway anatomies.
Another significant objective in laryngoscope interface evolution has been the development of disposable devices to address infection control concerns. Single-use laryngoscopes with comparable performance to reusable models have gained traction, particularly in emergency and pre-hospital settings.
The ongoing evolution of laryngoscope interfaces aims to address several key objectives: improving first-attempt success rates, reducing intubation time, minimizing complications, and enhancing operator confidence across various clinical scenarios. Future developments are likely to focus on smart, connected devices that can provide real-time guidance, collect performance data, and integrate with other medical systems for comprehensive airway management.
The early laryngoscopes featured simple, rigid designs with direct line-of-sight visualization. These traditional instruments, while effective, often required significant skill and experience to use effectively, particularly in challenging airway scenarios. The advent of fiber optic technology in the 1960s marked a significant milestone in laryngoscope interface evolution, allowing for improved illumination and the potential for indirect visualization.
The late 20th and early 21st centuries saw the introduction of video laryngoscopes, representing a paradigm shift in laryngoscope interfaces. These devices incorporate miniature cameras and high-resolution displays, providing a magnified, indirect view of the larynx. This technological leap aimed to reduce the learning curve for intubation and improve success rates, especially in difficult airway cases.
Recent trends in laryngoscope interface development have focused on ergonomic design, aiming to reduce operator strain during prolonged procedures. Adjustable handles, lightweight materials, and optimized blade shapes have been introduced to enhance user comfort and control. Additionally, the integration of advanced imaging technologies, such as augmented reality overlays and 3D visualization, is being explored to further assist operators in navigating complex airway anatomies.
Another significant objective in laryngoscope interface evolution has been the development of disposable devices to address infection control concerns. Single-use laryngoscopes with comparable performance to reusable models have gained traction, particularly in emergency and pre-hospital settings.
The ongoing evolution of laryngoscope interfaces aims to address several key objectives: improving first-attempt success rates, reducing intubation time, minimizing complications, and enhancing operator confidence across various clinical scenarios. Future developments are likely to focus on smart, connected devices that can provide real-time guidance, collect performance data, and integrate with other medical systems for comprehensive airway management.
Market Demand Analysis for Advanced Laryngoscopes
The market demand for advanced laryngoscopes has been steadily increasing, driven by several key factors in the healthcare industry. The growing prevalence of chronic respiratory diseases, coupled with an aging population, has led to a rise in the number of intubation procedures performed globally. This trend has created a substantial need for more sophisticated and user-friendly laryngoscope devices.
Healthcare providers are increasingly seeking laryngoscopes with improved operative interfaces to enhance procedural efficiency and patient outcomes. The demand for devices that offer better visualization, ergonomic design, and ease of use is particularly strong. Advanced laryngoscopes with integrated video systems and high-resolution displays are gaining traction, as they allow for better airway assessment and more precise intubation, especially in difficult cases.
The COVID-19 pandemic has further accelerated the demand for advanced laryngoscopes. The need for rapid and safe intubation of critically ill patients has highlighted the importance of devices that minimize the risk of viral transmission to healthcare workers. This has led to a surge in interest for laryngoscopes with disposable blades and those that allow for greater distance between the operator and the patient's airway.
In terms of market segmentation, hospitals remain the largest end-users of advanced laryngoscopes, followed by ambulatory surgical centers and emergency medical services. The increasing adoption of these devices in pre-hospital settings and smaller healthcare facilities is expanding the overall market size.
Geographically, North America and Europe currently dominate the advanced laryngoscope market, owing to their well-established healthcare infrastructure and higher healthcare spending. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth potential, driven by improving healthcare access and increasing investments in medical technology.
The market is also witnessing a shift towards more compact and portable laryngoscope designs, catering to the needs of emergency responders and healthcare providers in resource-limited settings. This trend is opening up new market opportunities and driving innovation in product design.
As healthcare systems worldwide focus on improving patient safety and reducing healthcare-associated infections, there is a growing demand for laryngoscopes with enhanced sterilization capabilities and single-use components. This trend is reshaping the market landscape and influencing product development strategies among manufacturers.
Overall, the market for advanced laryngoscopes with improved operative interfaces is poised for significant growth in the coming years, driven by technological advancements, changing healthcare needs, and the ongoing push for better patient outcomes and provider safety.
Healthcare providers are increasingly seeking laryngoscopes with improved operative interfaces to enhance procedural efficiency and patient outcomes. The demand for devices that offer better visualization, ergonomic design, and ease of use is particularly strong. Advanced laryngoscopes with integrated video systems and high-resolution displays are gaining traction, as they allow for better airway assessment and more precise intubation, especially in difficult cases.
The COVID-19 pandemic has further accelerated the demand for advanced laryngoscopes. The need for rapid and safe intubation of critically ill patients has highlighted the importance of devices that minimize the risk of viral transmission to healthcare workers. This has led to a surge in interest for laryngoscopes with disposable blades and those that allow for greater distance between the operator and the patient's airway.
In terms of market segmentation, hospitals remain the largest end-users of advanced laryngoscopes, followed by ambulatory surgical centers and emergency medical services. The increasing adoption of these devices in pre-hospital settings and smaller healthcare facilities is expanding the overall market size.
Geographically, North America and Europe currently dominate the advanced laryngoscope market, owing to their well-established healthcare infrastructure and higher healthcare spending. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth potential, driven by improving healthcare access and increasing investments in medical technology.
The market is also witnessing a shift towards more compact and portable laryngoscope designs, catering to the needs of emergency responders and healthcare providers in resource-limited settings. This trend is opening up new market opportunities and driving innovation in product design.
As healthcare systems worldwide focus on improving patient safety and reducing healthcare-associated infections, there is a growing demand for laryngoscopes with enhanced sterilization capabilities and single-use components. This trend is reshaping the market landscape and influencing product development strategies among manufacturers.
Overall, the market for advanced laryngoscopes with improved operative interfaces is poised for significant growth in the coming years, driven by technological advancements, changing healthcare needs, and the ongoing push for better patient outcomes and provider safety.
Current Challenges in Laryngoscope Operative Interfaces
Despite significant advancements in laryngoscope technology, several challenges persist in the development and implementation of operative interfaces. One of the primary issues is the limited field of view provided by traditional laryngoscopes. This constraint often leads to difficulties in visualizing the entire laryngeal structure, particularly in patients with complex anatomies or those undergoing procedures that require extensive manipulation of the airway.
Another significant challenge is the ergonomic design of laryngoscope handles and blades. Many practitioners report discomfort and fatigue during prolonged use, which can potentially impact the precision and efficiency of procedures. The need for improved ergonomics that can accommodate various hand sizes and preferences while maintaining optimal control and maneuverability remains a critical area for development.
The integration of advanced imaging technologies into laryngoscope operative interfaces presents both opportunities and challenges. While high-resolution cameras and video laryngoscopes have greatly enhanced visualization, issues such as image distortion, glare, and maintaining proper focus during dynamic procedures continue to pose difficulties. Additionally, the incorporation of these technologies often results in increased device complexity and cost, potentially limiting widespread adoption in resource-constrained settings.
Sterilization and infection control represent ongoing concerns in laryngoscope design. The intricate components of modern laryngoscopes, particularly those with integrated electronics, can be challenging to clean and sterilize effectively. Balancing the need for advanced functionality with ease of maintenance and infection prevention remains a significant hurdle for manufacturers and healthcare providers alike.
The development of intuitive and user-friendly interfaces for controlling various laryngoscope functions is another area of focus. As laryngoscopes become more sophisticated, incorporating features such as adjustable light intensity, image capture, and real-time data display, the complexity of user interfaces has increased. Designing interfaces that allow for seamless operation without distracting from the primary task of airway management is a persistent challenge.
Lastly, the integration of laryngoscope operative interfaces with other medical devices and hospital information systems presents both technical and interoperability challenges. Ensuring seamless data exchange, compatibility with existing infrastructure, and adherence to evolving healthcare IT standards are crucial considerations in the development of next-generation laryngoscope interfaces.
Another significant challenge is the ergonomic design of laryngoscope handles and blades. Many practitioners report discomfort and fatigue during prolonged use, which can potentially impact the precision and efficiency of procedures. The need for improved ergonomics that can accommodate various hand sizes and preferences while maintaining optimal control and maneuverability remains a critical area for development.
The integration of advanced imaging technologies into laryngoscope operative interfaces presents both opportunities and challenges. While high-resolution cameras and video laryngoscopes have greatly enhanced visualization, issues such as image distortion, glare, and maintaining proper focus during dynamic procedures continue to pose difficulties. Additionally, the incorporation of these technologies often results in increased device complexity and cost, potentially limiting widespread adoption in resource-constrained settings.
Sterilization and infection control represent ongoing concerns in laryngoscope design. The intricate components of modern laryngoscopes, particularly those with integrated electronics, can be challenging to clean and sterilize effectively. Balancing the need for advanced functionality with ease of maintenance and infection prevention remains a significant hurdle for manufacturers and healthcare providers alike.
The development of intuitive and user-friendly interfaces for controlling various laryngoscope functions is another area of focus. As laryngoscopes become more sophisticated, incorporating features such as adjustable light intensity, image capture, and real-time data display, the complexity of user interfaces has increased. Designing interfaces that allow for seamless operation without distracting from the primary task of airway management is a persistent challenge.
Lastly, the integration of laryngoscope operative interfaces with other medical devices and hospital information systems presents both technical and interoperability challenges. Ensuring seamless data exchange, compatibility with existing infrastructure, and adherence to evolving healthcare IT standards are crucial considerations in the development of next-generation laryngoscope interfaces.
State-of-the-Art Laryngoscope Interface Solutions
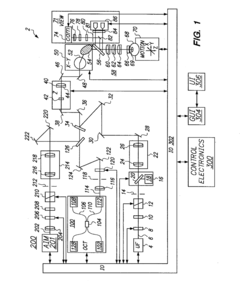
01 Video laryngoscope interfaces
Video laryngoscopes incorporate interfaces for displaying real-time images of the larynx during intubation procedures. These interfaces may include screens integrated into the handle or separate monitors, providing clear visualization to assist in proper tube placement. Some systems allow for image capture and recording for documentation or training purposes.- Video laryngoscope interfaces: Video laryngoscopes incorporate interfaces for displaying real-time images of the larynx during intubation procedures. These interfaces may include high-resolution screens, wireless transmission capabilities, and image processing features to enhance visualization for medical professionals.
- Control interfaces for laryngoscope operation: Laryngoscopes feature various control interfaces to enhance operability during procedures. These may include ergonomic handles with integrated buttons, touch-sensitive surfaces, or voice-activated controls for adjusting settings such as light intensity, image capture, or blade positioning.
- Data integration and connectivity: Modern laryngoscopes incorporate interfaces for data integration and connectivity with other medical systems. These interfaces enable the transfer of patient data, procedure recordings, and real-time collaboration with remote specialists, enhancing the overall efficacy of laryngoscopic procedures.
- Augmented reality and navigation interfaces: Advanced laryngoscope systems may feature augmented reality interfaces and navigation aids. These interfaces overlay real-time guidance, anatomical information, or procedural instructions onto the laryngoscope's view, assisting medical professionals in performing more precise and efficient intubations.
- User-customizable interfaces: Laryngoscopes with user-customizable interfaces allow medical professionals to tailor the device's settings and display preferences to their individual needs. These interfaces may include programmable buttons, adjustable screen layouts, and personalized alert systems to optimize the user experience during procedures.
02 Wireless connectivity in laryngoscopes
Modern laryngoscopes feature wireless connectivity options, enabling data transmission to external devices or systems. This allows for remote viewing of laryngoscope images, integration with hospital information systems, and potential telemedicine applications. Wireless interfaces may use various protocols such as Bluetooth or Wi-Fi for seamless communication.Expand Specific Solutions03 User interface for laryngoscope control
Laryngoscopes incorporate user interfaces for controlling various functions and settings. These may include touchscreens, buttons, or voice-activated controls to adjust parameters such as light intensity, image zoom, or recording functions. Intuitive interfaces aim to enhance usability and allow operators to focus on the intubation procedure.Expand Specific Solutions04 Integration with medical information systems
Laryngoscope systems can interface with broader medical information systems, allowing for seamless data exchange and integration. This may include connecting to electronic health records, picture archiving and communication systems (PACS), or other hospital databases to store patient information, images, and procedure details.Expand Specific Solutions05 Augmented reality interfaces for laryngoscopy
Advanced laryngoscope systems incorporate augmented reality (AR) interfaces to enhance visualization during procedures. These interfaces may overlay real-time guidance, anatomical markers, or other relevant information onto the laryngoscope view, assisting in navigation and improving procedural accuracy.Expand Specific Solutions
Key Players in Laryngoscope Technology
The laryngoscope operative interface market is in a growth phase, driven by increasing demand for minimally invasive procedures and technological advancements. The global market size is projected to expand significantly in the coming years. Technologically, the field is evolving rapidly, with companies like Zhejiang Youyi Medical Equipment Co Ltd and Karl Storz SE & Co. KG leading innovations in visualization and ergonomics. Established players such as Covidien AG and Teleflex Life Sciences LLC are competing with newer entrants like OptiMedica Corp., focusing on integrating advanced imaging and AI-assisted technologies. Research institutions like the University of Washington and Zhejiang University are contributing to technological advancements, potentially disrupting the current market dynamics.
Covidien AG
Technical Solution: Covidien (now part of Medtronic) has developed the McGrath™ Series video laryngoscopes, featuring a range of models for different clinical needs. Their systems incorporate high-resolution cameras and LED lighting for clear visualization. Covidien has introduced adjustable blade angulation in some models, allowing customization of the laryngeal view during intubation[9]. Recent innovations include integration with hospital information systems for automatic documentation and quality assurance purposes[10].
Strengths: Wide range of models catering to various clinical requirements. Integration with hospital systems enhances documentation capabilities. Weaknesses: Some models may have a steeper learning curve for users transitioning from traditional laryngoscopes.
Flexicare (Group) Ltd.
Technical Solution: Flexicare has developed the Vividtrac™ video laryngoscope, which features a unique single-use design that integrates the camera, light source, and blade into one disposable unit. This approach eliminates the need for sterilization between uses, reducing turnaround time and cross-contamination risks[7]. The system includes a reusable 3.5" LCD monitor with image capture and video recording capabilities. Flexicare has also implemented anti-fog technology in their latest models to maintain clear visualization during procedures[8].
Strengths: Fully disposable design eliminates sterilization needs. Compact and portable system suitable for various clinical settings. Weaknesses: Higher per-use cost due to disposable nature. Limited blade shape options compared to multi-use systems.
Innovative Interface Technologies for Laryngoscopes
Intubation device and system
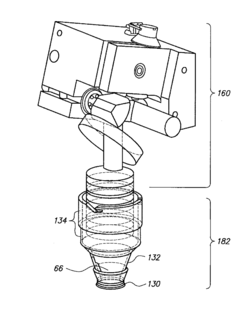
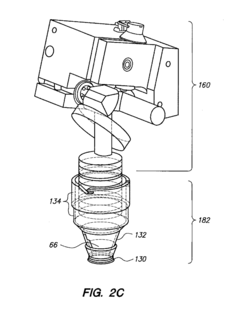
PatentPendingUS20230371805A1
Innovation
- A laryngoscopy system with a metal or metal alloy blade and an image display module that uses motion input control via an accelerometer, eliminating the need for external buttons and switches, and featuring a pivotable image display module and integral power source for reduced contamination and improved ease of use.
Patient interface for ophthalmologic diagnostic and interventional procedures
PatentActiveUS20170224533A1
Innovation
- A method involving a patient interface system with a housing, optical lens, and eye engagement assembly that uses vacuum seals and a tissue migration bolster structure to minimize eye distension, combined with a femtosecond laser for precise capsulorhexis creation, allowing for controlled and safe surgical procedures.
Ergonomic Considerations in Laryngoscope Development
Ergonomic considerations have become increasingly important in the development of laryngoscopes, as they directly impact the comfort, efficiency, and safety of medical professionals during intubation procedures. The design of laryngoscope handles and blades has evolved significantly to address the physical strain and potential injuries associated with prolonged use.
One key area of focus has been the optimization of handle shape and size. Manufacturers have introduced ergonomically contoured handles that provide a more natural grip, reducing hand fatigue and improving control during intubation. These designs often incorporate textured surfaces or rubberized coatings to enhance grip stability, especially in wet or gloved conditions.
Weight distribution has also been a critical factor in ergonomic improvements. Lighter materials, such as reinforced plastics and advanced alloys, are being used to reduce the overall weight of laryngoscopes without compromising durability. This reduction in weight helps minimize arm and wrist strain during extended procedures.
The angle between the handle and blade has been subject to extensive research and refinement. Optimized angles aim to reduce wrist flexion and ulnar deviation, promoting a more neutral wrist position during intubation. Some models now offer adjustable angles to accommodate different user preferences and patient anatomies.
Blade design has seen significant ergonomic advancements as well. Curved blades with improved profiles facilitate easier insertion and provide better visibility of the vocal cords. Some manufacturers have introduced disposable blades with ergonomic features, balancing hygiene concerns with user comfort.
The integration of video laryngoscopy has brought about new ergonomic challenges and solutions. Video laryngoscopes often feature screens positioned to allow for a more upright posture during intubation, reducing neck strain. The placement and design of controls for these devices have been optimized for ease of use and minimal hand movement.
Ergonomic considerations extend beyond the physical design to include cognitive ergonomics. User interfaces on video laryngoscopes have been simplified, with intuitive controls and clear visual feedback to reduce cognitive load during critical procedures. This approach aims to minimize errors and improve overall performance in high-stress situations.
Manufacturers are increasingly employing user-centered design processes, involving extensive feedback from anesthesiologists and other medical professionals. This collaborative approach ensures that ergonomic improvements are grounded in real-world clinical needs and preferences.
As laryngoscope technology continues to advance, ergonomic considerations will remain a crucial aspect of development. Future trends may include further personalization options, such as modular components that can be adjusted to individual user ergonomics, and the integration of haptic feedback to enhance sensory input during intubation procedures.
One key area of focus has been the optimization of handle shape and size. Manufacturers have introduced ergonomically contoured handles that provide a more natural grip, reducing hand fatigue and improving control during intubation. These designs often incorporate textured surfaces or rubberized coatings to enhance grip stability, especially in wet or gloved conditions.
Weight distribution has also been a critical factor in ergonomic improvements. Lighter materials, such as reinforced plastics and advanced alloys, are being used to reduce the overall weight of laryngoscopes without compromising durability. This reduction in weight helps minimize arm and wrist strain during extended procedures.
The angle between the handle and blade has been subject to extensive research and refinement. Optimized angles aim to reduce wrist flexion and ulnar deviation, promoting a more neutral wrist position during intubation. Some models now offer adjustable angles to accommodate different user preferences and patient anatomies.
Blade design has seen significant ergonomic advancements as well. Curved blades with improved profiles facilitate easier insertion and provide better visibility of the vocal cords. Some manufacturers have introduced disposable blades with ergonomic features, balancing hygiene concerns with user comfort.
The integration of video laryngoscopy has brought about new ergonomic challenges and solutions. Video laryngoscopes often feature screens positioned to allow for a more upright posture during intubation, reducing neck strain. The placement and design of controls for these devices have been optimized for ease of use and minimal hand movement.
Ergonomic considerations extend beyond the physical design to include cognitive ergonomics. User interfaces on video laryngoscopes have been simplified, with intuitive controls and clear visual feedback to reduce cognitive load during critical procedures. This approach aims to minimize errors and improve overall performance in high-stress situations.
Manufacturers are increasingly employing user-centered design processes, involving extensive feedback from anesthesiologists and other medical professionals. This collaborative approach ensures that ergonomic improvements are grounded in real-world clinical needs and preferences.
As laryngoscope technology continues to advance, ergonomic considerations will remain a crucial aspect of development. Future trends may include further personalization options, such as modular components that can be adjusted to individual user ergonomics, and the integration of haptic feedback to enhance sensory input during intubation procedures.
Regulatory Framework for Medical Device Interfaces
The regulatory framework for medical device interfaces, including laryngoscope operative interfaces, is a critical aspect of ensuring patient safety and device efficacy. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the development and approval of medical devices. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating medical devices, including laryngoscopes and their interfaces.
For laryngoscope operative interfaces, manufacturers must adhere to the FDA's Quality System Regulation (QSR), which outlines requirements for design controls, manufacturing processes, and post-market surveillance. The QSR emphasizes the importance of human factors engineering and usability testing to ensure that device interfaces are intuitive and minimize the risk of user error.
In the European Union, the Medical Device Regulation (MDR) governs the development and marketing of medical devices. The MDR places a strong emphasis on clinical evaluation and post-market surveillance, requiring manufacturers to demonstrate the safety and performance of their devices throughout their lifecycle. This includes the evaluation of user interfaces and their impact on device usability and safety.
International standards also play a crucial role in shaping the regulatory landscape for medical device interfaces. ISO 14971, which addresses risk management for medical devices, is particularly relevant for laryngoscope interfaces. This standard requires manufacturers to identify and mitigate potential risks associated with device use, including those related to the user interface.
The IEC 62366 series of standards focuses specifically on the application of usability engineering to medical devices. These standards provide guidance on how to incorporate usability considerations into the design and development process, ensuring that interfaces are optimized for their intended users and use environments.
As laryngoscope technology advances, regulatory bodies are adapting their frameworks to address new challenges. For instance, the integration of digital displays and augmented reality features in modern laryngoscopes has prompted regulators to consider additional requirements for software validation and cybersecurity. The FDA's guidance on "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" is increasingly relevant for laryngoscopes with digital interfaces.
Regulatory agencies are also placing greater emphasis on real-world evidence and post-market data collection. This shift allows for a more comprehensive understanding of how laryngoscope interfaces perform in clinical settings over time, potentially informing future regulatory decisions and design improvements.
For laryngoscope operative interfaces, manufacturers must adhere to the FDA's Quality System Regulation (QSR), which outlines requirements for design controls, manufacturing processes, and post-market surveillance. The QSR emphasizes the importance of human factors engineering and usability testing to ensure that device interfaces are intuitive and minimize the risk of user error.
In the European Union, the Medical Device Regulation (MDR) governs the development and marketing of medical devices. The MDR places a strong emphasis on clinical evaluation and post-market surveillance, requiring manufacturers to demonstrate the safety and performance of their devices throughout their lifecycle. This includes the evaluation of user interfaces and their impact on device usability and safety.
International standards also play a crucial role in shaping the regulatory landscape for medical device interfaces. ISO 14971, which addresses risk management for medical devices, is particularly relevant for laryngoscope interfaces. This standard requires manufacturers to identify and mitigate potential risks associated with device use, including those related to the user interface.
The IEC 62366 series of standards focuses specifically on the application of usability engineering to medical devices. These standards provide guidance on how to incorporate usability considerations into the design and development process, ensuring that interfaces are optimized for their intended users and use environments.
As laryngoscope technology advances, regulatory bodies are adapting their frameworks to address new challenges. For instance, the integration of digital displays and augmented reality features in modern laryngoscopes has prompted regulators to consider additional requirements for software validation and cybersecurity. The FDA's guidance on "Content of Premarket Submissions for Management of Cybersecurity in Medical Devices" is increasingly relevant for laryngoscopes with digital interfaces.
Regulatory agencies are also placing greater emphasis on real-world evidence and post-market data collection. This shift allows for a more comprehensive understanding of how laryngoscope interfaces perform in clinical settings over time, potentially informing future regulatory decisions and design improvements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!