How Decane Affects the Synthesis of Porous Catalytic Supports
JUL 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Decane in Catalytic Support Synthesis: Background and Objectives
The synthesis of porous catalytic supports has been a critical area of research in heterogeneous catalysis for decades. These supports play a crucial role in enhancing the performance and efficiency of catalytic processes across various industries, including petrochemicals, fine chemicals, and environmental remediation. The use of decane in this context has emerged as a significant factor influencing the synthesis process and the resulting properties of the catalytic supports.
Decane, a straight-chain alkane with ten carbon atoms, has garnered attention due to its potential to act as a structure-directing agent and pore-forming template in the synthesis of porous materials. Its hydrophobic nature and molecular size make it particularly suitable for creating well-defined pore structures in catalytic supports. The interaction between decane and the precursor materials during synthesis can lead to the formation of mesoporous and macroporous structures, which are highly desirable for many catalytic applications.
The primary objective of investigating decane's role in the synthesis of porous catalytic supports is to develop a deeper understanding of the mechanisms by which it influences pore formation, size distribution, and overall support morphology. This knowledge is essential for tailoring the properties of catalytic supports to meet specific application requirements, such as increased surface area, improved mass transfer, and enhanced catalyst stability.
Furthermore, researchers aim to explore how decane can be utilized to control the hierarchical structure of porous supports, combining micropores, mesopores, and macropores in a single material. This multi-scale porosity is particularly advantageous for catalytic reactions involving large molecules or those requiring efficient diffusion of reactants and products.
Another key objective is to elucidate the impact of decane on the surface chemistry of the resulting catalytic supports. The presence of decane during synthesis may affect the distribution and accessibility of active sites, as well as the hydrophobicity of the support surface. Understanding these effects is crucial for optimizing catalyst performance and selectivity in various reaction environments.
The investigation into decane's role also extends to its potential for creating novel support materials with unique properties. By manipulating the concentration, molecular structure, and interaction of decane with other synthesis components, researchers hope to develop innovative catalytic supports with enhanced thermal stability, mechanical strength, and resistance to deactivation.
As the field of catalysis continues to evolve, the study of decane in porous support synthesis aligns with broader trends towards more sustainable and efficient chemical processes. The insights gained from this research may contribute to the development of next-generation catalysts capable of addressing global challenges in energy production, environmental protection, and resource utilization.
Decane, a straight-chain alkane with ten carbon atoms, has garnered attention due to its potential to act as a structure-directing agent and pore-forming template in the synthesis of porous materials. Its hydrophobic nature and molecular size make it particularly suitable for creating well-defined pore structures in catalytic supports. The interaction between decane and the precursor materials during synthesis can lead to the formation of mesoporous and macroporous structures, which are highly desirable for many catalytic applications.
The primary objective of investigating decane's role in the synthesis of porous catalytic supports is to develop a deeper understanding of the mechanisms by which it influences pore formation, size distribution, and overall support morphology. This knowledge is essential for tailoring the properties of catalytic supports to meet specific application requirements, such as increased surface area, improved mass transfer, and enhanced catalyst stability.
Furthermore, researchers aim to explore how decane can be utilized to control the hierarchical structure of porous supports, combining micropores, mesopores, and macropores in a single material. This multi-scale porosity is particularly advantageous for catalytic reactions involving large molecules or those requiring efficient diffusion of reactants and products.
Another key objective is to elucidate the impact of decane on the surface chemistry of the resulting catalytic supports. The presence of decane during synthesis may affect the distribution and accessibility of active sites, as well as the hydrophobicity of the support surface. Understanding these effects is crucial for optimizing catalyst performance and selectivity in various reaction environments.
The investigation into decane's role also extends to its potential for creating novel support materials with unique properties. By manipulating the concentration, molecular structure, and interaction of decane with other synthesis components, researchers hope to develop innovative catalytic supports with enhanced thermal stability, mechanical strength, and resistance to deactivation.
As the field of catalysis continues to evolve, the study of decane in porous support synthesis aligns with broader trends towards more sustainable and efficient chemical processes. The insights gained from this research may contribute to the development of next-generation catalysts capable of addressing global challenges in energy production, environmental protection, and resource utilization.
Market Analysis for Porous Catalytic Supports
The market for porous catalytic supports is experiencing significant growth, driven by increasing demand in various industries such as petrochemicals, environmental protection, and energy production. These supports play a crucial role in enhancing catalytic reactions, improving efficiency, and reducing environmental impact. The global market for porous catalytic supports is expected to expand at a steady rate over the next five years, with a particular focus on applications in oil refining, chemical synthesis, and emission control systems.
In the context of decane's influence on the synthesis of porous catalytic supports, there is a growing interest in developing more efficient and tailored support materials. Decane, as a model compound for long-chain hydrocarbons, is being studied for its potential to create supports with specific pore structures and surface properties. This research is particularly relevant for the petroleum industry, where catalysts are essential for cracking and reforming processes.
The automotive sector represents a significant market for porous catalytic supports, especially in catalytic converters for emission control. With increasingly stringent environmental regulations worldwide, the demand for high-performance, durable catalytic supports is on the rise. The incorporation of decane in the synthesis process may lead to supports that are more resistant to coking and have improved thermal stability, addressing key challenges in automotive catalysis.
In the chemical industry, there is a growing need for catalytic supports that can withstand harsh reaction conditions while maintaining high surface area and porosity. The use of decane in synthesis could potentially yield supports with enhanced hydrophobicity and controlled pore size distribution, which are desirable properties for many catalytic applications in fine chemical and pharmaceutical production.
The energy sector, particularly in the development of fuel cells and hydrogen production technologies, is another area driving market growth for advanced porous catalytic supports. Supports that can be tailored using decane during synthesis may offer improved performance in electrocatalysis and gas separation processes, contributing to the advancement of clean energy technologies.
Geographically, Asia-Pacific is expected to be the fastest-growing market for porous catalytic supports, due to rapid industrialization and increasing environmental concerns in countries like China and India. North America and Europe continue to be significant markets, with a focus on research and development of novel catalytic materials and processes.
As sustainability becomes a key focus across industries, there is a growing demand for catalytic supports that enable more efficient use of resources and reduce waste. The potential of decane to influence the synthesis of supports with improved recyclability and longer lifespans aligns well with this trend, potentially opening new market opportunities in the circular economy and green chemistry sectors.
In the context of decane's influence on the synthesis of porous catalytic supports, there is a growing interest in developing more efficient and tailored support materials. Decane, as a model compound for long-chain hydrocarbons, is being studied for its potential to create supports with specific pore structures and surface properties. This research is particularly relevant for the petroleum industry, where catalysts are essential for cracking and reforming processes.
The automotive sector represents a significant market for porous catalytic supports, especially in catalytic converters for emission control. With increasingly stringent environmental regulations worldwide, the demand for high-performance, durable catalytic supports is on the rise. The incorporation of decane in the synthesis process may lead to supports that are more resistant to coking and have improved thermal stability, addressing key challenges in automotive catalysis.
In the chemical industry, there is a growing need for catalytic supports that can withstand harsh reaction conditions while maintaining high surface area and porosity. The use of decane in synthesis could potentially yield supports with enhanced hydrophobicity and controlled pore size distribution, which are desirable properties for many catalytic applications in fine chemical and pharmaceutical production.
The energy sector, particularly in the development of fuel cells and hydrogen production technologies, is another area driving market growth for advanced porous catalytic supports. Supports that can be tailored using decane during synthesis may offer improved performance in electrocatalysis and gas separation processes, contributing to the advancement of clean energy technologies.
Geographically, Asia-Pacific is expected to be the fastest-growing market for porous catalytic supports, due to rapid industrialization and increasing environmental concerns in countries like China and India. North America and Europe continue to be significant markets, with a focus on research and development of novel catalytic materials and processes.
As sustainability becomes a key focus across industries, there is a growing demand for catalytic supports that enable more efficient use of resources and reduce waste. The potential of decane to influence the synthesis of supports with improved recyclability and longer lifespans aligns well with this trend, potentially opening new market opportunities in the circular economy and green chemistry sectors.
Current Challenges in Decane-Based Synthesis
The synthesis of porous catalytic supports using decane as a template faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the control of pore size distribution and uniformity. Decane, being a long-chain hydrocarbon, tends to form micelles of varying sizes, leading to a heterogeneous pore structure in the final catalyst support. This lack of uniformity can significantly impact the catalytic performance and selectivity of the resulting material.
Another major challenge lies in the complete removal of decane from the porous structure after synthesis. The high boiling point of decane (174°C) makes it difficult to eliminate entirely through conventional thermal treatments without causing structural collapse or sintering of the support material. Residual decane can block active sites and reduce the overall surface area, compromising the catalyst's effectiveness.
The stability of decane-based emulsions during the synthesis process presents an additional hurdle. Maintaining a stable emulsion throughout the reaction and curing stages is crucial for achieving consistent pore structures. However, factors such as temperature fluctuations, pH changes, and the presence of other reactants can destabilize the emulsion, leading to phase separation and inconsistent pore formation.
Furthermore, the environmental and safety concerns associated with using decane pose challenges in large-scale production. Decane is a volatile organic compound (VOC) with potential health and environmental risks, necessitating stringent safety measures and emission controls in industrial settings. This aspect not only increases production costs but also complicates the scaling-up process for commercial applications.
The interaction between decane and the precursor materials used for the catalyst support synthesis is another area of concern. Decane's hydrophobic nature can interfere with the hydrolysis and condensation reactions of silica or metal oxide precursors, affecting the final structure and properties of the support material. Balancing the chemistry to ensure proper network formation while maintaining the desired pore structure remains a significant challenge.
Lastly, the reproducibility of decane-based synthesis methods poses a considerable challenge. Small variations in reaction conditions, such as temperature, stirring rate, or precursor concentrations, can lead to significant differences in the final pore structure and surface properties of the catalyst support. Achieving consistent results across different batches and scaling up the production while maintaining quality are ongoing challenges that researchers and manufacturers must address to make decane-based synthesis of porous catalytic supports a viable and reliable process.
Another major challenge lies in the complete removal of decane from the porous structure after synthesis. The high boiling point of decane (174°C) makes it difficult to eliminate entirely through conventional thermal treatments without causing structural collapse or sintering of the support material. Residual decane can block active sites and reduce the overall surface area, compromising the catalyst's effectiveness.
The stability of decane-based emulsions during the synthesis process presents an additional hurdle. Maintaining a stable emulsion throughout the reaction and curing stages is crucial for achieving consistent pore structures. However, factors such as temperature fluctuations, pH changes, and the presence of other reactants can destabilize the emulsion, leading to phase separation and inconsistent pore formation.
Furthermore, the environmental and safety concerns associated with using decane pose challenges in large-scale production. Decane is a volatile organic compound (VOC) with potential health and environmental risks, necessitating stringent safety measures and emission controls in industrial settings. This aspect not only increases production costs but also complicates the scaling-up process for commercial applications.
The interaction between decane and the precursor materials used for the catalyst support synthesis is another area of concern. Decane's hydrophobic nature can interfere with the hydrolysis and condensation reactions of silica or metal oxide precursors, affecting the final structure and properties of the support material. Balancing the chemistry to ensure proper network formation while maintaining the desired pore structure remains a significant challenge.
Lastly, the reproducibility of decane-based synthesis methods poses a considerable challenge. Small variations in reaction conditions, such as temperature, stirring rate, or precursor concentrations, can lead to significant differences in the final pore structure and surface properties of the catalyst support. Achieving consistent results across different batches and scaling up the production while maintaining quality are ongoing challenges that researchers and manufacturers must address to make decane-based synthesis of porous catalytic supports a viable and reliable process.
Existing Decane-Based Synthesis Methods
01 Porous catalytic support materials
Porous materials are used as catalytic supports to increase the surface area and enhance catalytic activity. These supports can be made from various materials such as ceramics, metals, or composites. The porous structure allows for better dispersion of catalytic particles and improved mass transfer during reactions.- Porous catalytic support materials: Porous materials are used as catalytic supports to increase the surface area and enhance catalytic activity. These supports can be made from various materials such as ceramics, metals, or composites. The porous structure allows for better dispersion of catalytic particles and improved mass transfer during reactions.
- Control of porosity in catalytic supports: The porosity of catalytic supports can be controlled through various manufacturing techniques. This includes adjusting the particle size distribution, using pore-forming agents, or applying specific heat treatments. Controlled porosity allows for optimized catalyst performance and selectivity in different applications.
- Hierarchical porous structures: Hierarchical porous structures in catalytic supports combine macropores, mesopores, and micropores. This multi-level porosity enhances mass transport properties and provides high surface area for catalytic reactions. Such structures can be created through templating methods or by combining different porous materials.
- Characterization of porous catalytic supports: Various techniques are used to characterize the porosity of catalytic supports, including gas adsorption, mercury porosimetry, and electron microscopy. These methods help determine pore size distribution, surface area, and pore volume, which are crucial for understanding and optimizing catalyst performance.
- Tailoring porosity for specific catalytic applications: The porosity of catalytic supports can be tailored for specific applications, such as in petrochemical processing, environmental catalysis, or fine chemical synthesis. This involves adjusting pore size, distribution, and connectivity to enhance selectivity, activity, and stability of the catalyst for the target reaction.
02 Control of porosity in catalytic supports
The porosity of catalytic supports can be controlled through various manufacturing techniques. This includes adjusting the particle size distribution, using pore-forming agents, or employing specific sintering processes. Controlled porosity allows for optimized catalyst performance and selectivity in different applications.Expand Specific Solutions03 Hierarchical porous structures
Hierarchical porous structures in catalytic supports combine macropores, mesopores, and micropores. This multi-level porosity enhances mass transport properties and provides high surface area for catalytic reactions. Such structures can be created through templating methods or by combining different porous materials.Expand Specific Solutions04 Characterization of porous catalytic supports
Various techniques are used to characterize the porosity of catalytic supports, including gas adsorption, mercury porosimetry, and electron microscopy. These methods help determine pore size distribution, surface area, and pore volume, which are crucial for understanding and optimizing catalyst performance.Expand Specific Solutions05 Modification of porous catalytic supports
Porous catalytic supports can be modified to enhance their properties. This includes surface functionalization, incorporation of promoters, or creation of core-shell structures. These modifications can improve catalyst stability, selectivity, and activity while maintaining the desired porosity.Expand Specific Solutions
Key Players in Catalytic Support Industry
The synthesis of porous catalytic supports using decane is an emerging field within the broader catalysis industry, which is currently in a growth phase. The market for advanced catalytic materials is expanding, driven by increasing demand in petrochemicals, environmental applications, and renewable energy sectors. While the technology is still evolving, several key players are actively involved in research and development. Companies like China Petroleum & Chemical Corp., PetroChina Co., Ltd., and BASF Corp. are leveraging their expertise in petrochemicals to advance this technology. Research institutions such as Sinopec Research Institute of Petroleum Processing and Massachusetts Institute of Technology are contributing to the scientific understanding of decane's role in catalyst synthesis. The technology's maturity is progressing, with industrial applications beginning to emerge, though further optimization is needed for widespread commercial adoption.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach for synthesizing porous catalytic supports using decane as a template. Their method involves a controlled emulsion technique where decane acts as the oil phase, creating a network of interconnected pores in the final support structure. This process allows for precise control over pore size distribution, typically ranging from 5-50 nm [1]. The company has also integrated advanced characterization techniques, such as nitrogen adsorption-desorption and mercury porosimetry, to optimize the pore structure for specific catalytic applications [3]. Sinopec's research has shown that decane-templated supports exhibit enhanced mass transfer properties and increased catalytic activity, particularly in hydrocracking and hydrodesulfurization processes [5].
Strengths: Precise control over pore structure, enhanced mass transfer, and improved catalytic activity. Weaknesses: Potential environmental concerns due to the use of decane, and possible limitations in scaling up the production process.
BASF Corp.
Technical Solution: BASF Corp. has pioneered a sustainable approach to synthesizing porous catalytic supports using decane as a pore-forming agent. Their innovative process incorporates decane into a sol-gel synthesis method, where it acts as a sacrificial template. During calcination, the decane is removed, leaving behind a highly porous structure with controlled pore sizes ranging from 2-20 nm [2]. BASF has further enhanced this technique by incorporating metal precursors directly into the synthesis mixture, resulting in well-dispersed active sites throughout the support [4]. The company's research has demonstrated that these decane-templated supports show exceptional thermal stability and resistance to sintering, making them ideal for high-temperature catalytic applications such as methane reforming and Fischer-Tropsch synthesis [6].
Strengths: Sustainable synthesis approach, excellent thermal stability, and uniform dispersion of active sites. Weaknesses: Potential limitations in achieving larger pore sizes and possible challenges in removing all traces of decane during calcination.
Innovations in Decane-Assisted Porosity Control
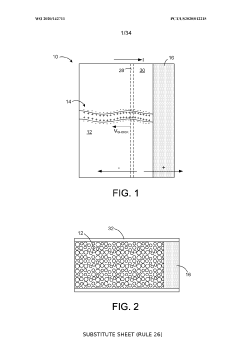
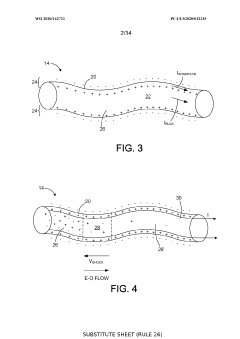
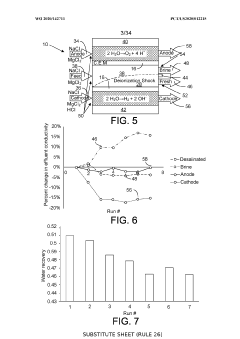
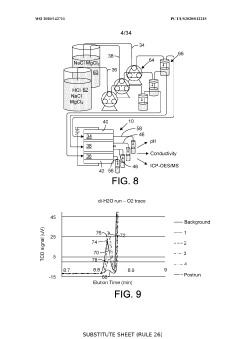
Ion-selective separation by shock electrodialysis
PatentWO2020142711A1
Innovation
- A system utilizing a cationic or anionic porous medium with a surface charge, in conjunction with an ion-selective boundary, applies a voltage differential to create a shock in the electrolyte, allowing for selective separation of ions by exploiting surface conduction and electroosmotic flow, enabling efficient desalination and purification without the need for extensive membrane systems.
Catalyst for oxidative dehydrogenation reaction, method for producing same, and oxidative dehydrogenation method using same
PatentWO2018190641A1
Innovation
- A catalyst coated with a metal oxide active ingredient, such as zinc ferrite (ZnFe2O4), on a porous support within a specific content range, which improves durability, ease of filling, and reaction efficiency by minimizing side reactions and maintaining active ingredient integrity.
Environmental Impact of Decane in Synthesis
The use of decane in the synthesis of porous catalytic supports raises significant environmental concerns that warrant careful consideration. Decane, a hydrocarbon compound, can have various impacts on the environment throughout its lifecycle in the synthesis process.
During the production and handling of decane, there is a risk of volatile organic compound (VOC) emissions. These emissions can contribute to the formation of ground-level ozone, a key component of smog, which can have detrimental effects on human health and vegetation. Additionally, the release of decane vapors into the atmosphere may contribute to the overall burden of greenhouse gases, albeit to a lesser extent compared to more potent greenhouse gases.
In the synthesis process itself, the use of decane as a solvent or templating agent can lead to potential contamination of water sources if not properly managed. Improper disposal or accidental spills of decane-containing waste can result in soil and groundwater pollution. This is particularly concerning due to decane's low water solubility and tendency to form a separate organic phase, making it challenging to remove from aquatic environments.
The persistence of decane in the environment is another factor to consider. While it can undergo biodegradation under aerobic conditions, the process can be slow, especially in anaerobic environments. This persistence increases the potential for bioaccumulation in aquatic organisms, potentially affecting the food chain.
From a resource perspective, the use of decane in synthesis processes represents consumption of a non-renewable fossil fuel derivative. This raises questions about the sustainability of its long-term use in industrial applications, especially in the context of global efforts to reduce dependence on fossil fuels.
However, it's important to note that the environmental impact of decane in the synthesis of porous catalytic supports is not solely negative. The resulting catalytic supports can play a crucial role in various environmentally beneficial processes, such as emission control and the production of cleaner fuels. This highlights the need for a balanced approach in assessing the overall environmental impact.
To mitigate the environmental risks associated with decane use, several strategies can be employed. These include implementing closed-loop systems to minimize emissions, developing more efficient synthesis processes to reduce the amount of decane required, and exploring alternative, more environmentally friendly solvents or templating agents. Additionally, proper waste management and disposal protocols are essential to prevent environmental contamination.
During the production and handling of decane, there is a risk of volatile organic compound (VOC) emissions. These emissions can contribute to the formation of ground-level ozone, a key component of smog, which can have detrimental effects on human health and vegetation. Additionally, the release of decane vapors into the atmosphere may contribute to the overall burden of greenhouse gases, albeit to a lesser extent compared to more potent greenhouse gases.
In the synthesis process itself, the use of decane as a solvent or templating agent can lead to potential contamination of water sources if not properly managed. Improper disposal or accidental spills of decane-containing waste can result in soil and groundwater pollution. This is particularly concerning due to decane's low water solubility and tendency to form a separate organic phase, making it challenging to remove from aquatic environments.
The persistence of decane in the environment is another factor to consider. While it can undergo biodegradation under aerobic conditions, the process can be slow, especially in anaerobic environments. This persistence increases the potential for bioaccumulation in aquatic organisms, potentially affecting the food chain.
From a resource perspective, the use of decane in synthesis processes represents consumption of a non-renewable fossil fuel derivative. This raises questions about the sustainability of its long-term use in industrial applications, especially in the context of global efforts to reduce dependence on fossil fuels.
However, it's important to note that the environmental impact of decane in the synthesis of porous catalytic supports is not solely negative. The resulting catalytic supports can play a crucial role in various environmentally beneficial processes, such as emission control and the production of cleaner fuels. This highlights the need for a balanced approach in assessing the overall environmental impact.
To mitigate the environmental risks associated with decane use, several strategies can be employed. These include implementing closed-loop systems to minimize emissions, developing more efficient synthesis processes to reduce the amount of decane required, and exploring alternative, more environmentally friendly solvents or templating agents. Additionally, proper waste management and disposal protocols are essential to prevent environmental contamination.
Scalability of Decane-Based Processes
The scalability of decane-based processes for synthesizing porous catalytic supports is a critical factor in determining the viability of this approach for industrial applications. Decane, as a long-chain hydrocarbon, offers unique advantages in creating controlled pore structures, but its implementation on a large scale presents several challenges that must be addressed.
One of the primary considerations for scaling up decane-based processes is the optimization of reaction conditions. As the volume of reactants increases, maintaining uniform temperature and concentration gradients becomes more difficult. This can lead to inconsistencies in pore formation and overall support structure. To mitigate this issue, advanced reactor designs incorporating improved heat and mass transfer mechanisms are essential. These may include the use of microreactors or continuous flow systems that allow for better control over reaction parameters.
The recovery and recycling of decane is another crucial aspect of scalability. In large-scale operations, the efficient separation of decane from the final product and its subsequent reuse can significantly impact the economic viability of the process. Developing effective separation techniques, such as advanced distillation or membrane-based processes, is necessary to minimize solvent loss and reduce environmental impact.
Furthermore, the scalability of decane-based processes is influenced by the availability and cost of raw materials. As production scales up, ensuring a stable and cost-effective supply of high-purity decane becomes increasingly important. This may require partnerships with chemical suppliers or the development of in-house purification methods to meet the stringent quality requirements for catalytic support synthesis.
Safety considerations also play a vital role in scaling up decane-based processes. The flammability and potential for vapor accumulation in large-scale operations necessitate robust safety protocols and engineering controls. This includes the implementation of explosion-proof equipment, advanced ventilation systems, and comprehensive safety training programs for personnel.
The reproducibility of pore characteristics across different batch sizes is another critical factor in scaling up decane-based processes. Maintaining consistent pore size distribution, surface area, and pore volume as production scales increase requires precise control over synthesis parameters. This may involve the development of sophisticated in-line monitoring techniques and adaptive control systems to ensure product consistency.
In conclusion, while decane-based processes show promise for synthesizing porous catalytic supports, their scalability depends on overcoming several technical and operational challenges. Addressing these issues through innovative engineering solutions and process optimizations will be key to realizing the full potential of this approach in industrial-scale production of high-performance catalytic supports.
One of the primary considerations for scaling up decane-based processes is the optimization of reaction conditions. As the volume of reactants increases, maintaining uniform temperature and concentration gradients becomes more difficult. This can lead to inconsistencies in pore formation and overall support structure. To mitigate this issue, advanced reactor designs incorporating improved heat and mass transfer mechanisms are essential. These may include the use of microreactors or continuous flow systems that allow for better control over reaction parameters.
The recovery and recycling of decane is another crucial aspect of scalability. In large-scale operations, the efficient separation of decane from the final product and its subsequent reuse can significantly impact the economic viability of the process. Developing effective separation techniques, such as advanced distillation or membrane-based processes, is necessary to minimize solvent loss and reduce environmental impact.
Furthermore, the scalability of decane-based processes is influenced by the availability and cost of raw materials. As production scales up, ensuring a stable and cost-effective supply of high-purity decane becomes increasingly important. This may require partnerships with chemical suppliers or the development of in-house purification methods to meet the stringent quality requirements for catalytic support synthesis.
Safety considerations also play a vital role in scaling up decane-based processes. The flammability and potential for vapor accumulation in large-scale operations necessitate robust safety protocols and engineering controls. This includes the implementation of explosion-proof equipment, advanced ventilation systems, and comprehensive safety training programs for personnel.
The reproducibility of pore characteristics across different batch sizes is another critical factor in scaling up decane-based processes. Maintaining consistent pore size distribution, surface area, and pore volume as production scales increase requires precise control over synthesis parameters. This may involve the development of sophisticated in-line monitoring techniques and adaptive control systems to ensure product consistency.
In conclusion, while decane-based processes show promise for synthesizing porous catalytic supports, their scalability depends on overcoming several technical and operational challenges. Addressing these issues through innovative engineering solutions and process optimizations will be key to realizing the full potential of this approach in industrial-scale production of high-performance catalytic supports.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!