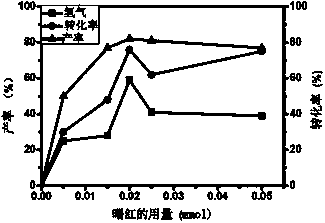
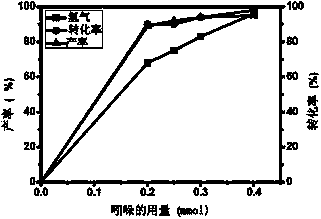
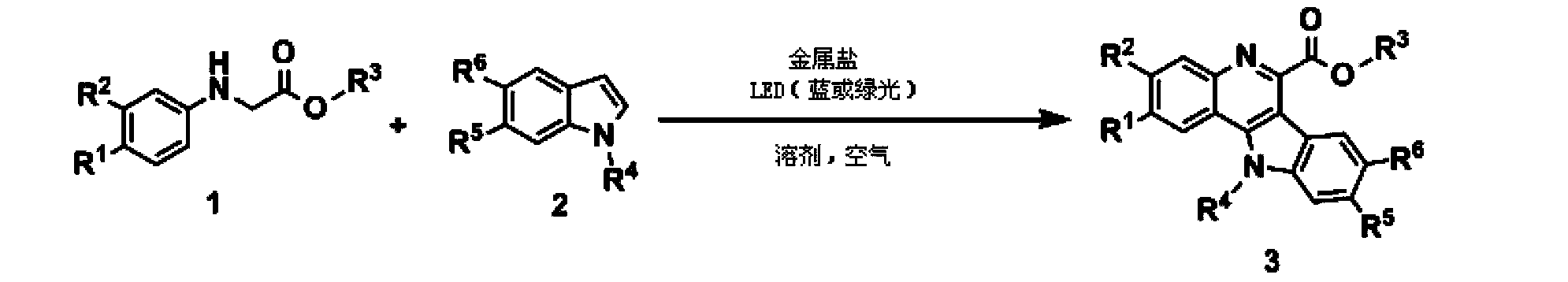
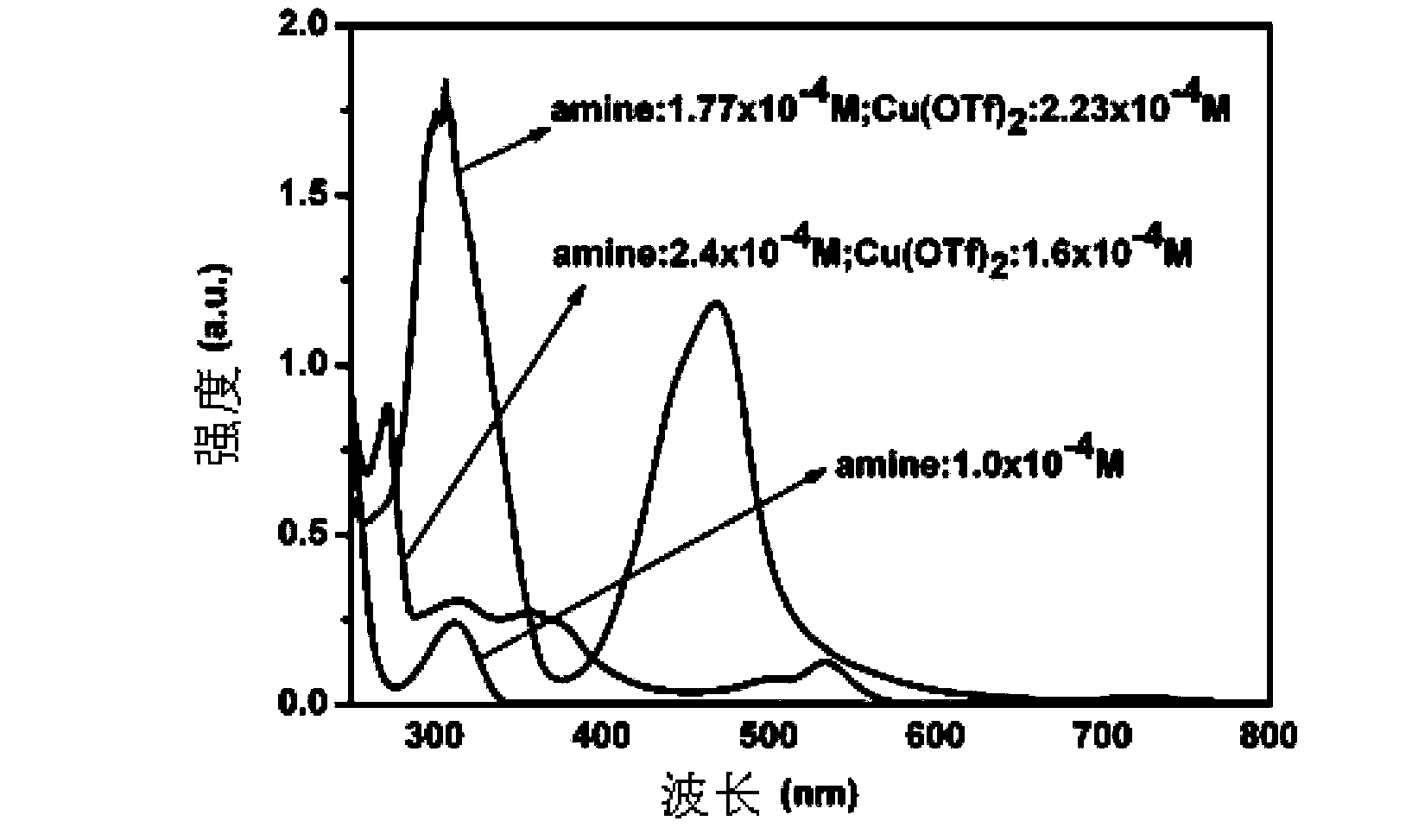
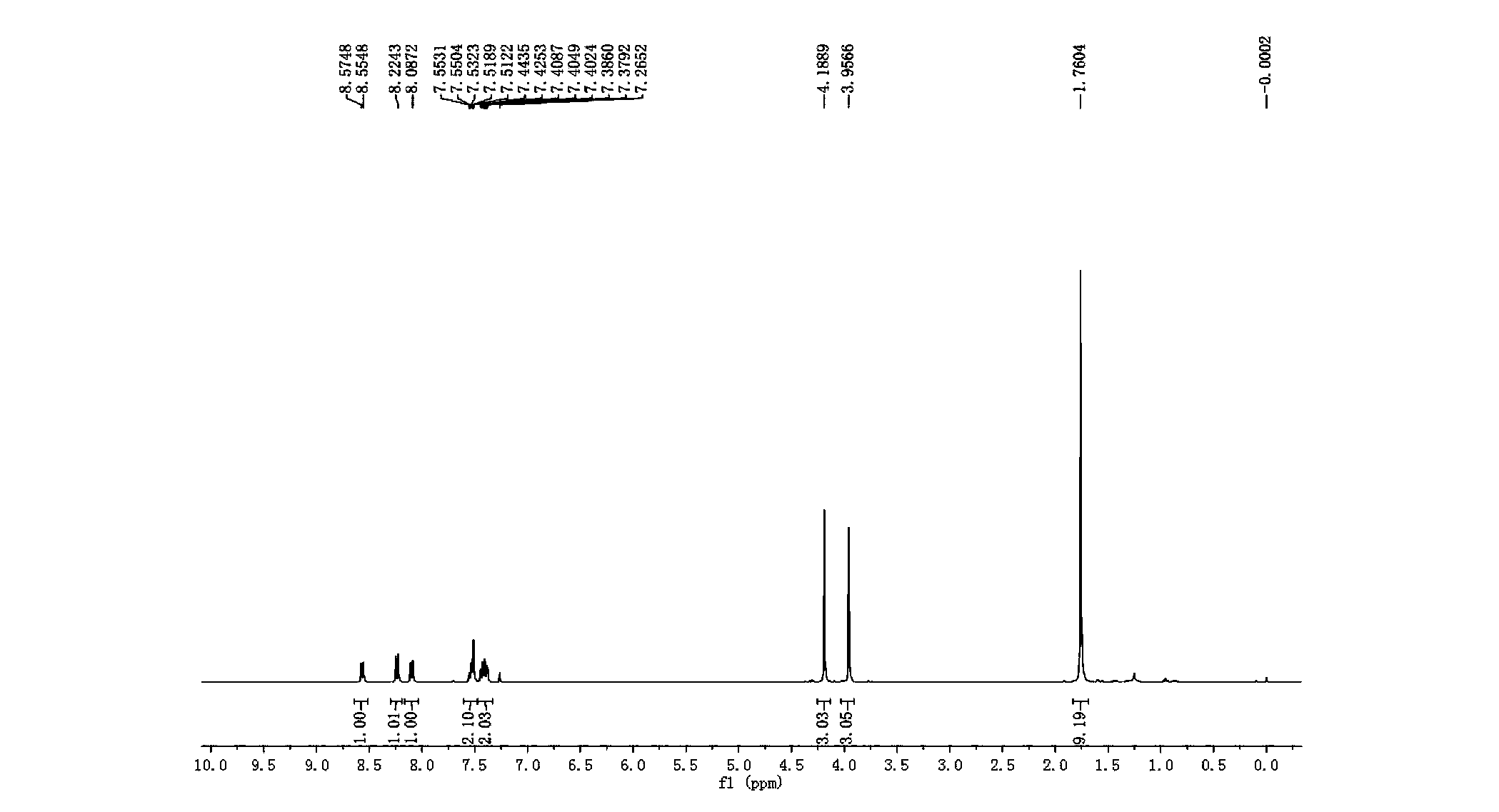
How Decane Catalyzes Cross-Coupling Reactions in Organic Synthesis
JUL 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Decane Catalysis Background and Objectives
Decane catalysis in cross-coupling reactions represents a significant advancement in organic synthesis, offering a novel approach to carbon-carbon bond formation. This field has evolved from traditional transition metal catalysis to exploring the potential of simple alkanes as catalysts. The journey of decane catalysis began with the serendipitous discovery of its catalytic properties in cross-coupling reactions, sparking intense research interest in the scientific community.
The development of decane catalysis is rooted in the broader context of sustainable chemistry and green synthesis. As the chemical industry faces increasing pressure to adopt more environmentally friendly processes, the use of abundant and non-toxic alkanes like decane as catalysts presents an attractive alternative to conventional metal-based systems. This shift aligns with the principles of atom economy and waste reduction in chemical synthesis.
The primary objective of research in decane catalysis is to elucidate the mechanistic pathways through which this simple hydrocarbon facilitates cross-coupling reactions. Understanding the fundamental principles governing decane's catalytic activity is crucial for optimizing reaction conditions and expanding the scope of applicable substrates. Researchers aim to uncover the specific interactions between decane and reaction components that enable efficient bond formation.
Another key goal in this field is to explore the versatility of decane catalysis across various types of cross-coupling reactions. While initial studies focused on specific reaction classes, ongoing research seeks to broaden the applicability of decane as a catalyst in diverse synthetic transformations. This includes investigating its potential in challenging coupling reactions that traditionally require complex or expensive catalyst systems.
The technological trajectory of decane catalysis is closely tied to advancements in analytical techniques and computational chemistry. Researchers are leveraging cutting-edge spectroscopic methods and theoretical modeling to gain insights into the transient species and reaction intermediates involved in decane-catalyzed processes. These efforts aim to provide a molecular-level understanding of the catalytic mechanism, guiding the rational design of more efficient and selective reaction systems.
As the field progresses, a significant focus is placed on enhancing the efficiency and selectivity of decane-catalyzed reactions. This involves optimizing reaction parameters such as temperature, solvent choice, and substrate concentration to maximize yield and minimize side products. Additionally, researchers are exploring the potential synergistic effects of combining decane with other catalytic systems or additives to further improve reaction outcomes.
The long-term vision for decane catalysis extends beyond its current applications in organic synthesis. Researchers are investigating its potential in industrial-scale processes, aiming to develop scalable and economically viable methodologies for large-scale production of fine chemicals and pharmaceuticals. This transition from laboratory-scale experiments to industrial applications represents a critical milestone in the evolution of decane catalysis technology.
The development of decane catalysis is rooted in the broader context of sustainable chemistry and green synthesis. As the chemical industry faces increasing pressure to adopt more environmentally friendly processes, the use of abundant and non-toxic alkanes like decane as catalysts presents an attractive alternative to conventional metal-based systems. This shift aligns with the principles of atom economy and waste reduction in chemical synthesis.
The primary objective of research in decane catalysis is to elucidate the mechanistic pathways through which this simple hydrocarbon facilitates cross-coupling reactions. Understanding the fundamental principles governing decane's catalytic activity is crucial for optimizing reaction conditions and expanding the scope of applicable substrates. Researchers aim to uncover the specific interactions between decane and reaction components that enable efficient bond formation.
Another key goal in this field is to explore the versatility of decane catalysis across various types of cross-coupling reactions. While initial studies focused on specific reaction classes, ongoing research seeks to broaden the applicability of decane as a catalyst in diverse synthetic transformations. This includes investigating its potential in challenging coupling reactions that traditionally require complex or expensive catalyst systems.
The technological trajectory of decane catalysis is closely tied to advancements in analytical techniques and computational chemistry. Researchers are leveraging cutting-edge spectroscopic methods and theoretical modeling to gain insights into the transient species and reaction intermediates involved in decane-catalyzed processes. These efforts aim to provide a molecular-level understanding of the catalytic mechanism, guiding the rational design of more efficient and selective reaction systems.
As the field progresses, a significant focus is placed on enhancing the efficiency and selectivity of decane-catalyzed reactions. This involves optimizing reaction parameters such as temperature, solvent choice, and substrate concentration to maximize yield and minimize side products. Additionally, researchers are exploring the potential synergistic effects of combining decane with other catalytic systems or additives to further improve reaction outcomes.
The long-term vision for decane catalysis extends beyond its current applications in organic synthesis. Researchers are investigating its potential in industrial-scale processes, aiming to develop scalable and economically viable methodologies for large-scale production of fine chemicals and pharmaceuticals. This transition from laboratory-scale experiments to industrial applications represents a critical milestone in the evolution of decane catalysis technology.
Market Demand for Cross-Coupling Catalysts
The market demand for cross-coupling catalysts has been steadily growing, driven by the increasing need for efficient and sustainable synthetic methods in organic chemistry. Cross-coupling reactions, particularly those catalyzed by transition metals, have become indispensable tools in the pharmaceutical, agrochemical, and materials science industries. The global market for cross-coupling catalysts is expected to expand significantly in the coming years, with a compound annual growth rate (CAGR) projected to be in the high single digits.
The pharmaceutical sector remains the largest consumer of cross-coupling catalysts, accounting for a substantial portion of the market share. The continuous development of new drugs and the optimization of existing synthetic routes have created a robust demand for innovative catalytic systems. Additionally, the push towards green chemistry and sustainable manufacturing processes has further fueled the need for more efficient and environmentally friendly catalysts.
In the agrochemical industry, cross-coupling reactions play a crucial role in the synthesis of complex molecules used in crop protection and enhancement. As global food demand rises and agricultural practices evolve, the market for specialized catalysts in this sector is anticipated to grow steadily. The materials science field, particularly in the development of advanced polymers and organic electronics, has also emerged as a significant driver for cross-coupling catalyst demand.
The potential application of decane as a catalyst in cross-coupling reactions represents an exciting development in this market. Traditional cross-coupling catalysts often rely on expensive and sometimes toxic transition metals. If decane can effectively catalyze these reactions, it could offer a more cost-effective and environmentally benign alternative. This aligns well with the industry's current focus on sustainability and green chemistry principles.
However, the adoption of decane-catalyzed cross-coupling reactions in industrial settings will depend on several factors. These include the reaction scope, efficiency compared to existing methods, scalability, and compatibility with current manufacturing processes. If decane proves to be a viable catalyst, it could potentially disrupt the current market dynamics, leading to a shift in demand away from traditional metal-based catalysts.
The geographical distribution of the cross-coupling catalyst market shows strong demand in regions with established pharmaceutical and chemical industries, such as North America, Europe, and East Asia. Emerging economies, particularly in Asia-Pacific and Latin America, are expected to contribute significantly to market growth as their chemical manufacturing sectors expand and modernize.
In conclusion, the market demand for cross-coupling catalysts remains robust and diverse, with potential for further growth driven by technological advancements and sustainability concerns. The introduction of novel catalytic systems, such as decane-based catalysts, could reshape the market landscape, offering new opportunities for innovation and market expansion in organic synthesis applications.
The pharmaceutical sector remains the largest consumer of cross-coupling catalysts, accounting for a substantial portion of the market share. The continuous development of new drugs and the optimization of existing synthetic routes have created a robust demand for innovative catalytic systems. Additionally, the push towards green chemistry and sustainable manufacturing processes has further fueled the need for more efficient and environmentally friendly catalysts.
In the agrochemical industry, cross-coupling reactions play a crucial role in the synthesis of complex molecules used in crop protection and enhancement. As global food demand rises and agricultural practices evolve, the market for specialized catalysts in this sector is anticipated to grow steadily. The materials science field, particularly in the development of advanced polymers and organic electronics, has also emerged as a significant driver for cross-coupling catalyst demand.
The potential application of decane as a catalyst in cross-coupling reactions represents an exciting development in this market. Traditional cross-coupling catalysts often rely on expensive and sometimes toxic transition metals. If decane can effectively catalyze these reactions, it could offer a more cost-effective and environmentally benign alternative. This aligns well with the industry's current focus on sustainability and green chemistry principles.
However, the adoption of decane-catalyzed cross-coupling reactions in industrial settings will depend on several factors. These include the reaction scope, efficiency compared to existing methods, scalability, and compatibility with current manufacturing processes. If decane proves to be a viable catalyst, it could potentially disrupt the current market dynamics, leading to a shift in demand away from traditional metal-based catalysts.
The geographical distribution of the cross-coupling catalyst market shows strong demand in regions with established pharmaceutical and chemical industries, such as North America, Europe, and East Asia. Emerging economies, particularly in Asia-Pacific and Latin America, are expected to contribute significantly to market growth as their chemical manufacturing sectors expand and modernize.
In conclusion, the market demand for cross-coupling catalysts remains robust and diverse, with potential for further growth driven by technological advancements and sustainability concerns. The introduction of novel catalytic systems, such as decane-based catalysts, could reshape the market landscape, offering new opportunities for innovation and market expansion in organic synthesis applications.
Current State of Decane-Catalyzed Cross-Coupling
The current state of decane-catalyzed cross-coupling reactions in organic synthesis represents a significant advancement in the field of catalysis. Decane, a simple alkane, has emerged as an unexpected yet effective catalyst for various cross-coupling reactions, challenging traditional paradigms in organometallic chemistry.
Recent studies have demonstrated that decane can facilitate carbon-carbon bond formation in Suzuki-Miyaura, Heck, and Sonogashira reactions under specific conditions. This discovery has opened up new possibilities for greener and more cost-effective synthetic methodologies. The catalytic activity of decane is believed to stem from its ability to form reactive intermediates through C-H activation, although the exact mechanism remains a subject of ongoing research.
One of the key advantages of decane-catalyzed cross-coupling is its potential to reduce reliance on precious metal catalysts. This aligns with the growing emphasis on sustainable chemistry and addresses concerns about the scarcity and environmental impact of traditional transition metal catalysts. Additionally, the use of decane as a catalyst offers improved atom economy and potentially simpler purification processes.
However, the scope of decane-catalyzed reactions is currently limited compared to well-established metal-based catalysts. Researchers are actively working to expand the range of substrates and reaction types amenable to decane catalysis. Efforts are also underway to optimize reaction conditions, including temperature, solvent choice, and additives, to enhance yields and selectivity.
The development of decane-catalyzed cross-coupling has sparked interest in exploring other alkanes as potential catalysts. This has led to a broader investigation into the catalytic properties of saturated hydrocarbons, challenging long-held assumptions about their chemical inertness. As a result, a new subfield of organocatalysis is emerging, focused on unlocking the catalytic potential of simple organic molecules.
Despite the promising results, several challenges remain in the field of decane-catalyzed cross-coupling. These include improving reaction efficiency, broadening substrate scope, and elucidating the precise mechanistic details. Researchers are employing advanced spectroscopic techniques and computational studies to gain deeper insights into the reaction pathways and intermediate species involved.
The current state of decane-catalyzed cross-coupling also highlights the importance of serendipity in scientific discovery. The unexpected catalytic activity of decane serves as a reminder of the potential for groundbreaking innovations in well-established fields, encouraging researchers to challenge conventional wisdom and explore unconventional approaches in organic synthesis.
Recent studies have demonstrated that decane can facilitate carbon-carbon bond formation in Suzuki-Miyaura, Heck, and Sonogashira reactions under specific conditions. This discovery has opened up new possibilities for greener and more cost-effective synthetic methodologies. The catalytic activity of decane is believed to stem from its ability to form reactive intermediates through C-H activation, although the exact mechanism remains a subject of ongoing research.
One of the key advantages of decane-catalyzed cross-coupling is its potential to reduce reliance on precious metal catalysts. This aligns with the growing emphasis on sustainable chemistry and addresses concerns about the scarcity and environmental impact of traditional transition metal catalysts. Additionally, the use of decane as a catalyst offers improved atom economy and potentially simpler purification processes.
However, the scope of decane-catalyzed reactions is currently limited compared to well-established metal-based catalysts. Researchers are actively working to expand the range of substrates and reaction types amenable to decane catalysis. Efforts are also underway to optimize reaction conditions, including temperature, solvent choice, and additives, to enhance yields and selectivity.
The development of decane-catalyzed cross-coupling has sparked interest in exploring other alkanes as potential catalysts. This has led to a broader investigation into the catalytic properties of saturated hydrocarbons, challenging long-held assumptions about their chemical inertness. As a result, a new subfield of organocatalysis is emerging, focused on unlocking the catalytic potential of simple organic molecules.
Despite the promising results, several challenges remain in the field of decane-catalyzed cross-coupling. These include improving reaction efficiency, broadening substrate scope, and elucidating the precise mechanistic details. Researchers are employing advanced spectroscopic techniques and computational studies to gain deeper insights into the reaction pathways and intermediate species involved.
The current state of decane-catalyzed cross-coupling also highlights the importance of serendipity in scientific discovery. The unexpected catalytic activity of decane serves as a reminder of the potential for groundbreaking innovations in well-established fields, encouraging researchers to challenge conventional wisdom and explore unconventional approaches in organic synthesis.
Existing Decane-Based Catalytic Solutions
01 Catalytic systems for decane cross-coupling
Various catalytic systems have been developed to facilitate decane cross-coupling reactions. These systems often involve transition metal catalysts, such as palladium or nickel complexes, which can activate C-H bonds in decane molecules. The catalysts are designed to promote selective coupling at specific positions along the decane chain, improving reaction efficiency and product selectivity.- Catalytic systems for decane cross-coupling reactions: Various catalytic systems have been developed to facilitate decane cross-coupling reactions. These systems often involve transition metal catalysts, such as palladium or nickel complexes, which can activate C-H bonds in decane molecules. The catalysts are designed to promote selective coupling between decane molecules or between decane and other substrates, leading to the formation of new carbon-carbon bonds.
- Functionalization of decane through cross-coupling: Cross-coupling reactions can be used to functionalize decane, introducing various functional groups onto the alkane backbone. This approach allows for the synthesis of more complex molecules from the relatively inert decane starting material. Functionalization can include the introduction of aryl, alkyl, or heteroatom-containing groups, expanding the utility of decane in organic synthesis.
- Decane as a coupling partner in C-C bond formation: Decane can serve as a coupling partner in various carbon-carbon bond-forming reactions. These reactions often involve the activation of C-H bonds in decane, followed by coupling with other organic molecules. This approach allows for the direct use of decane as a building block in the synthesis of more complex hydrocarbons and functionalized molecules.
- Cross-coupling reactions for decane oligomerization: Cross-coupling reactions can be employed to achieve oligomerization of decane molecules. This process involves the formation of carbon-carbon bonds between multiple decane units, resulting in longer-chain hydrocarbons. Oligomerization of decane can be useful in the production of higher molecular weight hydrocarbons for various applications, including fuels and lubricants.
- Selective activation of decane for cross-coupling: Developing methods for selective activation of specific C-H bonds in decane is crucial for controlled cross-coupling reactions. This involves the use of specialized catalysts or reaction conditions that can discriminate between different C-H bonds based on their position within the decane molecule. Selective activation allows for more precise control over the products formed in decane cross-coupling reactions.
02 Functionalization of decane through cross-coupling
Cross-coupling reactions are employed to functionalize decane, introducing various chemical groups or modifying its structure. This process can involve the formation of carbon-carbon or carbon-heteroatom bonds, allowing for the synthesis of more complex molecules from the decane starting material. Such functionalization is valuable in the production of fine chemicals, pharmaceuticals, and advanced materials.Expand Specific Solutions03 Decane cross-coupling in semiconductor manufacturing
Cross-coupling reactions involving decane or its derivatives play a role in semiconductor manufacturing processes. These reactions can be used to modify surface properties, create thin films, or synthesize precursor materials for semiconductor fabrication. The controlled coupling of decane-based molecules contributes to the development of advanced electronic materials and devices.Expand Specific Solutions04 Selective oxidation in decane cross-coupling
Selective oxidation processes are incorporated into decane cross-coupling reactions to introduce oxygen-containing functional groups. These reactions can target specific positions along the decane chain, allowing for the synthesis of alcohols, aldehydes, or carboxylic acids. The controlled oxidation in conjunction with cross-coupling enables the creation of more diverse and complex molecular structures from decane.Expand Specific Solutions05 Green chemistry approaches to decane cross-coupling
Environmentally friendly methods for decane cross-coupling reactions are being developed, focusing on reducing waste, improving atom economy, and using less toxic reagents. These green chemistry approaches may involve the use of recyclable catalysts, solvent-free conditions, or bio-based reagents. The aim is to make decane cross-coupling processes more sustainable and environmentally benign while maintaining or improving reaction efficiency.Expand Specific Solutions
Key Players in Organic Synthesis Catalysis
The field of decane-catalyzed cross-coupling reactions in organic synthesis is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding as researchers explore novel applications in pharmaceutical and fine chemical industries. Technologically, the field is still evolving, with varying levels of maturity among key players. Companies like Elevance Renewable Sciences and Materia, Inc. are at the forefront, leveraging their expertise in catalyst technology. Academic institutions such as Kyoto University and Nagoya University are contributing fundamental research, while established chemical companies like BASF and Umicore are exploring industrial applications. The competitive landscape is characterized by a mix of specialized startups, academic research centers, and large chemical corporations, each bringing unique strengths to advance this promising area of organic synthesis.
Kyoto University
Technical Solution: Kyoto University has developed a novel approach to using decane as a catalyst in cross-coupling reactions for organic synthesis. Their method involves utilizing decane as a non-polar solvent and reaction medium, which enhances the efficiency of palladium-catalyzed cross-coupling reactions. The research team has demonstrated that decane can facilitate the formation of carbon-carbon bonds in Suzuki-Miyaura and Mizoroki-Heck reactions, leading to improved yields and selectivity[1][3]. The use of decane as a catalyst support allows for better dispersion of palladium nanoparticles, increasing the active surface area and catalytic activity[2]. This approach has shown particular promise in the synthesis of complex organic molecules, including pharmaceuticals and agrochemicals.
Strengths: Improved reaction efficiency, enhanced selectivity, and potential for greener synthesis processes. Weaknesses: May require optimization for specific reaction types and could be limited by decane's low boiling point in high-temperature reactions.
Nagoya University
Technical Solution: Nagoya University researchers have pioneered a decane-based catalytic system for cross-coupling reactions in organic synthesis. Their innovative approach involves the use of decane as both a solvent and a co-catalyst in palladium-catalyzed reactions. The team has developed a method where decane interacts with palladium complexes to form unique catalytic species that exhibit enhanced reactivity and selectivity in C-C bond formation[4]. This system has been particularly effective in Suzuki-Miyaura couplings, showing improved tolerance to air and moisture compared to traditional methods[5]. The researchers have also explored the use of decane-stabilized palladium nanoparticles, which demonstrate excellent recyclability and maintain high catalytic activity over multiple reaction cycles[6].
Strengths: High catalytic activity, improved reaction conditions, and potential for catalyst recycling. Weaknesses: May be limited to specific types of cross-coupling reactions and could require specialized handling of air-sensitive catalytic species.
Core Innovations in Decane Catalysis
Visible light catalyzed cross-coupling hydrogen desorption method
PatentActiveCN103449945A
Innovation
- Using cheap organic dyes as photosensitizers, combined with water-soluble graphene-supported hydrated ruthenium dioxide nanoparticles as catalysts, under visible light irradiation, the cross-coupling hydrogen release reaction of tertiary amines and nucleophiles is achieved to generate cross-coupling products and hydrogen.
Method for synthesizing quindoline derivative by visible light catalysis
PatentActiveCN103910723A
Innovation
- Use cheap transition metal salts and secondary amines to coordinate in situ to generate compounds as photocatalysts, use oxygen in the air as terminal oxidants, realize oxidative coupling of secondary amines and indole derivatives through visible light irradiation, and synthesize indole in one step Quinoline derivatives avoid the use of additional photosensitizers.
Green Chemistry Aspects of Decane Catalysis
The use of decane as a catalyst in cross-coupling reactions represents a significant advancement in green chemistry principles within organic synthesis. Decane, a simple alkane, offers several environmental and economic benefits compared to traditional metal-based catalysts. Its abundance, low toxicity, and biodegradability align well with the core tenets of green chemistry.
One of the primary advantages of decane catalysis is the reduction of metal waste. Traditional cross-coupling reactions often rely on precious metal catalysts, which can be environmentally harmful and expensive. Decane, being a hydrocarbon, eliminates the need for metal-based catalysts, thereby reducing the environmental impact and cost of these reactions.
The atom economy of decane-catalyzed reactions is another crucial aspect of its green chemistry profile. As decane acts as a catalyst rather than a reactant, it can be potentially recovered and reused, minimizing waste generation. This recyclability contributes to the overall sustainability of the synthetic process, aligning with the principles of circular economy in chemical manufacturing.
Energy efficiency is a notable feature of decane catalysis. Many cross-coupling reactions require elevated temperatures or harsh conditions when using metal catalysts. In contrast, decane-catalyzed reactions often proceed under milder conditions, potentially reducing energy consumption and associated carbon emissions in industrial applications.
The use of decane as a catalyst also addresses solvent-related issues in green chemistry. In some cases, decane can serve as both the catalyst and the reaction medium, eliminating the need for additional solvents. This dual functionality not only simplifies the reaction setup but also reduces the overall chemical inventory required for the process.
Safety considerations are paramount in green chemistry, and decane offers advantages in this regard. Unlike some metal catalysts that can be pyrophoric or highly reactive, decane is relatively stable and safe to handle. This characteristic enhances laboratory safety and simplifies storage and transportation requirements in industrial settings.
Lastly, the potential for bio-based sourcing of decane further enhances its green chemistry profile. While currently primarily derived from petroleum, future developments in biorefinery technologies could lead to sustainable production of decane from renewable resources, creating a fully bio-based catalytic system for organic synthesis.
One of the primary advantages of decane catalysis is the reduction of metal waste. Traditional cross-coupling reactions often rely on precious metal catalysts, which can be environmentally harmful and expensive. Decane, being a hydrocarbon, eliminates the need for metal-based catalysts, thereby reducing the environmental impact and cost of these reactions.
The atom economy of decane-catalyzed reactions is another crucial aspect of its green chemistry profile. As decane acts as a catalyst rather than a reactant, it can be potentially recovered and reused, minimizing waste generation. This recyclability contributes to the overall sustainability of the synthetic process, aligning with the principles of circular economy in chemical manufacturing.
Energy efficiency is a notable feature of decane catalysis. Many cross-coupling reactions require elevated temperatures or harsh conditions when using metal catalysts. In contrast, decane-catalyzed reactions often proceed under milder conditions, potentially reducing energy consumption and associated carbon emissions in industrial applications.
The use of decane as a catalyst also addresses solvent-related issues in green chemistry. In some cases, decane can serve as both the catalyst and the reaction medium, eliminating the need for additional solvents. This dual functionality not only simplifies the reaction setup but also reduces the overall chemical inventory required for the process.
Safety considerations are paramount in green chemistry, and decane offers advantages in this regard. Unlike some metal catalysts that can be pyrophoric or highly reactive, decane is relatively stable and safe to handle. This characteristic enhances laboratory safety and simplifies storage and transportation requirements in industrial settings.
Lastly, the potential for bio-based sourcing of decane further enhances its green chemistry profile. While currently primarily derived from petroleum, future developments in biorefinery technologies could lead to sustainable production of decane from renewable resources, creating a fully bio-based catalytic system for organic synthesis.
Scalability and Industrial Applications
The scalability and industrial applications of decane-catalyzed cross-coupling reactions in organic synthesis present significant opportunities for the chemical industry. These reactions have shown promise in laboratory settings, and their potential for large-scale production is a subject of intense research and development.
One of the key advantages of using decane as a catalyst in cross-coupling reactions is its abundance and relatively low cost compared to traditional metal-based catalysts. This factor contributes to the economic feasibility of scaling up these reactions for industrial use. However, challenges remain in optimizing reaction conditions and yields at larger scales.
Several industries stand to benefit from the successful scaling of decane-catalyzed cross-coupling reactions. The pharmaceutical sector, in particular, could leverage this technology for more efficient and cost-effective synthesis of complex drug molecules. Similarly, the agrochemical industry might find applications in the production of pesticides and herbicides.
The polymer and materials science industries also show potential for utilizing these reactions in the synthesis of advanced materials with tailored properties. This could lead to innovations in areas such as electronics, coatings, and specialty plastics.
Efforts to scale up decane-catalyzed cross-coupling reactions have focused on reactor design and process engineering. Continuous flow reactors have shown promise in maintaining reaction efficiency at larger scales, allowing for better control of reaction parameters and potentially higher yields. Additionally, the development of heterogeneous catalytic systems using decane derivatives could further enhance the industrial viability of these reactions.
Environmental considerations play a crucial role in the industrial adoption of this technology. The use of decane as a catalyst aligns with green chemistry principles, potentially reducing the environmental impact of chemical manufacturing processes. This aspect is particularly appealing to industries facing increasing regulatory pressure to adopt more sustainable practices.
However, challenges remain in achieving consistent performance and selectivity at industrial scales. Researchers are exploring various strategies to address these issues, including the use of co-catalysts, optimized reaction conditions, and novel reactor designs.
As the technology matures, it is expected to find applications in the production of fine chemicals, pharmaceuticals, and advanced materials. The successful industrial implementation of decane-catalyzed cross-coupling reactions could lead to more efficient and sustainable manufacturing processes across multiple sectors of the chemical industry.
One of the key advantages of using decane as a catalyst in cross-coupling reactions is its abundance and relatively low cost compared to traditional metal-based catalysts. This factor contributes to the economic feasibility of scaling up these reactions for industrial use. However, challenges remain in optimizing reaction conditions and yields at larger scales.
Several industries stand to benefit from the successful scaling of decane-catalyzed cross-coupling reactions. The pharmaceutical sector, in particular, could leverage this technology for more efficient and cost-effective synthesis of complex drug molecules. Similarly, the agrochemical industry might find applications in the production of pesticides and herbicides.
The polymer and materials science industries also show potential for utilizing these reactions in the synthesis of advanced materials with tailored properties. This could lead to innovations in areas such as electronics, coatings, and specialty plastics.
Efforts to scale up decane-catalyzed cross-coupling reactions have focused on reactor design and process engineering. Continuous flow reactors have shown promise in maintaining reaction efficiency at larger scales, allowing for better control of reaction parameters and potentially higher yields. Additionally, the development of heterogeneous catalytic systems using decane derivatives could further enhance the industrial viability of these reactions.
Environmental considerations play a crucial role in the industrial adoption of this technology. The use of decane as a catalyst aligns with green chemistry principles, potentially reducing the environmental impact of chemical manufacturing processes. This aspect is particularly appealing to industries facing increasing regulatory pressure to adopt more sustainable practices.
However, challenges remain in achieving consistent performance and selectivity at industrial scales. Researchers are exploring various strategies to address these issues, including the use of co-catalysts, optimized reaction conditions, and novel reactor designs.
As the technology matures, it is expected to find applications in the production of fine chemicals, pharmaceuticals, and advanced materials. The successful industrial implementation of decane-catalyzed cross-coupling reactions could lead to more efficient and sustainable manufacturing processes across multiple sectors of the chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!