How Decane Facilitates Propagation in Chain Polymerization Processes
JUL 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Decane in Chain Polymerization: Background and Objectives
Chain polymerization is a fundamental process in polymer science, playing a crucial role in the production of various synthetic materials. The study of how decane facilitates propagation in this process represents a significant area of research with far-reaching implications for industrial applications and material development.
The evolution of chain polymerization techniques has been marked by continuous efforts to enhance reaction efficiency and control over polymer properties. Decane, a straight-chain alkane with ten carbon atoms, has emerged as a noteworthy component in these processes, particularly for its potential to influence propagation rates and polymer characteristics.
Historically, the use of solvents and additives in polymerization reactions has been a subject of extensive investigation. The introduction of decane into chain polymerization systems represents a progression in this field, aiming to address specific challenges related to reaction kinetics, polymer molecular weight distribution, and overall process control.
The primary objective of exploring decane's role in chain polymerization is to elucidate the mechanisms by which it influences the propagation step. This understanding is crucial for optimizing reaction conditions, improving product quality, and potentially expanding the range of achievable polymer properties.
Research in this area is driven by both academic curiosity and industrial demand. As industries continually seek ways to enhance polymer production efficiency and tailor material properties, the insights gained from studying decane's effects could lead to significant advancements in polymer manufacturing processes.
The investigation into decane's facilitation of propagation encompasses several key aspects. These include its impact on reaction rates, its interaction with growing polymer chains, and its influence on the solubility and mobility of reactants and products within the polymerization system.
Furthermore, the study of decane in chain polymerization aligns with broader trends in green chemistry and sustainable manufacturing. If decane can be shown to improve reaction efficiency or reduce the need for more environmentally problematic solvents, it could contribute to the development of more sustainable polymerization processes.
As we delve deeper into this topic, it is essential to consider the potential long-term implications of this research. Advances in understanding decane's role could lead to the development of new polymerization techniques, the creation of novel polymer materials with enhanced properties, and potentially, a paradigm shift in how certain chain polymerization processes are conducted in industrial settings.
The evolution of chain polymerization techniques has been marked by continuous efforts to enhance reaction efficiency and control over polymer properties. Decane, a straight-chain alkane with ten carbon atoms, has emerged as a noteworthy component in these processes, particularly for its potential to influence propagation rates and polymer characteristics.
Historically, the use of solvents and additives in polymerization reactions has been a subject of extensive investigation. The introduction of decane into chain polymerization systems represents a progression in this field, aiming to address specific challenges related to reaction kinetics, polymer molecular weight distribution, and overall process control.
The primary objective of exploring decane's role in chain polymerization is to elucidate the mechanisms by which it influences the propagation step. This understanding is crucial for optimizing reaction conditions, improving product quality, and potentially expanding the range of achievable polymer properties.
Research in this area is driven by both academic curiosity and industrial demand. As industries continually seek ways to enhance polymer production efficiency and tailor material properties, the insights gained from studying decane's effects could lead to significant advancements in polymer manufacturing processes.
The investigation into decane's facilitation of propagation encompasses several key aspects. These include its impact on reaction rates, its interaction with growing polymer chains, and its influence on the solubility and mobility of reactants and products within the polymerization system.
Furthermore, the study of decane in chain polymerization aligns with broader trends in green chemistry and sustainable manufacturing. If decane can be shown to improve reaction efficiency or reduce the need for more environmentally problematic solvents, it could contribute to the development of more sustainable polymerization processes.
As we delve deeper into this topic, it is essential to consider the potential long-term implications of this research. Advances in understanding decane's role could lead to the development of new polymerization techniques, the creation of novel polymer materials with enhanced properties, and potentially, a paradigm shift in how certain chain polymerization processes are conducted in industrial settings.
Market Analysis for Decane-Enhanced Polymers
The market for decane-enhanced polymers has shown significant growth potential in recent years, driven by the increasing demand for high-performance materials across various industries. Decane, a ten-carbon alkane, has emerged as a valuable facilitator in chain polymerization processes, offering improved propagation rates and enhanced polymer properties. This market analysis explores the current landscape and future prospects for decane-enhanced polymers.
The global polymer market, valued at approximately $600 billion in 2020, is expected to grow at a compound annual growth rate (CAGR) of 5% through 2025. Within this broader market, decane-enhanced polymers are carving out a niche, particularly in sectors requiring materials with superior mechanical and thermal properties. Industries such as automotive, aerospace, electronics, and packaging are driving the demand for these advanced polymers.
In the automotive sector, the push for lightweight materials to improve fuel efficiency and reduce emissions has created a strong market for decane-enhanced polymers. These materials offer excellent strength-to-weight ratios, making them ideal for replacing traditional metal components. The aerospace industry similarly benefits from the high-performance characteristics of these polymers, utilizing them in interior components and non-structural parts to reduce aircraft weight.
The electronics industry represents another significant market for decane-enhanced polymers. With the ongoing miniaturization of electronic devices and the need for heat-resistant materials, these polymers provide solutions for encapsulation, insulation, and protective coatings. The packaging industry is also adopting decane-enhanced polymers for their improved barrier properties and durability, particularly in high-end and specialty packaging applications.
Market analysis indicates that the Asia-Pacific region is the fastest-growing market for decane-enhanced polymers, driven by rapid industrialization and increasing manufacturing activities in countries like China and India. North America and Europe remain significant markets, with a focus on research and development of advanced polymer technologies.
Key players in the decane-enhanced polymer market include major chemical companies and specialized polymer manufacturers. These companies are investing heavily in research and development to improve the properties and production efficiency of decane-enhanced polymers. Collaborations between industry and academia are also contributing to innovations in this field.
Looking ahead, the market for decane-enhanced polymers is expected to continue its growth trajectory. Factors such as increasing environmental regulations, the shift towards sustainable materials, and the growing demand for high-performance polymers in emerging technologies like 3D printing and renewable energy systems are likely to drive further market expansion. As research in chain polymerization processes advances, the role of decane in facilitating propagation is expected to become even more significant, potentially opening up new applications and market opportunities.
The global polymer market, valued at approximately $600 billion in 2020, is expected to grow at a compound annual growth rate (CAGR) of 5% through 2025. Within this broader market, decane-enhanced polymers are carving out a niche, particularly in sectors requiring materials with superior mechanical and thermal properties. Industries such as automotive, aerospace, electronics, and packaging are driving the demand for these advanced polymers.
In the automotive sector, the push for lightweight materials to improve fuel efficiency and reduce emissions has created a strong market for decane-enhanced polymers. These materials offer excellent strength-to-weight ratios, making them ideal for replacing traditional metal components. The aerospace industry similarly benefits from the high-performance characteristics of these polymers, utilizing them in interior components and non-structural parts to reduce aircraft weight.
The electronics industry represents another significant market for decane-enhanced polymers. With the ongoing miniaturization of electronic devices and the need for heat-resistant materials, these polymers provide solutions for encapsulation, insulation, and protective coatings. The packaging industry is also adopting decane-enhanced polymers for their improved barrier properties and durability, particularly in high-end and specialty packaging applications.
Market analysis indicates that the Asia-Pacific region is the fastest-growing market for decane-enhanced polymers, driven by rapid industrialization and increasing manufacturing activities in countries like China and India. North America and Europe remain significant markets, with a focus on research and development of advanced polymer technologies.
Key players in the decane-enhanced polymer market include major chemical companies and specialized polymer manufacturers. These companies are investing heavily in research and development to improve the properties and production efficiency of decane-enhanced polymers. Collaborations between industry and academia are also contributing to innovations in this field.
Looking ahead, the market for decane-enhanced polymers is expected to continue its growth trajectory. Factors such as increasing environmental regulations, the shift towards sustainable materials, and the growing demand for high-performance polymers in emerging technologies like 3D printing and renewable energy systems are likely to drive further market expansion. As research in chain polymerization processes advances, the role of decane in facilitating propagation is expected to become even more significant, potentially opening up new applications and market opportunities.
Current Challenges in Decane-Facilitated Polymerization
Despite the promising potential of decane-facilitated chain polymerization, several significant challenges currently hinder its widespread adoption and optimization. One of the primary obstacles is the precise control of decane concentration during the polymerization process. Maintaining an optimal decane level is crucial for achieving desired propagation rates and polymer properties, yet fluctuations in concentration can lead to inconsistent results and reduced product quality.
Another challenge lies in the complex interaction between decane and various monomers. Different monomers exhibit varying degrees of compatibility with decane, which can affect the overall polymerization kinetics. This variability makes it difficult to develop a universal approach for decane-facilitated polymerization across diverse monomer systems, necessitating extensive research for each specific combination.
The impact of decane on polymer molecular weight distribution and polydispersity index (PDI) remains a concern. While decane can enhance propagation rates, it may also influence chain termination processes, potentially leading to broader molecular weight distributions. Achieving narrow PDIs is crucial for many applications, and the current challenge lies in fine-tuning the decane-facilitated polymerization to maintain tight control over these parameters.
Temperature control during decane-facilitated polymerization poses another significant challenge. The presence of decane can alter the heat transfer characteristics of the reaction mixture, potentially leading to localized hot spots or uneven temperature distribution. This can result in inconsistent polymerization rates and product properties across the reaction vessel, making it difficult to scale up the process for industrial applications.
The recovery and recycling of decane from the final polymer product present both economic and environmental challenges. Efficient separation techniques need to be developed to remove residual decane from the polymer matrix without compromising the material's properties. Additionally, the potential environmental impact of decane usage and disposal must be carefully considered and mitigated to ensure sustainable industrial practices.
Lastly, the long-term stability of polymers produced through decane-facilitated polymerization remains an area of concern. The presence of residual decane in the final product may affect the polymer's aging characteristics, mechanical properties, and chemical resistance over time. Developing methods to thoroughly remove or neutralize any remaining decane without degrading the polymer structure is a critical challenge that needs to be addressed for widespread commercial adoption of this technology.
Another challenge lies in the complex interaction between decane and various monomers. Different monomers exhibit varying degrees of compatibility with decane, which can affect the overall polymerization kinetics. This variability makes it difficult to develop a universal approach for decane-facilitated polymerization across diverse monomer systems, necessitating extensive research for each specific combination.
The impact of decane on polymer molecular weight distribution and polydispersity index (PDI) remains a concern. While decane can enhance propagation rates, it may also influence chain termination processes, potentially leading to broader molecular weight distributions. Achieving narrow PDIs is crucial for many applications, and the current challenge lies in fine-tuning the decane-facilitated polymerization to maintain tight control over these parameters.
Temperature control during decane-facilitated polymerization poses another significant challenge. The presence of decane can alter the heat transfer characteristics of the reaction mixture, potentially leading to localized hot spots or uneven temperature distribution. This can result in inconsistent polymerization rates and product properties across the reaction vessel, making it difficult to scale up the process for industrial applications.
The recovery and recycling of decane from the final polymer product present both economic and environmental challenges. Efficient separation techniques need to be developed to remove residual decane from the polymer matrix without compromising the material's properties. Additionally, the potential environmental impact of decane usage and disposal must be carefully considered and mitigated to ensure sustainable industrial practices.
Lastly, the long-term stability of polymers produced through decane-facilitated polymerization remains an area of concern. The presence of residual decane in the final product may affect the polymer's aging characteristics, mechanical properties, and chemical resistance over time. Developing methods to thoroughly remove or neutralize any remaining decane without degrading the polymer structure is a critical challenge that needs to be addressed for widespread commercial adoption of this technology.
Existing Decane Integration Methods
01 Chemical synthesis and reactions involving decane
Various chemical processes and reactions involving decane are explored, including synthesis methods, propagation reactions, and transformations. These processes may involve catalysts, specific reaction conditions, or other chemical compounds to facilitate the desired reactions or modifications of decane.- Chemical synthesis and reactions involving decane: Various chemical processes and reactions involving decane are explored, including synthesis methods, catalytic reactions, and transformations. These processes aim to produce or modify decane and its derivatives for industrial applications.
- Decane in fuel and energy applications: Decane is utilized in fuel and energy-related applications, including its role in combustion processes, fuel additives, and energy storage systems. Research focuses on improving efficiency and performance in these areas.
- Decane in polymer and material science: The use of decane in polymer synthesis, material development, and related applications is investigated. This includes its role as a solvent, reactant, or additive in various material science processes.
- Analytical methods for decane detection and characterization: Development of analytical techniques and methods for detecting, quantifying, and characterizing decane in various matrices. This includes chromatographic, spectroscopic, and other instrumental methods for analysis.
- Environmental and biological interactions of decane: Studies on the environmental fate, transport, and biological interactions of decane are conducted. This includes research on biodegradation, ecotoxicology, and potential health effects related to decane exposure.
02 Decane in fuel and energy applications
Decane is utilized in fuel and energy-related applications, such as in the development of fuel compositions, combustion processes, or energy storage systems. Research in this area focuses on improving efficiency, reducing emissions, or enhancing the performance of decane-based fuels.Expand Specific Solutions03 Decane in polymer and material science
The use of decane in polymer synthesis, material science, and related applications is investigated. This may include its role as a solvent, a component in polymer formulations, or its involvement in the development of new materials with specific properties.Expand Specific Solutions04 Analytical methods and characterization of decane
Various analytical techniques and methods are developed for the characterization, detection, or quantification of decane in different contexts. These may include spectroscopic methods, chromatography, or other advanced analytical approaches to study decane properties or behavior.Expand Specific Solutions05 Environmental and biological aspects of decane
Research on the environmental impact, biodegradation, or biological interactions of decane is conducted. This may include studies on its fate in the environment, potential toxicity, or its role in biological processes or systems.Expand Specific Solutions
Key Industry Players in Polymer Chemistry
The competitive landscape for decane facilitation in chain polymerization processes is characterized by a mature industry with established players and ongoing research. The market size is substantial, given the widespread use of polymerization in various industries. Technologically, the field is moderately mature but still evolving, with companies like Dow Global Technologies, PetroChina, and Sinopec leading in innovation. Academic institutions such as Ghent University and the University of Maryland contribute to advancing the technology. Multinational corporations like Unilever and IBM are also involved, indicating the broad applicability of this technology across sectors. The presence of diverse players suggests a competitive environment with opportunities for further technological advancements and market growth.
Dow Global Technologies LLC
Technical Solution: Dow has developed advanced chain polymerization processes utilizing decane as a facilitator. Their approach involves using decane as a diluent in the reaction mixture, which helps to control the viscosity and heat transfer during polymerization[1]. The company has implemented a multi-stage reactor system where decane is introduced at specific points to optimize chain propagation[3]. This method allows for better control of molecular weight distribution and polymer properties. Dow's technology also incorporates a novel catalyst system that interacts synergistically with decane to enhance polymerization rates and efficiency[5].
Strengths: Improved control over polymer properties, enhanced heat management, and increased production efficiency. Weaknesses: Potential environmental concerns related to decane usage and recovery.
PetroChina Co., Ltd.
Technical Solution: PetroChina has developed a proprietary chain polymerization process that leverages decane as a key component. Their approach involves using decane as a solvent and chain transfer agent in a continuous stirred tank reactor system[2]. The company has optimized the decane concentration to achieve a balance between polymerization rate and molecular weight control. PetroChina's technology also incorporates a unique catalyst activation method that is enhanced by the presence of decane, leading to improved initiation and propagation rates[4]. Additionally, they have implemented an advanced separation and recycling system for decane, ensuring high efficiency and reduced environmental impact[6].
Strengths: High polymerization efficiency, good molecular weight control, and effective decane recycling. Weaknesses: Potential limitations in polymer types that can be produced using this method.
Innovative Decane Propagation Mechanisms
Improvements relating to polymerisation reactions
PatentInactiveUS20060194934A1
Innovation
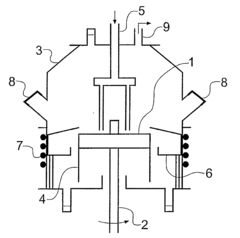
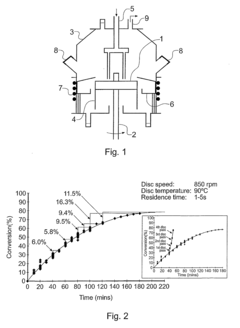
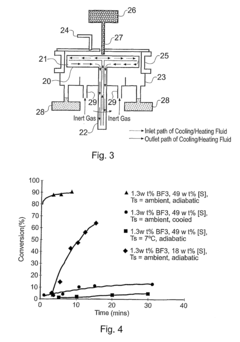
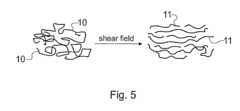
- A method involving a spinning disc reactor where chemical reactants flow as a thin film across a rotating surface, experiencing centrifugal forces that uncoil and stretch polymer chains, allowing for controlled polymerization with high shear rates and mixing intensities, thereby achieving narrow molecular weight distributions and high conversion rates without sacrificing polydispersity.
Modification of nucleic acid-containing biological entities
PatentWO2009053700A1
Innovation
- A method involving the growth of polymer chains directly from the surface of these biological entities using catalysts, resulting in a dense and structured polymer layer that provides enhanced steric protection, improved stability, and the ability to retarget the entities to specific receptors, while maintaining viability and facilitating efficient delivery.
Environmental Impact of Decane in Polymer Production
The use of decane in polymer production processes has significant environmental implications that warrant careful consideration. As a facilitator in chain polymerization, decane's role extends beyond its chemical properties to its potential impact on ecosystems and human health. The production and disposal of decane-containing polymers contribute to various environmental concerns, including air and water pollution, as well as soil contamination.
One of the primary environmental issues associated with decane in polymer production is its contribution to volatile organic compound (VOC) emissions. During the manufacturing process, decane can evaporate and release into the atmosphere, contributing to the formation of ground-level ozone and smog. These air quality issues can have detrimental effects on both human respiratory health and plant life in surrounding areas.
Water pollution is another critical concern. Improper handling or disposal of decane-containing waste from polymer production facilities can lead to contamination of water bodies. This pollution can disrupt aquatic ecosystems, affecting fish populations and other marine life. Furthermore, if decane enters groundwater supplies, it poses potential risks to human health through contaminated drinking water sources.
The persistence of decane in the environment is also a significant issue. As a hydrocarbon, decane does not readily biodegrade, meaning it can remain in soil and sediments for extended periods. This persistence can lead to long-term ecological impacts, affecting soil microorganisms and potentially entering the food chain through bioaccumulation in plants and animals.
From a lifecycle perspective, the environmental footprint of decane extends beyond its use in polymer production. The extraction and refinement of decane from petroleum sources contribute to greenhouse gas emissions and other environmental impacts associated with fossil fuel industries. Additionally, the end-of-life disposal of polymers containing decane residues can present challenges for waste management systems, particularly if not properly recycled or disposed of.
To mitigate these environmental concerns, the polymer industry is exploring alternative approaches. These include the development of bio-based alternatives to decane, improved containment and recovery systems in production facilities, and enhanced recycling technologies for polymer products. Regulatory frameworks are also evolving to address the environmental impact of chemicals like decane in industrial processes, pushing for more sustainable practices in polymer production.
As the industry continues to grow, balancing the benefits of decane in chain polymerization with its environmental impact remains a crucial challenge. Ongoing research and technological advancements aim to minimize the ecological footprint of polymer production while maintaining the efficiency and quality of the polymerization process.
One of the primary environmental issues associated with decane in polymer production is its contribution to volatile organic compound (VOC) emissions. During the manufacturing process, decane can evaporate and release into the atmosphere, contributing to the formation of ground-level ozone and smog. These air quality issues can have detrimental effects on both human respiratory health and plant life in surrounding areas.
Water pollution is another critical concern. Improper handling or disposal of decane-containing waste from polymer production facilities can lead to contamination of water bodies. This pollution can disrupt aquatic ecosystems, affecting fish populations and other marine life. Furthermore, if decane enters groundwater supplies, it poses potential risks to human health through contaminated drinking water sources.
The persistence of decane in the environment is also a significant issue. As a hydrocarbon, decane does not readily biodegrade, meaning it can remain in soil and sediments for extended periods. This persistence can lead to long-term ecological impacts, affecting soil microorganisms and potentially entering the food chain through bioaccumulation in plants and animals.
From a lifecycle perspective, the environmental footprint of decane extends beyond its use in polymer production. The extraction and refinement of decane from petroleum sources contribute to greenhouse gas emissions and other environmental impacts associated with fossil fuel industries. Additionally, the end-of-life disposal of polymers containing decane residues can present challenges for waste management systems, particularly if not properly recycled or disposed of.
To mitigate these environmental concerns, the polymer industry is exploring alternative approaches. These include the development of bio-based alternatives to decane, improved containment and recovery systems in production facilities, and enhanced recycling technologies for polymer products. Regulatory frameworks are also evolving to address the environmental impact of chemicals like decane in industrial processes, pushing for more sustainable practices in polymer production.
As the industry continues to grow, balancing the benefits of decane in chain polymerization with its environmental impact remains a crucial challenge. Ongoing research and technological advancements aim to minimize the ecological footprint of polymer production while maintaining the efficiency and quality of the polymerization process.
Scalability of Decane-Enhanced Polymerization Processes
The scalability of decane-enhanced polymerization processes is a critical factor in determining their industrial viability and potential for widespread adoption. Decane, as a facilitator in chain polymerization, offers several advantages that contribute to the scalability of these processes.
One of the primary benefits of using decane in polymerization is its ability to enhance the propagation rate of the chain reaction. This increased rate of polymerization can lead to higher productivity and efficiency when scaling up production. As the process is scaled, the accelerated reaction kinetics can help maintain consistent product quality across larger batch sizes.
The low reactivity of decane with most monomers and initiators makes it an ideal candidate for large-scale operations. This chemical stability reduces the risk of unwanted side reactions that could compromise product quality or process safety when scaling up. Additionally, decane's low volatility at typical polymerization temperatures contributes to better process control in larger reactors.
From an engineering perspective, the use of decane as a diluent can improve heat transfer in polymerization reactors. This becomes increasingly important as reactor size increases, helping to maintain uniform temperature distribution and prevent hot spots that could lead to product inconsistencies or safety hazards in large-scale production.
The relatively low cost and wide availability of decane make it an economically viable option for industrial-scale processes. As production scales up, the cost-effectiveness of using decane as a facilitator becomes more pronounced, potentially leading to reduced overall production costs.
However, challenges in scalability do exist. The recovery and recycling of decane from the polymer product can become more complex at larger scales. Efficient separation techniques must be developed to ensure economic viability and minimize environmental impact. Additionally, the increased volumes of decane required for large-scale production necessitate careful consideration of storage, handling, and safety protocols.
Despite these challenges, the potential for decane-enhanced polymerization processes to be scaled up effectively is significant. Ongoing research and development efforts are focused on optimizing reactor designs, improving separation technologies, and enhancing process control strategies to fully leverage the benefits of decane in industrial-scale polymerization operations.
One of the primary benefits of using decane in polymerization is its ability to enhance the propagation rate of the chain reaction. This increased rate of polymerization can lead to higher productivity and efficiency when scaling up production. As the process is scaled, the accelerated reaction kinetics can help maintain consistent product quality across larger batch sizes.
The low reactivity of decane with most monomers and initiators makes it an ideal candidate for large-scale operations. This chemical stability reduces the risk of unwanted side reactions that could compromise product quality or process safety when scaling up. Additionally, decane's low volatility at typical polymerization temperatures contributes to better process control in larger reactors.
From an engineering perspective, the use of decane as a diluent can improve heat transfer in polymerization reactors. This becomes increasingly important as reactor size increases, helping to maintain uniform temperature distribution and prevent hot spots that could lead to product inconsistencies or safety hazards in large-scale production.
The relatively low cost and wide availability of decane make it an economically viable option for industrial-scale processes. As production scales up, the cost-effectiveness of using decane as a facilitator becomes more pronounced, potentially leading to reduced overall production costs.
However, challenges in scalability do exist. The recovery and recycling of decane from the polymer product can become more complex at larger scales. Efficient separation techniques must be developed to ensure economic viability and minimize environmental impact. Additionally, the increased volumes of decane required for large-scale production necessitate careful consideration of storage, handling, and safety protocols.
Despite these challenges, the potential for decane-enhanced polymerization processes to be scaled up effectively is significant. Ongoing research and development efforts are focused on optimizing reactor designs, improving separation technologies, and enhancing process control strategies to fully leverage the benefits of decane in industrial-scale polymerization operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!