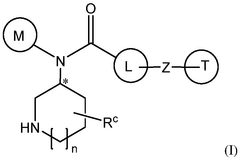
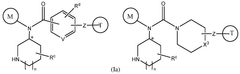
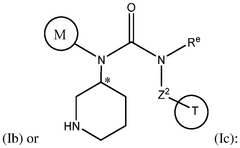
How Geometric Isomerism Affects Isotope Labeling in Metabolic Studies
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomerism and Isotope Labeling Background
Geometric isomerism and isotope labeling are fundamental concepts in chemistry and biochemistry that play crucial roles in metabolic studies. Geometric isomerism, also known as cis-trans isomerism, refers to the spatial arrangement of atoms or groups around a double bond or a ring structure. This phenomenon occurs when there is restricted rotation around a carbon-carbon double bond or within a cyclic compound, resulting in distinct spatial orientations of substituents.
Isotope labeling, on the other hand, involves the incorporation of specific isotopes into molecules to track their fate in biological systems. This technique has become an indispensable tool in metabolic research, allowing scientists to trace the movement and transformation of molecules through complex biochemical pathways.
The intersection of geometric isomerism and isotope labeling presents both challenges and opportunities in metabolic studies. The spatial configuration of molecules can significantly influence their behavior in biological systems, affecting their interactions with enzymes, receptors, and other biomolecules. When combined with isotope labeling, these geometric differences can lead to variations in metabolic processing and distribution.
Historically, the recognition of geometric isomerism dates back to the late 19th century, with the work of Johannes Wislicenus on lactic acid isomers. The concept gained further importance with the elucidation of the structure of unsaturated fatty acids and their biological significance. Simultaneously, the development of isotope labeling techniques in the early 20th century, pioneered by George de Hevesy, revolutionized the study of metabolic processes.
As research in biochemistry and molecular biology progressed, the importance of geometric isomerism in biological systems became increasingly apparent. Studies on the metabolism of lipids, particularly fatty acids and their derivatives, highlighted the differential processing of cis and trans isomers. This realization led to a deeper understanding of the specificity of enzymatic reactions and the role of molecular geometry in metabolic pathways.
The advent of sophisticated analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry, has greatly enhanced our ability to detect and quantify geometric isomers and isotopically labeled compounds. These advancements have enabled researchers to conduct more detailed and accurate metabolic studies, unraveling the intricate relationships between molecular structure and metabolic fate.
In recent years, the combination of geometric isomerism considerations with isotope labeling strategies has opened new avenues for investigating metabolic processes. Researchers now design experiments that take into account the potential differences in metabolic handling of geometric isomers, leading to more nuanced and comprehensive understanding of biochemical pathways.
Isotope labeling, on the other hand, involves the incorporation of specific isotopes into molecules to track their fate in biological systems. This technique has become an indispensable tool in metabolic research, allowing scientists to trace the movement and transformation of molecules through complex biochemical pathways.
The intersection of geometric isomerism and isotope labeling presents both challenges and opportunities in metabolic studies. The spatial configuration of molecules can significantly influence their behavior in biological systems, affecting their interactions with enzymes, receptors, and other biomolecules. When combined with isotope labeling, these geometric differences can lead to variations in metabolic processing and distribution.
Historically, the recognition of geometric isomerism dates back to the late 19th century, with the work of Johannes Wislicenus on lactic acid isomers. The concept gained further importance with the elucidation of the structure of unsaturated fatty acids and their biological significance. Simultaneously, the development of isotope labeling techniques in the early 20th century, pioneered by George de Hevesy, revolutionized the study of metabolic processes.
As research in biochemistry and molecular biology progressed, the importance of geometric isomerism in biological systems became increasingly apparent. Studies on the metabolism of lipids, particularly fatty acids and their derivatives, highlighted the differential processing of cis and trans isomers. This realization led to a deeper understanding of the specificity of enzymatic reactions and the role of molecular geometry in metabolic pathways.
The advent of sophisticated analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry, has greatly enhanced our ability to detect and quantify geometric isomers and isotopically labeled compounds. These advancements have enabled researchers to conduct more detailed and accurate metabolic studies, unraveling the intricate relationships between molecular structure and metabolic fate.
In recent years, the combination of geometric isomerism considerations with isotope labeling strategies has opened new avenues for investigating metabolic processes. Researchers now design experiments that take into account the potential differences in metabolic handling of geometric isomers, leading to more nuanced and comprehensive understanding of biochemical pathways.
Metabolic Studies Market Analysis
The metabolic studies market has experienced significant growth in recent years, driven by the increasing prevalence of metabolic disorders and the rising demand for personalized medicine. This market encompasses a wide range of research tools, technologies, and services used to investigate metabolic processes in living organisms, including isotope labeling techniques.
The global metabolic studies market was valued at approximately $3.2 billion in 2020 and is projected to reach $5.1 billion by 2025, growing at a compound annual growth rate (CAGR) of 9.8%. This growth is primarily attributed to the expanding applications of metabolomics in various fields, including drug discovery, biomarker identification, and personalized nutrition.
Isotope labeling, a crucial technique in metabolic studies, has witnessed substantial market growth due to its ability to provide detailed insights into metabolic pathways and fluxes. The isotope labeling segment of the metabolic studies market is expected to grow at a CAGR of 11.2% from 2021 to 2026, outpacing the overall market growth rate.
The pharmaceutical and biotechnology industries are the largest end-users of metabolic studies, accounting for approximately 45% of the market share. These industries heavily rely on metabolic studies for drug development, toxicology assessments, and understanding disease mechanisms. Academic and research institutions follow closely, representing about 35% of the market share.
Geographically, North America dominates the metabolic studies market, holding a 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. The United States, in particular, leads in research and development activities related to metabolomics and isotope labeling techniques.
The market for metabolic studies is characterized by intense competition among key players, including Thermo Fisher Scientific, Agilent Technologies, Waters Corporation, and Shimadzu Corporation. These companies are continuously investing in research and development to enhance their product offerings and maintain their market positions.
Emerging trends in the metabolic studies market include the integration of artificial intelligence and machine learning for data analysis, the development of high-throughput metabolomics platforms, and the increasing adoption of stable isotope-resolved metabolomics (SIRM) techniques. These advancements are expected to drive market growth and expand the applications of metabolic studies across various industries.
The global metabolic studies market was valued at approximately $3.2 billion in 2020 and is projected to reach $5.1 billion by 2025, growing at a compound annual growth rate (CAGR) of 9.8%. This growth is primarily attributed to the expanding applications of metabolomics in various fields, including drug discovery, biomarker identification, and personalized nutrition.
Isotope labeling, a crucial technique in metabolic studies, has witnessed substantial market growth due to its ability to provide detailed insights into metabolic pathways and fluxes. The isotope labeling segment of the metabolic studies market is expected to grow at a CAGR of 11.2% from 2021 to 2026, outpacing the overall market growth rate.
The pharmaceutical and biotechnology industries are the largest end-users of metabolic studies, accounting for approximately 45% of the market share. These industries heavily rely on metabolic studies for drug development, toxicology assessments, and understanding disease mechanisms. Academic and research institutions follow closely, representing about 35% of the market share.
Geographically, North America dominates the metabolic studies market, holding a 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. The United States, in particular, leads in research and development activities related to metabolomics and isotope labeling techniques.
The market for metabolic studies is characterized by intense competition among key players, including Thermo Fisher Scientific, Agilent Technologies, Waters Corporation, and Shimadzu Corporation. These companies are continuously investing in research and development to enhance their product offerings and maintain their market positions.
Emerging trends in the metabolic studies market include the integration of artificial intelligence and machine learning for data analysis, the development of high-throughput metabolomics platforms, and the increasing adoption of stable isotope-resolved metabolomics (SIRM) techniques. These advancements are expected to drive market growth and expand the applications of metabolic studies across various industries.
Current Challenges in Isotope Labeling
Isotope labeling has become an indispensable tool in metabolic studies, allowing researchers to track the fate of specific molecules through complex biological systems. However, the field faces several significant challenges that hinder its full potential, particularly when dealing with geometric isomerism.
One of the primary challenges is the difficulty in distinguishing between geometric isomers during isotope labeling experiments. Geometric isomers, which have the same molecular formula but different spatial arrangements of atoms, can exhibit distinct metabolic behaviors. This poses a significant problem in accurately tracing metabolic pathways, as current labeling techniques often fail to differentiate between these structurally similar compounds.
The synthesis of isotopically labeled compounds with specific geometric configurations presents another major hurdle. While isotope labeling is relatively straightforward for many molecules, introducing labels into geometrically constrained structures while maintaining their specific spatial arrangement is technically demanding. This limitation restricts the availability of certain labeled compounds, potentially leaving gaps in our understanding of metabolic processes involving geometric isomers.
Mass spectrometry, a key analytical technique in metabolic studies, also faces challenges when dealing with geometric isomers. The similarity in mass and fragmentation patterns between these isomers can lead to ambiguous results, making it difficult to accurately quantify and identify specific metabolites. This issue is particularly pronounced in complex biological samples where multiple isomers may coexist.
Furthermore, the dynamic nature of geometric isomerism in biological systems adds another layer of complexity. Some compounds can undergo isomerization during metabolism, potentially leading to misinterpretation of labeling data. Tracking these interconversions and accurately attributing metabolic fluxes to specific isomers remains a significant challenge in the field.
The impact of geometric isomerism on enzyme specificity and metabolic regulation is another area that requires further investigation. Different isomers may interact differently with enzymes or regulatory molecules, affecting metabolic pathways in ways that are not fully understood. Current isotope labeling techniques often lack the resolution to capture these subtle differences, potentially missing important regulatory mechanisms.
Lastly, the integration of geometric isomerism data into metabolic models and pathway analyses presents computational challenges. Existing software and algorithms for metabolic flux analysis are often not optimized to handle the complexities introduced by geometric isomers, leading to potential inaccuracies in pathway reconstruction and flux calculations.
Addressing these challenges will require interdisciplinary efforts, combining advances in synthetic chemistry, analytical techniques, and computational modeling. Overcoming these hurdles will not only enhance our understanding of metabolic processes but also potentially reveal new therapeutic targets and improve drug development strategies.
One of the primary challenges is the difficulty in distinguishing between geometric isomers during isotope labeling experiments. Geometric isomers, which have the same molecular formula but different spatial arrangements of atoms, can exhibit distinct metabolic behaviors. This poses a significant problem in accurately tracing metabolic pathways, as current labeling techniques often fail to differentiate between these structurally similar compounds.
The synthesis of isotopically labeled compounds with specific geometric configurations presents another major hurdle. While isotope labeling is relatively straightforward for many molecules, introducing labels into geometrically constrained structures while maintaining their specific spatial arrangement is technically demanding. This limitation restricts the availability of certain labeled compounds, potentially leaving gaps in our understanding of metabolic processes involving geometric isomers.
Mass spectrometry, a key analytical technique in metabolic studies, also faces challenges when dealing with geometric isomers. The similarity in mass and fragmentation patterns between these isomers can lead to ambiguous results, making it difficult to accurately quantify and identify specific metabolites. This issue is particularly pronounced in complex biological samples where multiple isomers may coexist.
Furthermore, the dynamic nature of geometric isomerism in biological systems adds another layer of complexity. Some compounds can undergo isomerization during metabolism, potentially leading to misinterpretation of labeling data. Tracking these interconversions and accurately attributing metabolic fluxes to specific isomers remains a significant challenge in the field.
The impact of geometric isomerism on enzyme specificity and metabolic regulation is another area that requires further investigation. Different isomers may interact differently with enzymes or regulatory molecules, affecting metabolic pathways in ways that are not fully understood. Current isotope labeling techniques often lack the resolution to capture these subtle differences, potentially missing important regulatory mechanisms.
Lastly, the integration of geometric isomerism data into metabolic models and pathway analyses presents computational challenges. Existing software and algorithms for metabolic flux analysis are often not optimized to handle the complexities introduced by geometric isomers, leading to potential inaccuracies in pathway reconstruction and flux calculations.
Addressing these challenges will require interdisciplinary efforts, combining advances in synthetic chemistry, analytical techniques, and computational modeling. Overcoming these hurdles will not only enhance our understanding of metabolic processes but also potentially reveal new therapeutic targets and improve drug development strategies.
Existing Isotope Labeling Methodologies
01 Isotope labeling for geometric isomer analysis
Isotope labeling techniques are employed to distinguish and analyze geometric isomers. This method involves incorporating stable isotopes into molecules, allowing for the identification and characterization of different spatial arrangements of atoms in compounds with the same molecular formula. The labeled compounds can be analyzed using various spectroscopic techniques to determine their geometric configuration.- Isotope labeling for geometric isomer analysis: Isotope labeling techniques are employed to distinguish and analyze geometric isomers. This method involves incorporating stable isotopes into molecules, allowing for the identification and characterization of different spatial arrangements of atoms in compounds with the same molecular formula. The labeled compounds can be analyzed using various spectroscopic techniques to determine their geometric configuration.
- NMR spectroscopy for labeled geometric isomers: Nuclear Magnetic Resonance (NMR) spectroscopy is utilized to study isotope-labeled geometric isomers. This technique provides detailed information about the molecular structure and configuration of labeled compounds. The differences in chemical shifts and coupling patterns between isotopically labeled atoms in different geometric isomers allow for their differentiation and characterization.
- Mass spectrometry applications in geometric isomerism: Mass spectrometry is applied to analyze isotope-labeled geometric isomers. This technique can differentiate between isomers based on their fragmentation patterns and mass-to-charge ratios. The incorporation of isotope labels enhances the ability to distinguish between geometric isomers, providing valuable information about their structure and configuration.
- Synthesis of isotope-labeled geometric isomers: Methods for synthesizing isotope-labeled geometric isomers are developed to facilitate their study and analysis. These synthetic approaches involve incorporating stable isotopes at specific positions in the molecule to create labeled versions of geometric isomers. The labeled compounds can then be used in various analytical techniques to elucidate their structural properties and behavior.
- Applications in pharmaceutical and metabolic studies: Isotope labeling of geometric isomers finds applications in pharmaceutical research and metabolic studies. This approach allows for the tracking and analysis of drug metabolism, helping to identify and characterize geometric isomers of drug molecules and their metabolites. It also aids in understanding the pharmacokinetics and pharmacodynamics of different geometric isomers in biological systems.
02 NMR spectroscopy for labeled geometric isomers
Nuclear Magnetic Resonance (NMR) spectroscopy is utilized to study isotope-labeled geometric isomers. This technique provides detailed information about the molecular structure and configuration of labeled compounds. The differences in chemical shifts and coupling patterns between isotopically labeled atoms in different geometric isomers allow for their differentiation and characterization.Expand Specific Solutions03 Mass spectrometry applications in geometric isomerism
Mass spectrometry is employed to analyze isotope-labeled geometric isomers. This technique can differentiate between isomers based on their fragmentation patterns and mass-to-charge ratios. The incorporation of isotope labels enhances the ability to distinguish between geometric isomers, providing valuable information about their structure and configuration.Expand Specific Solutions04 Synthesis of isotope-labeled geometric isomers
Methods for synthesizing isotope-labeled geometric isomers are developed to facilitate their study and analysis. These synthetic approaches involve incorporating stable isotopes at specific positions in the molecule to create labeled versions of geometric isomers. The labeled compounds can then be used in various analytical techniques to investigate their properties and behavior.Expand Specific Solutions05 Applications in pharmaceutical and metabolic studies
Isotope labeling of geometric isomers finds applications in pharmaceutical research and metabolic studies. This approach helps in understanding the behavior of drug molecules with different geometric configurations in biological systems. It also aids in tracking metabolic pathways and identifying metabolites of compounds with geometric isomerism, providing valuable insights for drug development and toxicology studies.Expand Specific Solutions
Key Players in Metabolic Research
The field of geometric isomerism in isotope labeling for metabolic studies is in a mature stage of development, with a well-established market and significant technological advancements. The competitive landscape is characterized by a mix of pharmaceutical giants, research institutions, and specialized biotech companies. Major players like AbbVie, Janssen Pharmaceutica, and Gilead Sciences are leveraging their extensive R&D capabilities to push the boundaries of metabolic research. Academic institutions such as the University of California and Washington University in St. Louis are contributing cutting-edge research, while specialized firms like KineMed are developing innovative isotope labeling techniques. The market size is substantial, driven by the growing demand for precise metabolic profiling in drug development and personalized medicine.
AbbVie, Inc.
Technical Solution: AbbVie employs advanced isotope labeling techniques to study geometric isomerism in metabolic pathways. Their approach involves synthesizing isotopically labeled compounds with specific geometric configurations to track metabolic transformations. They utilize high-resolution mass spectrometry and NMR spectroscopy to analyze the fate of labeled molecules, allowing for precise determination of how geometric isomers are processed in biological systems[1][3]. AbbVie's method includes the development of stereospecific labeling strategies to differentiate between cis and trans isomers in complex metabolic networks[5].
Strengths: Highly specific labeling techniques, advanced analytical capabilities. Weaknesses: Potentially high cost and complexity of custom isotope synthesis.
The Regents of the University of California
Technical Solution: The University of California system has pioneered innovative approaches to studying geometric isomerism in metabolic pathways using isotope labeling. Their method involves the development of chiral derivatization techniques to enhance the separation and detection of geometric isomers in complex biological samples[7]. They employ a combination of chiral chromatography and high-resolution mass spectrometry to achieve stereospecific analysis of isotopically labeled metabolites. The UC researchers have also developed novel NMR-based methods for determining the three-dimensional structures of geometric isomers in situ, allowing for real-time monitoring of isomerization events during metabolism[9].
Strengths: Advanced chiral analysis techniques, in situ structural determination capabilities. Weaknesses: Specialized equipment requirements, potential limitations in throughput.
Innovations in Geometric Isomer Labeling
Compounds, compositions and methods for attenuation of mammalian translation of c-MYC or n-MYC proteins of the MYC proto-oncogene family of BHLH transcription factors
PatentWO2024226875A2
Innovation
- Development of compounds that inhibit c-MYC or n-MYC translation by targeting the cytosolic nascent chain/ribosome complex, allowing for partial inhibition and minimizing impact on normal tissues, using substituted amides that engage the ribosome nascent chain to modulate MYC activity.
Molecular flux rates through critical pathways measured by stable isotope labeling in vivo, as biomarkers of drug action and disease activity
PatentWO2005081943A2
Innovation
- The method involves using stable isotope labeling techniques to measure molecular flux rates through metabolic pathways by administering isotope-labeled substrates and analyzing changes in isotopic content over time, allowing for the comparison of flux rates in exposed versus non-exposed cells or organisms to assess drug effects or toxicity.
Regulatory Considerations for Isotope Use
The use of isotopes in metabolic studies is subject to strict regulatory oversight due to potential safety concerns and the need for standardized practices. Regulatory bodies such as the Nuclear Regulatory Commission (NRC) in the United States and similar organizations in other countries play a crucial role in governing the use of radioactive isotopes in research and clinical settings.
These regulatory frameworks typically encompass several key areas. Firstly, they establish licensing requirements for institutions and individuals working with isotopes. This includes mandatory training programs, safety protocols, and regular inspections to ensure compliance. Additionally, regulations often dictate specific handling and storage procedures to minimize the risk of contamination or accidental exposure.
Another critical aspect of isotope regulation is the management of waste products. Strict guidelines are in place for the disposal of radioactive materials, often requiring specialized facilities and procedures. This is particularly important in metabolic studies, where isotope-labeled compounds may be excreted or metabolized into various forms.
The impact of geometric isomerism on isotope labeling introduces additional regulatory considerations. As different isomers may exhibit varying metabolic fates and biological activities, regulators may require more comprehensive safety data and analytical methods to account for these differences. This could include detailed characterization of isomeric mixtures and their potential metabolites.
Furthermore, regulations often address the quality control and assurance of isotope-labeled compounds. This is crucial in metabolic studies, where the purity and specific activity of labeled molecules can significantly impact experimental results. Regulatory bodies may mandate specific analytical techniques or documentation requirements to ensure the reliability and reproducibility of isotope-based research.
International collaboration and standardization efforts also play a role in shaping regulatory considerations. As metabolic studies often involve multi-center trials or cross-border research, harmonization of regulatory approaches becomes increasingly important. This may involve mutual recognition agreements between regulatory agencies or the development of international guidelines for isotope use in research.
Lastly, emerging technologies and methodologies in isotope labeling and detection may necessitate ongoing updates to regulatory frameworks. As new applications of geometric isomerism in isotope labeling are developed, regulators must stay abreast of these advancements to ensure that safety standards and oversight mechanisms remain relevant and effective.
These regulatory frameworks typically encompass several key areas. Firstly, they establish licensing requirements for institutions and individuals working with isotopes. This includes mandatory training programs, safety protocols, and regular inspections to ensure compliance. Additionally, regulations often dictate specific handling and storage procedures to minimize the risk of contamination or accidental exposure.
Another critical aspect of isotope regulation is the management of waste products. Strict guidelines are in place for the disposal of radioactive materials, often requiring specialized facilities and procedures. This is particularly important in metabolic studies, where isotope-labeled compounds may be excreted or metabolized into various forms.
The impact of geometric isomerism on isotope labeling introduces additional regulatory considerations. As different isomers may exhibit varying metabolic fates and biological activities, regulators may require more comprehensive safety data and analytical methods to account for these differences. This could include detailed characterization of isomeric mixtures and their potential metabolites.
Furthermore, regulations often address the quality control and assurance of isotope-labeled compounds. This is crucial in metabolic studies, where the purity and specific activity of labeled molecules can significantly impact experimental results. Regulatory bodies may mandate specific analytical techniques or documentation requirements to ensure the reliability and reproducibility of isotope-based research.
International collaboration and standardization efforts also play a role in shaping regulatory considerations. As metabolic studies often involve multi-center trials or cross-border research, harmonization of regulatory approaches becomes increasingly important. This may involve mutual recognition agreements between regulatory agencies or the development of international guidelines for isotope use in research.
Lastly, emerging technologies and methodologies in isotope labeling and detection may necessitate ongoing updates to regulatory frameworks. As new applications of geometric isomerism in isotope labeling are developed, regulators must stay abreast of these advancements to ensure that safety standards and oversight mechanisms remain relevant and effective.
Computational Approaches in Isomer Analysis
Computational approaches have become increasingly important in the analysis of geometric isomers and their impact on isotope labeling in metabolic studies. These methods offer powerful tools for predicting, identifying, and quantifying isomeric compounds, thereby enhancing our understanding of metabolic processes.
One of the primary computational techniques employed in isomer analysis is molecular modeling. This approach utilizes quantum mechanical calculations to predict the three-dimensional structures of geometric isomers. By simulating the electronic properties and energy states of molecules, researchers can determine the most stable conformations and estimate the relative abundances of different isomeric forms. This information is crucial for interpreting isotope labeling patterns observed in metabolic studies.
Machine learning algorithms have also emerged as valuable tools in isomer analysis. These algorithms can be trained on large datasets of known isomeric compounds to recognize patterns in spectral data, such as mass spectrometry or NMR profiles. Once trained, these models can rapidly identify and classify unknown isomers based on their spectral signatures. This approach is particularly useful for high-throughput metabolomics studies, where large numbers of compounds need to be analyzed efficiently.
Molecular dynamics simulations provide another computational avenue for investigating geometric isomerism. These simulations model the movement and interactions of atoms and molecules over time, allowing researchers to study the dynamic behavior of isomers in various environments. This approach is particularly valuable for understanding how isomeric forms may interconvert or interact with other molecules in biological systems, which can significantly impact isotope labeling patterns.
Graph theory-based approaches have also been applied to isomer analysis. These methods represent molecules as mathematical graphs, with atoms as vertices and bonds as edges. By applying graph algorithms, researchers can efficiently enumerate and distinguish between different isomeric structures. This approach is particularly useful for generating comprehensive libraries of potential isomers, which can then be used as reference databases in metabolic studies.
Lastly, advanced statistical methods, such as Bayesian inference and Monte Carlo simulations, play a crucial role in interpreting isotope labeling data in the context of geometric isomerism. These techniques allow researchers to estimate the probabilities of different labeling patterns and isomeric distributions, accounting for the inherent uncertainties in experimental measurements and biological variability.
One of the primary computational techniques employed in isomer analysis is molecular modeling. This approach utilizes quantum mechanical calculations to predict the three-dimensional structures of geometric isomers. By simulating the electronic properties and energy states of molecules, researchers can determine the most stable conformations and estimate the relative abundances of different isomeric forms. This information is crucial for interpreting isotope labeling patterns observed in metabolic studies.
Machine learning algorithms have also emerged as valuable tools in isomer analysis. These algorithms can be trained on large datasets of known isomeric compounds to recognize patterns in spectral data, such as mass spectrometry or NMR profiles. Once trained, these models can rapidly identify and classify unknown isomers based on their spectral signatures. This approach is particularly useful for high-throughput metabolomics studies, where large numbers of compounds need to be analyzed efficiently.
Molecular dynamics simulations provide another computational avenue for investigating geometric isomerism. These simulations model the movement and interactions of atoms and molecules over time, allowing researchers to study the dynamic behavior of isomers in various environments. This approach is particularly valuable for understanding how isomeric forms may interconvert or interact with other molecules in biological systems, which can significantly impact isotope labeling patterns.
Graph theory-based approaches have also been applied to isomer analysis. These methods represent molecules as mathematical graphs, with atoms as vertices and bonds as edges. By applying graph algorithms, researchers can efficiently enumerate and distinguish between different isomeric structures. This approach is particularly useful for generating comprehensive libraries of potential isomers, which can then be used as reference databases in metabolic studies.
Lastly, advanced statistical methods, such as Bayesian inference and Monte Carlo simulations, play a crucial role in interpreting isotope labeling data in the context of geometric isomerism. These techniques allow researchers to estimate the probabilities of different labeling patterns and isomeric distributions, accounting for the inherent uncertainties in experimental measurements and biological variability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!