How Geometric Isomers Affect Cellular Uptake of Nanoparticles
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanoparticle Isomers and Cellular Uptake Overview
Nanoparticle isomers have emerged as a crucial factor in the field of nanomedicine, particularly in their interaction with cellular systems. These geometric variations of nanoparticles with identical chemical compositions can significantly influence their cellular uptake, a key determinant in the efficacy of nanoparticle-based drug delivery systems and diagnostic tools.
The concept of geometric isomerism in nanoparticles encompasses a wide range of structural variations, including differences in shape, aspect ratio, and surface curvature. These subtle alterations can lead to profound changes in how nanoparticles interact with cell membranes, proteins, and intracellular components, ultimately affecting their internalization and distribution within cells.
Recent studies have demonstrated that the shape of nanoparticles plays a critical role in their cellular uptake. For instance, rod-shaped nanoparticles often exhibit higher uptake rates compared to their spherical counterparts, attributed to their increased surface area and unique interaction with cell membranes. Similarly, the aspect ratio of rod-like nanoparticles has been shown to influence their internalization pathway and intracellular fate.
Surface curvature is another geometric factor that significantly impacts cellular uptake. Nanoparticles with high surface curvature, such as small spheres or sharp-edged structures, can more easily penetrate cell membranes compared to those with lower curvature. This phenomenon is partly due to the differential wrapping of cell membranes around nanoparticles of varying curvatures.
The influence of geometric isomers on cellular uptake extends beyond mere internalization rates. Different isomers can trigger distinct cellular responses, activate various endocytic pathways, and even target specific subcellular compartments. This selectivity opens up new possibilities for tailoring nanoparticle designs to achieve desired therapeutic outcomes or diagnostic accuracy.
Understanding the relationship between nanoparticle geometry and cellular uptake is crucial for advancing the field of nanomedicine. It enables the rational design of nanoparticles with optimized shapes and structures for specific biomedical applications. Moreover, this knowledge can be leveraged to enhance drug delivery efficiency, improve cellular targeting, and minimize undesired side effects in nanoparticle-based therapies.
As research in this area progresses, the complexity of geometric isomer effects on cellular uptake continues to unfold. Factors such as surface chemistry, particle size, and the physiological environment interact with geometric properties, creating a multifaceted landscape of nanoparticle-cell interactions. This intricate interplay underscores the need for comprehensive studies and sophisticated modeling approaches to fully elucidate the impact of geometric isomers on cellular uptake of nanoparticles.
The concept of geometric isomerism in nanoparticles encompasses a wide range of structural variations, including differences in shape, aspect ratio, and surface curvature. These subtle alterations can lead to profound changes in how nanoparticles interact with cell membranes, proteins, and intracellular components, ultimately affecting their internalization and distribution within cells.
Recent studies have demonstrated that the shape of nanoparticles plays a critical role in their cellular uptake. For instance, rod-shaped nanoparticles often exhibit higher uptake rates compared to their spherical counterparts, attributed to their increased surface area and unique interaction with cell membranes. Similarly, the aspect ratio of rod-like nanoparticles has been shown to influence their internalization pathway and intracellular fate.
Surface curvature is another geometric factor that significantly impacts cellular uptake. Nanoparticles with high surface curvature, such as small spheres or sharp-edged structures, can more easily penetrate cell membranes compared to those with lower curvature. This phenomenon is partly due to the differential wrapping of cell membranes around nanoparticles of varying curvatures.
The influence of geometric isomers on cellular uptake extends beyond mere internalization rates. Different isomers can trigger distinct cellular responses, activate various endocytic pathways, and even target specific subcellular compartments. This selectivity opens up new possibilities for tailoring nanoparticle designs to achieve desired therapeutic outcomes or diagnostic accuracy.
Understanding the relationship between nanoparticle geometry and cellular uptake is crucial for advancing the field of nanomedicine. It enables the rational design of nanoparticles with optimized shapes and structures for specific biomedical applications. Moreover, this knowledge can be leveraged to enhance drug delivery efficiency, improve cellular targeting, and minimize undesired side effects in nanoparticle-based therapies.
As research in this area progresses, the complexity of geometric isomer effects on cellular uptake continues to unfold. Factors such as surface chemistry, particle size, and the physiological environment interact with geometric properties, creating a multifaceted landscape of nanoparticle-cell interactions. This intricate interplay underscores the need for comprehensive studies and sophisticated modeling approaches to fully elucidate the impact of geometric isomers on cellular uptake of nanoparticles.
Market Demand for Targeted Drug Delivery
The market demand for targeted drug delivery systems utilizing nanoparticles has been steadily increasing due to their potential to revolutionize therapeutic approaches across various medical fields. This growing interest is driven by the ability of nanoparticles to enhance drug efficacy while minimizing side effects, a crucial factor in improving patient outcomes and quality of life.
In oncology, the demand for targeted nanoparticle-based drug delivery is particularly high. Cancer treatments often suffer from poor specificity, leading to severe side effects. Nanoparticles offer a solution by selectively accumulating in tumor tissues through the enhanced permeability and retention (EPR) effect. This targeted approach not only improves therapeutic efficacy but also reduces systemic toxicity, addressing a significant unmet need in cancer treatment.
The pharmaceutical industry has recognized the potential of nanoparticle-based drug delivery systems, leading to increased investment in research and development. Major pharmaceutical companies are actively pursuing partnerships with nanotechnology firms to incorporate these advanced delivery systems into their drug pipelines. This trend is expected to continue, driving market growth and innovation in the coming years.
Beyond oncology, there is a growing demand for targeted drug delivery in other therapeutic areas such as neurology, cardiovascular diseases, and infectious diseases. In neurology, nanoparticles capable of crossing the blood-brain barrier offer new possibilities for treating neurological disorders. Similarly, in cardiovascular medicine, targeted delivery of therapeutics to atherosclerotic plaques could significantly improve treatment outcomes for heart disease patients.
The global market for targeted drug delivery systems is projected to experience substantial growth. Factors contributing to this growth include the rising prevalence of chronic diseases, increasing healthcare expenditure, and advancements in nanotechnology. Additionally, the growing aging population worldwide is expected to further drive demand for more effective and less toxic treatment options.
Regulatory agencies have also recognized the potential of nanoparticle-based drug delivery systems, leading to the development of specific guidelines for their evaluation and approval. This regulatory clarity is expected to facilitate the commercialization of nanoparticle-based therapeutics, further stimulating market growth and investment in this field.
As research continues to uncover the impact of geometric isomers on cellular uptake of nanoparticles, the demand for more sophisticated and tailored drug delivery systems is likely to increase. This knowledge could lead to the development of highly specific nanocarriers, capable of targeting particular cell types or tissues with unprecedented precision, thus opening new avenues for personalized medicine and expanding the market for targeted drug delivery systems.
In oncology, the demand for targeted nanoparticle-based drug delivery is particularly high. Cancer treatments often suffer from poor specificity, leading to severe side effects. Nanoparticles offer a solution by selectively accumulating in tumor tissues through the enhanced permeability and retention (EPR) effect. This targeted approach not only improves therapeutic efficacy but also reduces systemic toxicity, addressing a significant unmet need in cancer treatment.
The pharmaceutical industry has recognized the potential of nanoparticle-based drug delivery systems, leading to increased investment in research and development. Major pharmaceutical companies are actively pursuing partnerships with nanotechnology firms to incorporate these advanced delivery systems into their drug pipelines. This trend is expected to continue, driving market growth and innovation in the coming years.
Beyond oncology, there is a growing demand for targeted drug delivery in other therapeutic areas such as neurology, cardiovascular diseases, and infectious diseases. In neurology, nanoparticles capable of crossing the blood-brain barrier offer new possibilities for treating neurological disorders. Similarly, in cardiovascular medicine, targeted delivery of therapeutics to atherosclerotic plaques could significantly improve treatment outcomes for heart disease patients.
The global market for targeted drug delivery systems is projected to experience substantial growth. Factors contributing to this growth include the rising prevalence of chronic diseases, increasing healthcare expenditure, and advancements in nanotechnology. Additionally, the growing aging population worldwide is expected to further drive demand for more effective and less toxic treatment options.
Regulatory agencies have also recognized the potential of nanoparticle-based drug delivery systems, leading to the development of specific guidelines for their evaluation and approval. This regulatory clarity is expected to facilitate the commercialization of nanoparticle-based therapeutics, further stimulating market growth and investment in this field.
As research continues to uncover the impact of geometric isomers on cellular uptake of nanoparticles, the demand for more sophisticated and tailored drug delivery systems is likely to increase. This knowledge could lead to the development of highly specific nanocarriers, capable of targeting particular cell types or tissues with unprecedented precision, thus opening new avenues for personalized medicine and expanding the market for targeted drug delivery systems.
Current Challenges in Nanoparticle-Cell Interactions
The field of nanoparticle-cell interactions faces several significant challenges that hinder the full realization of nanoparticle potential in biomedical applications. One of the primary obstacles is the complexity of cellular uptake mechanisms, which vary depending on the nanoparticle's physicochemical properties and the target cell type. This variability makes it difficult to predict and control nanoparticle internalization, limiting the efficacy of targeted drug delivery and diagnostic imaging.
Another major challenge lies in understanding the influence of nanoparticle geometry on cellular uptake. While size and surface chemistry have been extensively studied, the impact of shape and geometric isomerism remains less explored. Researchers struggle to establish clear correlations between nanoparticle geometry and cellular internalization rates, as well as intracellular trafficking pathways. This knowledge gap impedes the design of optimized nanoparticles for specific biomedical applications.
The biological identity of nanoparticles in physiological environments presents another hurdle. Upon entering biological fluids, nanoparticles rapidly acquire a protein corona, which can significantly alter their surface properties and cellular interactions. Predicting and controlling the composition of this protein corona remains challenging, as it depends on numerous factors including nanoparticle geometry, surface chemistry, and the specific biological environment.
Toxicity concerns continue to be a significant challenge in nanoparticle-cell interactions. While many nanoparticles show promise in vitro, translating these results to in vivo applications often reveals unexpected toxicity issues. Understanding the long-term effects of nanoparticle exposure on cellular function and overall organism health remains a critical area of investigation.
The development of standardized protocols for assessing nanoparticle-cell interactions is another ongoing challenge. The lack of universally accepted methods for characterizing nanoparticle uptake, intracellular distribution, and biological effects hampers the comparison of results across different studies and impedes progress in the field.
Lastly, the challenge of scaling up nanoparticle production while maintaining consistent physicochemical properties, including geometric isomerism, presents a significant hurdle for clinical translation. Ensuring batch-to-batch reproducibility in terms of size, shape, and surface properties is crucial for reliable cellular uptake and biological effects, yet remains difficult to achieve at larger scales.
Another major challenge lies in understanding the influence of nanoparticle geometry on cellular uptake. While size and surface chemistry have been extensively studied, the impact of shape and geometric isomerism remains less explored. Researchers struggle to establish clear correlations between nanoparticle geometry and cellular internalization rates, as well as intracellular trafficking pathways. This knowledge gap impedes the design of optimized nanoparticles for specific biomedical applications.
The biological identity of nanoparticles in physiological environments presents another hurdle. Upon entering biological fluids, nanoparticles rapidly acquire a protein corona, which can significantly alter their surface properties and cellular interactions. Predicting and controlling the composition of this protein corona remains challenging, as it depends on numerous factors including nanoparticle geometry, surface chemistry, and the specific biological environment.
Toxicity concerns continue to be a significant challenge in nanoparticle-cell interactions. While many nanoparticles show promise in vitro, translating these results to in vivo applications often reveals unexpected toxicity issues. Understanding the long-term effects of nanoparticle exposure on cellular function and overall organism health remains a critical area of investigation.
The development of standardized protocols for assessing nanoparticle-cell interactions is another ongoing challenge. The lack of universally accepted methods for characterizing nanoparticle uptake, intracellular distribution, and biological effects hampers the comparison of results across different studies and impedes progress in the field.
Lastly, the challenge of scaling up nanoparticle production while maintaining consistent physicochemical properties, including geometric isomerism, presents a significant hurdle for clinical translation. Ensuring batch-to-batch reproducibility in terms of size, shape, and surface properties is crucial for reliable cellular uptake and biological effects, yet remains difficult to achieve at larger scales.
Existing Strategies for Enhancing Cellular Uptake
01 Nanoparticle design for enhanced cellular uptake
Optimizing nanoparticle properties such as size, shape, surface charge, and composition to improve cellular internalization. This includes developing strategies to overcome biological barriers and enhance targeting to specific cell types or intracellular compartments.- Nanoparticle design for enhanced cellular uptake: Optimizing nanoparticle properties such as size, shape, surface charge, and composition to improve cellular internalization. This includes the development of targeted nanoparticles that can specifically interact with cell surface receptors to facilitate uptake.
- Mechanisms of nanoparticle cellular uptake: Investigation of various cellular uptake pathways for nanoparticles, including endocytosis, phagocytosis, and direct penetration. Understanding these mechanisms helps in designing more effective nanoparticle-based drug delivery systems.
- Intracellular trafficking and fate of nanoparticles: Studying the intracellular movement and processing of nanoparticles after uptake, including their localization in specific organelles and potential degradation or exocytosis. This knowledge is crucial for developing nanoparticles that can effectively deliver their payload to target sites within cells.
- Enhancing nanoparticle cellular uptake through surface modifications: Modifying nanoparticle surfaces with various ligands, polymers, or biomolecules to improve their interaction with cell membranes and increase uptake efficiency. This includes strategies such as PEGylation, antibody conjugation, and cell-penetrating peptide functionalization.
- Evaluation and quantification of nanoparticle cellular uptake: Development and application of techniques to measure and analyze nanoparticle internalization by cells. This includes imaging methods, flow cytometry, and spectroscopic techniques to assess uptake efficiency and intracellular distribution of nanoparticles.
02 Functionalization of nanoparticles for improved uptake
Modifying nanoparticle surfaces with ligands, antibodies, or other biomolecules to facilitate receptor-mediated endocytosis or specific cellular targeting. This approach can increase the efficiency and selectivity of nanoparticle uptake by cells.Expand Specific Solutions03 Mechanisms of nanoparticle cellular internalization
Investigating the various pathways and mechanisms by which nanoparticles enter cells, including endocytosis, phagocytosis, and direct membrane penetration. Understanding these processes helps in designing more effective nanoparticle-based drug delivery systems.Expand Specific Solutions04 Intracellular trafficking and fate of nanoparticles
Studying the movement and distribution of nanoparticles within cells after uptake, including their localization in specific organelles and potential exocytosis. This knowledge is crucial for developing nanoparticles that can effectively deliver their payload to desired intracellular targets.Expand Specific Solutions05 Nanoparticle-based drug delivery systems
Developing nanocarriers for improved drug delivery, focusing on enhancing cellular uptake to increase therapeutic efficacy. This includes designing nanoparticles that can overcome biological barriers, protect drugs from degradation, and release their payload at specific intracellular locations.Expand Specific Solutions
Key Players in Nanomedicine Research
The field of geometric isomers affecting cellular uptake of nanoparticles is in an early developmental stage, with significant potential for growth. The market size is expanding as researchers explore applications in drug delivery and targeted therapies. While the technology is still maturing, several key players are advancing the field. The University of California, MIT, and Northwestern University are leading academic institutions conducting cutting-edge research. Companies like Nanobiotix and Spago Nanomedical are developing nanoparticle-based therapies, leveraging geometric isomer effects. Collaboration between academia and industry is driving innovation, with organizations like the French National Center for Scientific Research contributing to fundamental understanding. As the technology progresses, we can expect increased commercial interest and potential breakthroughs in nanomedicine applications.
The Regents of the University of California
Technical Solution: The University of California system has made significant contributions to understanding how geometric isomers affect nanoparticle cellular uptake. Their research teams have developed a library of nanoparticles with varying geometric isomers, systematically studying their interactions with different cell types[4]. They have pioneered the use of high-throughput screening methods to rapidly assess the impact of geometric isomers on cellular internalization rates and pathways[5]. UC researchers have also explored the influence of nanoparticle shape anisotropy on biodistribution and tumor targeting efficiency in vivo, providing valuable insights for cancer nanomedicine[6].
Strengths: Comprehensive nanoparticle library, high-throughput screening capabilities, and in vivo studies for translational research. Weaknesses: Potential variability in results across different UC campuses and research groups.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach to studying geometric isomers' effect on nanoparticle cellular uptake. They utilize advanced imaging techniques, such as super-resolution microscopy, to visualize the interaction between nanoparticles and cell membranes at the nanoscale level[1]. Their research focuses on designing nanoparticles with specific geometric isomers that can enhance cellular internalization. MIT's team has engineered shape-shifting nanoparticles that can change their geometry in response to cellular environmental cues, potentially improving targeted drug delivery[2]. They have also investigated the role of surface chemistry in conjunction with geometric isomers to optimize nanoparticle-cell interactions[3].
Strengths: Cutting-edge imaging technologies, innovative shape-shifting nanoparticle design, and comprehensive approach combining geometry and surface chemistry. Weaknesses: Potential challenges in scaling up production of complex nanoparticle designs for clinical applications.
Innovations in Geometric Isomer-Based Nanoparticles
Nanoparticles for selective tissue or cellular uptake
PatentPendingUS20220339294A1
Innovation
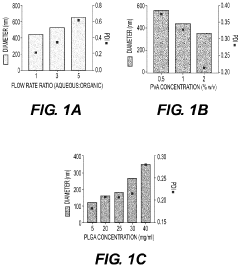
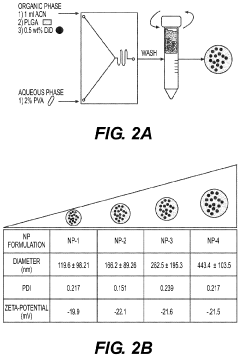
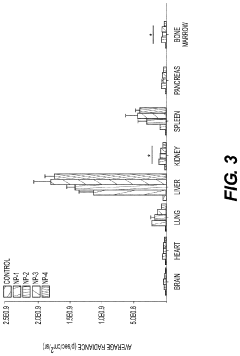
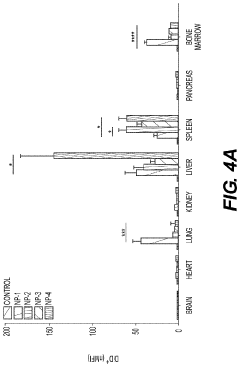
- Development of biodegradable polymeric nanoparticles with controlled sizes between 70 nm and 220 nm, manufactured using a microfluidic system, which are selectively taken up by lung cells and bone marrow cells without the need for targeting agents, allowing for the delivery of therapeutic, diagnostic, and prophylactic agents.
Regulatory Considerations for Nanomedicines
The regulatory landscape for nanomedicines is complex and evolving, reflecting the unique challenges posed by these innovative therapeutic approaches. Regulatory bodies worldwide, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have developed specific guidelines to address the safety, efficacy, and quality of nanomedicines. These guidelines take into account the distinctive properties of nanoparticles, such as their size, surface characteristics, and potential for geometric isomerism.
One of the primary regulatory considerations for nanomedicines is the characterization of the nanoparticles themselves. This includes detailed analysis of particle size distribution, shape, surface properties, and stability. For geometric isomers, regulatory agencies require comprehensive data on the different isomeric forms present in the formulation, their relative proportions, and their individual impacts on cellular uptake and therapeutic efficacy.
Safety assessment is another critical aspect of the regulatory process for nanomedicines. Regulatory bodies demand extensive preclinical and clinical studies to evaluate the potential toxicity and immunogenicity of nanoparticles. This includes assessing how geometric isomers may differentially interact with biological systems, potentially leading to varied safety profiles. Long-term safety studies are often required to address concerns about the persistence of nanoparticles in the body.
Manufacturing and quality control present unique challenges in the regulatory framework for nanomedicines. Regulatory agencies expect robust and reproducible manufacturing processes that can consistently produce nanoparticles with well-defined geometric properties. This includes implementing appropriate analytical methods to characterize and quantify different geometric isomers throughout the manufacturing process.
The regulatory pathway for nanomedicines often involves a case-by-case approach, given the diversity of nanoparticle designs and applications. Regulatory agencies may require additional data or studies specific to the geometric isomers present in a given formulation. This could include investigations into how different isomers affect biodistribution, pharmacokinetics, and cellular uptake mechanisms.
As the field of nanomedicine advances, regulatory frameworks continue to evolve. There is an ongoing dialogue between regulatory agencies, researchers, and industry to refine guidelines and address emerging challenges. This includes developing standardized methods for characterizing geometric isomers and assessing their impact on nanoparticle behavior in biological systems.
One of the primary regulatory considerations for nanomedicines is the characterization of the nanoparticles themselves. This includes detailed analysis of particle size distribution, shape, surface properties, and stability. For geometric isomers, regulatory agencies require comprehensive data on the different isomeric forms present in the formulation, their relative proportions, and their individual impacts on cellular uptake and therapeutic efficacy.
Safety assessment is another critical aspect of the regulatory process for nanomedicines. Regulatory bodies demand extensive preclinical and clinical studies to evaluate the potential toxicity and immunogenicity of nanoparticles. This includes assessing how geometric isomers may differentially interact with biological systems, potentially leading to varied safety profiles. Long-term safety studies are often required to address concerns about the persistence of nanoparticles in the body.
Manufacturing and quality control present unique challenges in the regulatory framework for nanomedicines. Regulatory agencies expect robust and reproducible manufacturing processes that can consistently produce nanoparticles with well-defined geometric properties. This includes implementing appropriate analytical methods to characterize and quantify different geometric isomers throughout the manufacturing process.
The regulatory pathway for nanomedicines often involves a case-by-case approach, given the diversity of nanoparticle designs and applications. Regulatory agencies may require additional data or studies specific to the geometric isomers present in a given formulation. This could include investigations into how different isomers affect biodistribution, pharmacokinetics, and cellular uptake mechanisms.
As the field of nanomedicine advances, regulatory frameworks continue to evolve. There is an ongoing dialogue between regulatory agencies, researchers, and industry to refine guidelines and address emerging challenges. This includes developing standardized methods for characterizing geometric isomers and assessing their impact on nanoparticle behavior in biological systems.
Toxicological Implications of Nanoparticle Isomers
The toxicological implications of nanoparticle isomers are a critical aspect of nanotechnology safety research. Geometric isomers of nanoparticles can exhibit significantly different biological behaviors, potentially leading to varied toxicological profiles.
One of the primary concerns is the differential cellular uptake of nanoparticle isomers. Studies have shown that the shape and orientation of nanoparticles can greatly influence their interaction with cell membranes and subsequent internalization. For instance, rod-shaped nanoparticles may have different uptake rates compared to their spherical counterparts, even when composed of the same material.
The toxicity of nanoparticle isomers can also vary due to differences in their surface reactivity. Geometric variations can expose different atomic arrangements on the particle surface, potentially altering their chemical reactivity and interaction with biological molecules. This can lead to varying degrees of oxidative stress, inflammation, or DNA damage within cells.
Furthermore, the biodistribution of nanoparticle isomers may differ, affecting their accumulation in specific organs or tissues. This can result in organ-specific toxicity profiles that are dependent on the geometric configuration of the nanoparticles. For example, certain isomers may more readily cross the blood-brain barrier, potentially leading to neurotoxic effects.
The immune system response to nanoparticle isomers is another crucial consideration. Different geometric configurations can trigger varied immune reactions, ranging from mild activation to severe inflammatory responses. This variability in immune recognition and processing can have significant implications for the overall toxicological impact of nanoparticles in biological systems.
Long-term persistence and degradation of nanoparticle isomers in the body are also important factors in assessing their toxicological implications. The geometric structure can influence the stability and degradation rate of nanoparticles, potentially leading to prolonged exposure or the generation of toxic byproducts during breakdown.
Understanding these toxicological implications is essential for the safe design and application of nanoparticles in various fields, including medicine, environmental remediation, and consumer products. It underscores the need for comprehensive toxicological assessments that consider not only the chemical composition but also the geometric isomerism of nanoparticles.
One of the primary concerns is the differential cellular uptake of nanoparticle isomers. Studies have shown that the shape and orientation of nanoparticles can greatly influence their interaction with cell membranes and subsequent internalization. For instance, rod-shaped nanoparticles may have different uptake rates compared to their spherical counterparts, even when composed of the same material.
The toxicity of nanoparticle isomers can also vary due to differences in their surface reactivity. Geometric variations can expose different atomic arrangements on the particle surface, potentially altering their chemical reactivity and interaction with biological molecules. This can lead to varying degrees of oxidative stress, inflammation, or DNA damage within cells.
Furthermore, the biodistribution of nanoparticle isomers may differ, affecting their accumulation in specific organs or tissues. This can result in organ-specific toxicity profiles that are dependent on the geometric configuration of the nanoparticles. For example, certain isomers may more readily cross the blood-brain barrier, potentially leading to neurotoxic effects.
The immune system response to nanoparticle isomers is another crucial consideration. Different geometric configurations can trigger varied immune reactions, ranging from mild activation to severe inflammatory responses. This variability in immune recognition and processing can have significant implications for the overall toxicological impact of nanoparticles in biological systems.
Long-term persistence and degradation of nanoparticle isomers in the body are also important factors in assessing their toxicological implications. The geometric structure can influence the stability and degradation rate of nanoparticles, potentially leading to prolonged exposure or the generation of toxic byproducts during breakdown.
Understanding these toxicological implications is essential for the safe design and application of nanoparticles in various fields, including medicine, environmental remediation, and consumer products. It underscores the need for comprehensive toxicological assessments that consider not only the chemical composition but also the geometric isomerism of nanoparticles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!