How Geometric Isomers Affect Transmembrane Ion Channel Activity
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Channel Isomers
Ion channels are integral membrane proteins that facilitate the passage of ions across biological membranes. These channels play crucial roles in various physiological processes, including nerve signaling, muscle contraction, and hormone secretion. The activity of ion channels can be significantly influenced by their geometric isomers, which are molecules with the same chemical formula but different spatial arrangements of atoms.
Geometric isomers of ion channels can arise from various structural modifications, such as changes in protein folding, subunit assembly, or post-translational modifications. These isomeric forms can exhibit distinct functional properties, leading to differences in ion selectivity, gating mechanisms, and overall channel activity. The impact of geometric isomers on ion channel function is a subject of intense research due to its implications for understanding cellular physiology and developing targeted therapeutic interventions.
One of the most well-studied examples of geometric isomerism affecting ion channel activity is the case of voltage-gated sodium channels. These channels exist in different conformational states, including closed, open, and inactivated. The transition between these states is influenced by the spatial arrangement of key structural elements, such as the voltage-sensing domain and the pore-forming region. Alterations in the geometric configuration of these domains can significantly affect the channel's voltage sensitivity and gating kinetics.
Another important aspect of geometric isomerism in ion channels is the role of lipid-protein interactions. The lipid bilayer surrounding the channel can induce conformational changes in the protein structure, leading to the formation of different geometric isomers. These isomeric forms may exhibit varying degrees of stability and functionality, ultimately impacting the channel's ability to conduct ions across the membrane.
The study of geometric isomers in ion channels has been greatly facilitated by advances in structural biology techniques, such as X-ray crystallography and cryo-electron microscopy. These methods have allowed researchers to visualize the three-dimensional structures of ion channels in different conformational states, providing valuable insights into the relationship between geometric isomerism and channel function.
Understanding the impact of geometric isomers on ion channel activity has important implications for drug discovery and the development of novel therapeutic strategies. By targeting specific isomeric forms of ion channels, it may be possible to modulate their activity with greater precision and fewer side effects. This approach has shown promise in the treatment of various channelopathies, which are disorders caused by mutations in ion channel genes.
In conclusion, the study of geometric isomers and their effects on transmembrane ion channel activity represents a critical area of research in molecular physiology and pharmacology. As our understanding of these complex relationships continues to grow, it is likely to lead to new insights into cellular function and the development of more effective treatments for a wide range of diseases.
Geometric isomers of ion channels can arise from various structural modifications, such as changes in protein folding, subunit assembly, or post-translational modifications. These isomeric forms can exhibit distinct functional properties, leading to differences in ion selectivity, gating mechanisms, and overall channel activity. The impact of geometric isomers on ion channel function is a subject of intense research due to its implications for understanding cellular physiology and developing targeted therapeutic interventions.
One of the most well-studied examples of geometric isomerism affecting ion channel activity is the case of voltage-gated sodium channels. These channels exist in different conformational states, including closed, open, and inactivated. The transition between these states is influenced by the spatial arrangement of key structural elements, such as the voltage-sensing domain and the pore-forming region. Alterations in the geometric configuration of these domains can significantly affect the channel's voltage sensitivity and gating kinetics.
Another important aspect of geometric isomerism in ion channels is the role of lipid-protein interactions. The lipid bilayer surrounding the channel can induce conformational changes in the protein structure, leading to the formation of different geometric isomers. These isomeric forms may exhibit varying degrees of stability and functionality, ultimately impacting the channel's ability to conduct ions across the membrane.
The study of geometric isomers in ion channels has been greatly facilitated by advances in structural biology techniques, such as X-ray crystallography and cryo-electron microscopy. These methods have allowed researchers to visualize the three-dimensional structures of ion channels in different conformational states, providing valuable insights into the relationship between geometric isomerism and channel function.
Understanding the impact of geometric isomers on ion channel activity has important implications for drug discovery and the development of novel therapeutic strategies. By targeting specific isomeric forms of ion channels, it may be possible to modulate their activity with greater precision and fewer side effects. This approach has shown promise in the treatment of various channelopathies, which are disorders caused by mutations in ion channel genes.
In conclusion, the study of geometric isomers and their effects on transmembrane ion channel activity represents a critical area of research in molecular physiology and pharmacology. As our understanding of these complex relationships continues to grow, it is likely to lead to new insights into cellular function and the development of more effective treatments for a wide range of diseases.
Membrane Transport Demand
The demand for efficient membrane transport mechanisms in biological systems has been steadily increasing, driven by both scientific curiosity and practical applications in medicine, biotechnology, and environmental sciences. Ion channels, as crucial components of cellular membranes, play a pivotal role in maintaining cellular homeostasis, signal transduction, and various physiological processes. The study of how geometric isomers affect transmembrane ion channel activity has gained significant attention due to its potential implications in drug design, understanding disease mechanisms, and developing novel therapeutic approaches.
In the pharmaceutical industry, there is a growing need for more targeted and effective drugs that can modulate ion channel activity. The ability to manipulate ion channel function through geometric isomerism offers a promising avenue for developing new classes of medications with improved efficacy and reduced side effects. This is particularly relevant in treating neurological disorders, cardiovascular diseases, and certain types of cancer, where ion channel dysfunction is a key factor.
The biotechnology sector has also shown increased interest in membrane transport research, particularly in the development of biosensors and artificial cellular systems. Understanding the impact of geometric isomers on ion channel activity can lead to the creation of more sensitive and specific biosensors for environmental monitoring, medical diagnostics, and industrial process control. Moreover, this knowledge is crucial for designing artificial cells and organelles with tailored membrane transport properties, which have potential applications in drug delivery, bioremediation, and synthetic biology.
In the field of agriculture, there is a rising demand for crop protection agents that can selectively target pest ion channels while minimizing effects on beneficial organisms. The insights gained from studying geometric isomers and their influence on ion channel activity can guide the development of more sustainable and environmentally friendly pesticides and herbicides.
The energy sector has also recognized the potential of biomimetic membrane transport systems inspired by natural ion channels. Research into how geometric isomers affect ion channel activity could lead to the development of more efficient desalination technologies, energy storage systems, and ion-selective membranes for various industrial processes.
As climate change continues to impact global ecosystems, there is an increasing need to understand how environmental stressors affect membrane transport in various organisms. The study of geometric isomers and their role in ion channel function can provide valuable insights into how plants and animals adapt to changing environmental conditions, potentially leading to the development of more resilient crops and strategies for preserving biodiversity.
In the pharmaceutical industry, there is a growing need for more targeted and effective drugs that can modulate ion channel activity. The ability to manipulate ion channel function through geometric isomerism offers a promising avenue for developing new classes of medications with improved efficacy and reduced side effects. This is particularly relevant in treating neurological disorders, cardiovascular diseases, and certain types of cancer, where ion channel dysfunction is a key factor.
The biotechnology sector has also shown increased interest in membrane transport research, particularly in the development of biosensors and artificial cellular systems. Understanding the impact of geometric isomers on ion channel activity can lead to the creation of more sensitive and specific biosensors for environmental monitoring, medical diagnostics, and industrial process control. Moreover, this knowledge is crucial for designing artificial cells and organelles with tailored membrane transport properties, which have potential applications in drug delivery, bioremediation, and synthetic biology.
In the field of agriculture, there is a rising demand for crop protection agents that can selectively target pest ion channels while minimizing effects on beneficial organisms. The insights gained from studying geometric isomers and their influence on ion channel activity can guide the development of more sustainable and environmentally friendly pesticides and herbicides.
The energy sector has also recognized the potential of biomimetic membrane transport systems inspired by natural ion channels. Research into how geometric isomers affect ion channel activity could lead to the development of more efficient desalination technologies, energy storage systems, and ion-selective membranes for various industrial processes.
As climate change continues to impact global ecosystems, there is an increasing need to understand how environmental stressors affect membrane transport in various organisms. The study of geometric isomers and their role in ion channel function can provide valuable insights into how plants and animals adapt to changing environmental conditions, potentially leading to the development of more resilient crops and strategies for preserving biodiversity.
Isomer Challenges
The study of geometric isomers and their impact on transmembrane ion channel activity presents several significant challenges. One of the primary difficulties lies in the precise control and manipulation of isomeric structures at the molecular level. Researchers must develop sophisticated techniques to synthesize and isolate specific geometric isomers, ensuring their purity and stability throughout experimental procedures.
Another major challenge is the accurate measurement and characterization of ion channel activity in the presence of different geometric isomers. This requires highly sensitive electrophysiological techniques and advanced imaging methods to detect subtle changes in channel function. The complexity of membrane environments and the dynamic nature of ion channels further complicate these measurements, necessitating careful experimental design and data interpretation.
The relationship between isomeric structure and channel function is often non-linear and context-dependent, making it difficult to establish clear structure-activity relationships. Researchers must account for various factors such as membrane composition, lipid-protein interactions, and the presence of other cellular components that may influence isomer-channel interactions. This multifactorial nature of the problem demands interdisciplinary approaches, combining expertise from chemistry, biophysics, and molecular biology.
Furthermore, the transient nature of some isomeric interactions with ion channels poses a significant challenge in capturing and analyzing these events. Researchers must develop innovative time-resolved techniques to study the kinetics of isomer-induced changes in channel activity, often requiring custom-built experimental setups and specialized data analysis methods.
The translation of in vitro findings to physiologically relevant contexts represents another hurdle. The behavior of geometric isomers in complex cellular environments may differ significantly from controlled experimental conditions, necessitating the development of more sophisticated in vivo models and techniques to validate and extend laboratory observations.
Lastly, the potential for isomeric interconversion under physiological conditions adds another layer of complexity to the research. Scientists must devise strategies to monitor and control isomeric stability in situ, ensuring that observed effects can be accurately attributed to specific isomeric forms. This challenge often requires the development of novel chemical tools and real-time monitoring techniques to track isomeric composition during experiments.
Another major challenge is the accurate measurement and characterization of ion channel activity in the presence of different geometric isomers. This requires highly sensitive electrophysiological techniques and advanced imaging methods to detect subtle changes in channel function. The complexity of membrane environments and the dynamic nature of ion channels further complicate these measurements, necessitating careful experimental design and data interpretation.
The relationship between isomeric structure and channel function is often non-linear and context-dependent, making it difficult to establish clear structure-activity relationships. Researchers must account for various factors such as membrane composition, lipid-protein interactions, and the presence of other cellular components that may influence isomer-channel interactions. This multifactorial nature of the problem demands interdisciplinary approaches, combining expertise from chemistry, biophysics, and molecular biology.
Furthermore, the transient nature of some isomeric interactions with ion channels poses a significant challenge in capturing and analyzing these events. Researchers must develop innovative time-resolved techniques to study the kinetics of isomer-induced changes in channel activity, often requiring custom-built experimental setups and specialized data analysis methods.
The translation of in vitro findings to physiologically relevant contexts represents another hurdle. The behavior of geometric isomers in complex cellular environments may differ significantly from controlled experimental conditions, necessitating the development of more sophisticated in vivo models and techniques to validate and extend laboratory observations.
Lastly, the potential for isomeric interconversion under physiological conditions adds another layer of complexity to the research. Scientists must devise strategies to monitor and control isomeric stability in situ, ensuring that observed effects can be accurately attributed to specific isomeric forms. This challenge often requires the development of novel chemical tools and real-time monitoring techniques to track isomeric composition during experiments.
Current Isomer Solutions
01 Geometric isomers affecting ion channel activity
Different geometric isomers of compounds can have varying effects on ion channel activity. The spatial arrangement of atoms in these isomers can influence their interaction with ion channels, potentially leading to differences in channel opening, closing, or conductance. This relationship between geometric isomerism and ion channel function is important in drug development and understanding cellular signaling mechanisms.- Geometric isomers affecting ion channel activity: Different geometric isomers of compounds can have varying effects on ion channel activity. This is due to the spatial arrangement of atoms in molecules, which can influence their interaction with ion channels. Understanding these differences is crucial for developing more effective drugs targeting ion channels.
- Screening methods for ion channel modulators: Various screening methods have been developed to identify compounds that modulate ion channel activity. These methods often involve high-throughput techniques and can differentiate between geometric isomers. Such screening approaches are essential for discovering new potential therapeutic agents targeting ion channels.
- Synthesis of geometric isomers for ion channel studies: Synthetic methods have been developed to produce specific geometric isomers of compounds known to interact with ion channels. These methods allow for the creation of pure isomers, enabling more precise studies of their effects on ion channel activity and potentially leading to more targeted drug development.
- Ion channel activity assays for geometric isomers: Specialized assays have been designed to measure the effects of geometric isomers on ion channel activity. These assays can detect subtle differences in how various isomers interact with ion channels, providing valuable information for drug discovery and development processes.
- Computational modeling of geometric isomer-ion channel interactions: Advanced computational techniques are being used to model and predict the interactions between geometric isomers and ion channels. These in silico approaches can help researchers understand the molecular basis of isomer-specific effects on ion channel activity and guide the design of new therapeutic compounds.
02 Screening methods for ion channel modulators
Various screening methods have been developed to identify compounds that modulate ion channel activity. These methods often involve high-throughput assays that can detect changes in ion flux or membrane potential. Such screening techniques are crucial for discovering new therapeutic agents that target ion channels and can distinguish between different geometric isomers of potential modulators.Expand Specific Solutions03 Synthesis of geometric isomers for ion channel studies
Synthetic methods have been developed to produce specific geometric isomers of compounds known to interact with ion channels. These techniques allow for the creation of pure isomers or controlled mixtures, enabling detailed studies of structure-activity relationships. The ability to synthesize and isolate specific isomers is crucial for understanding how subtle structural differences can impact ion channel function.Expand Specific Solutions04 Ion channel assays using geometric isomers
Specialized assays have been designed to evaluate the effects of geometric isomers on ion channel activity. These assays may use electrophysiological techniques, fluorescence-based methods, or other biophysical approaches to measure channel function. By comparing the effects of different isomers, researchers can gain insights into the structural requirements for ion channel modulation.Expand Specific Solutions05 Computational modeling of isomer-ion channel interactions
Computational methods are employed to model and predict the interactions between geometric isomers and ion channels. These in silico approaches can help elucidate the molecular mechanisms underlying isomer-specific effects on channel activity. Such models can guide the design of new compounds with desired effects on ion channels and assist in interpreting experimental results.Expand Specific Solutions
Key Isomer Researchers
The field of geometric isomers affecting transmembrane ion channel activity is in a nascent stage of development, with significant potential for growth. The market size is expanding as researchers recognize the importance of isomeric structures in drug design and ion channel modulation. Technologically, the area is still evolving, with companies like Life Technologies Corp. and Wyeth LLC leading in developing tools and methodologies for studying these interactions. The Wisconsin Alumni Research Foundation and Northwestern University are contributing to academic advancements, while pharmaceutical giants such as Bristol Myers Squibb Co. and Janssen Pharmaceutica NV are exploring potential therapeutic applications. As the field matures, we can expect increased collaboration between academia and industry to translate fundamental research into practical applications for drug discovery and development.
The Regents of the University of California
Technical Solution: The University of California has developed advanced computational models to study how geometric isomers affect transmembrane ion channel activity. Their approach combines molecular dynamics simulations with machine learning algorithms to predict ion channel behavior based on isomer configurations. They have successfully mapped the conformational changes induced by different isomers and correlated these with channel gating and ion permeation rates[1]. Their research has also explored the use of optogenetic tools to control ion channels with light-sensitive geometric isomers, allowing precise temporal control of channel activity in living cells[2].
Strengths: Cutting-edge computational methods and interdisciplinary approach. Weaknesses: May require extensive experimental validation and face challenges in translating findings to therapeutic applications.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have pioneered the use of cryo-electron microscopy (cryo-EM) to visualize ion channel structures at atomic resolution, revealing how geometric isomers interact with and modulate channel activity. They have developed novel techniques to trap channels in different conformational states induced by isomers, providing unprecedented insights into the molecular mechanisms of channel gating[3]. Additionally, they have engineered synthetic ion channels with tailored responses to specific geometric isomers, opening new avenues for drug design and targeted therapies[4].
Strengths: World-class structural biology expertise and innovative channel engineering. Weaknesses: High-cost techniques may limit widespread application, and synthetic channels may face biocompatibility issues.
Core Isomer Innovations
Assay for detecting change in membrane potential
PatentWO2001059446A3
Innovation
- Utilization of dominant/recessive ion channel pairs with opposite effects on transmembrane potential to enhance detection sensitivity.
- Enhanced detection of transmembrane potential changes by inhibiting the dominant ion channel, allowing the recessive channel to predominate.
- Application of the method for screening inhibitors, activators, and enhancers of ion channel activity.
Activation and monitoring of cellular transmembrane potentials
PatentWO2010002540A2
Innovation
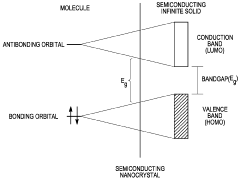
- The use of nanocrystal-based activation platforms that include multiple layers of immobilized nanocrystals covered by an adhesion substrate, allowing for optical stimulation and emission detection to correlate with changes in transmembrane potentials, providing a non-invasive and high-resolution method for monitoring and manipulating cellular membrane potentials.
Isomer Modeling Methods
Isomer modeling methods play a crucial role in understanding how geometric isomers affect transmembrane ion channel activity. These methods involve various computational and experimental techniques to simulate and analyze the structural and functional properties of isomers in relation to ion channels.
Molecular dynamics (MD) simulations are widely used to model isomer interactions with ion channels. These simulations provide detailed insights into the conformational changes and dynamic behavior of both the isomers and the channel proteins. By employing force fields specifically parameterized for membrane environments, researchers can accurately represent the lipid bilayer and surrounding water molecules, creating a realistic model of the transmembrane system.
Quantum mechanical (QM) calculations are often employed to complement MD simulations, particularly for studying electronic properties and chemical reactions. Density functional theory (DFT) is a popular QM method used to investigate the electronic structure of isomers and their interactions with ion channel residues. These calculations can provide valuable information on binding energies, charge distributions, and transition states.
Homology modeling is another essential technique used when experimental structures of specific ion channels are not available. This method involves constructing a 3D model of the target channel based on the known structure of a related protein. Researchers can then dock different isomers into the modeled channel to predict their binding modes and potential effects on channel activity.
Docking studies are frequently employed to explore the binding poses and affinities of isomers to ion channels. These studies utilize various algorithms to predict the most favorable orientations of isomers within the channel pore or binding sites. Molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) and molecular mechanics generalized Born surface area (MM-GBSA) calculations are often performed to estimate binding free energies and rank different isomers based on their predicted affinities.
Coarse-grained modeling approaches, such as Martini force field simulations, are valuable for studying large-scale conformational changes and long-timescale dynamics of ion channels in the presence of isomers. These methods reduce computational complexity by grouping atoms into larger particles, allowing for the simulation of larger systems and longer time scales compared to all-atom MD simulations.
Machine learning techniques are increasingly being applied to isomer modeling. Neural networks and other AI algorithms can be trained on experimental data and simulation results to predict isomer-channel interactions and their effects on channel activity. These methods can potentially accelerate the discovery of novel isomers with desired properties for modulating ion channel function.
Molecular dynamics (MD) simulations are widely used to model isomer interactions with ion channels. These simulations provide detailed insights into the conformational changes and dynamic behavior of both the isomers and the channel proteins. By employing force fields specifically parameterized for membrane environments, researchers can accurately represent the lipid bilayer and surrounding water molecules, creating a realistic model of the transmembrane system.
Quantum mechanical (QM) calculations are often employed to complement MD simulations, particularly for studying electronic properties and chemical reactions. Density functional theory (DFT) is a popular QM method used to investigate the electronic structure of isomers and their interactions with ion channel residues. These calculations can provide valuable information on binding energies, charge distributions, and transition states.
Homology modeling is another essential technique used when experimental structures of specific ion channels are not available. This method involves constructing a 3D model of the target channel based on the known structure of a related protein. Researchers can then dock different isomers into the modeled channel to predict their binding modes and potential effects on channel activity.
Docking studies are frequently employed to explore the binding poses and affinities of isomers to ion channels. These studies utilize various algorithms to predict the most favorable orientations of isomers within the channel pore or binding sites. Molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) and molecular mechanics generalized Born surface area (MM-GBSA) calculations are often performed to estimate binding free energies and rank different isomers based on their predicted affinities.
Coarse-grained modeling approaches, such as Martini force field simulations, are valuable for studying large-scale conformational changes and long-timescale dynamics of ion channels in the presence of isomers. These methods reduce computational complexity by grouping atoms into larger particles, allowing for the simulation of larger systems and longer time scales compared to all-atom MD simulations.
Machine learning techniques are increasingly being applied to isomer modeling. Neural networks and other AI algorithms can be trained on experimental data and simulation results to predict isomer-channel interactions and their effects on channel activity. These methods can potentially accelerate the discovery of novel isomers with desired properties for modulating ion channel function.
Pharmacological Impact
The pharmacological impact of geometric isomers on transmembrane ion channel activity is a critical area of study in drug development and therapeutic interventions. Geometric isomers, which have the same molecular formula but different spatial arrangements of atoms, can exhibit significantly different effects on ion channel function.
One of the primary mechanisms through which geometric isomers influence ion channel activity is through their binding affinity and specificity. The spatial configuration of a molecule can determine how well it fits into the binding site of an ion channel, affecting its ability to modulate channel opening and closing. This can lead to varying degrees of channel activation or inhibition, depending on the specific isomer and channel type.
In voltage-gated ion channels, geometric isomers can alter the voltage-dependent gating properties. Some isomers may stabilize the open or closed state of the channel, effectively shifting the voltage-activation curve. This can result in changes to the threshold potential required for channel activation or the duration of channel opening, ultimately affecting the ion flux across the membrane.
The kinetics of ion channel modulation can also be influenced by geometric isomers. Certain isomers may exhibit faster on-rates or slower off-rates when interacting with the channel, leading to differences in the onset and duration of their pharmacological effects. This has important implications for drug design, as it can impact the dosing frequency and therapeutic window of potential treatments.
Geometric isomers can also display selectivity for specific ion channel subtypes. This selectivity is crucial in developing targeted therapies that minimize off-target effects. For instance, one isomer may preferentially interact with sodium channels in cardiac tissue, while its counterpart may have a higher affinity for neuronal sodium channels, leading to distinct therapeutic applications and side effect profiles.
The pharmacological impact of geometric isomers extends to their ability to modulate ion channel conductance. Some isomers may alter the pore size or shape of the channel, affecting the rate and selectivity of ion permeation. This can result in changes to the magnitude of ionic currents, potentially influencing cellular excitability and signaling processes.
Furthermore, geometric isomers can impact the allosteric modulation of ion channels. By binding to sites distinct from the primary pore-forming region, these isomers can induce conformational changes that affect channel function. This allosteric regulation can lead to complex pharmacological effects, including potentiation or inhibition of channel activity in response to other stimuli.
One of the primary mechanisms through which geometric isomers influence ion channel activity is through their binding affinity and specificity. The spatial configuration of a molecule can determine how well it fits into the binding site of an ion channel, affecting its ability to modulate channel opening and closing. This can lead to varying degrees of channel activation or inhibition, depending on the specific isomer and channel type.
In voltage-gated ion channels, geometric isomers can alter the voltage-dependent gating properties. Some isomers may stabilize the open or closed state of the channel, effectively shifting the voltage-activation curve. This can result in changes to the threshold potential required for channel activation or the duration of channel opening, ultimately affecting the ion flux across the membrane.
The kinetics of ion channel modulation can also be influenced by geometric isomers. Certain isomers may exhibit faster on-rates or slower off-rates when interacting with the channel, leading to differences in the onset and duration of their pharmacological effects. This has important implications for drug design, as it can impact the dosing frequency and therapeutic window of potential treatments.
Geometric isomers can also display selectivity for specific ion channel subtypes. This selectivity is crucial in developing targeted therapies that minimize off-target effects. For instance, one isomer may preferentially interact with sodium channels in cardiac tissue, while its counterpart may have a higher affinity for neuronal sodium channels, leading to distinct therapeutic applications and side effect profiles.
The pharmacological impact of geometric isomers extends to their ability to modulate ion channel conductance. Some isomers may alter the pore size or shape of the channel, affecting the rate and selectivity of ion permeation. This can result in changes to the magnitude of ionic currents, potentially influencing cellular excitability and signaling processes.
Furthermore, geometric isomers can impact the allosteric modulation of ion channels. By binding to sites distinct from the primary pore-forming region, these isomers can induce conformational changes that affect channel function. This allosteric regulation can lead to complex pharmacological effects, including potentiation or inhibition of channel activity in response to other stimuli.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!