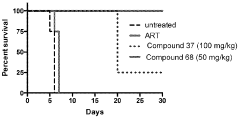
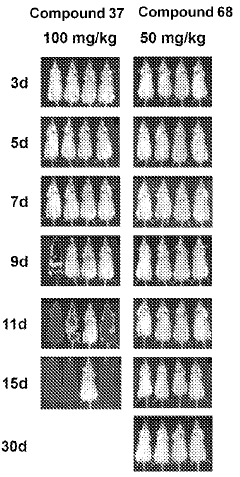
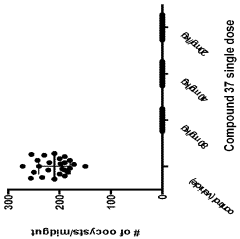
How Geometric Isomers Alter Chemical Signal Transduction Pathways
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers in Signal Transduction: Background
Geometric isomers play a crucial role in chemical signal transduction pathways, significantly influencing biological processes at the molecular level. These structural variants of molecules, which possess identical molecular formulas but different spatial arrangements of atoms, can dramatically alter the way signals are transmitted within cells and organisms.
The concept of geometric isomerism dates back to the late 19th century, with early observations in organic chemistry. However, its importance in biological systems was not fully appreciated until the mid-20th century. The discovery of cis-trans isomerism in retinal, a key component in the visual cycle, marked a turning point in understanding how subtle molecular changes can profoundly impact physiological processes.
In the context of signal transduction, geometric isomers often serve as molecular switches, capable of triggering or inhibiting specific cellular responses. This property is particularly evident in hormone signaling, neurotransmitter function, and photoreceptor mechanisms. For instance, the light-induced isomerization of 11-cis-retinal to all-trans-retinal in rhodopsin is the primary event in visual phototransduction.
The field of signal transduction has evolved significantly over the past few decades, with geometric isomerism emerging as a critical factor in understanding the intricacies of cellular communication. Advances in structural biology, particularly X-ray crystallography and NMR spectroscopy, have provided detailed insights into how isomeric changes affect protein-ligand interactions and subsequent signaling cascades.
Recent research has highlighted the role of geometric isomers in various physiological processes, including circadian rhythm regulation, plant growth responses, and even in the mechanisms of certain diseases. For example, the cis-trans isomerization of proline residues in proteins has been implicated in the pathogenesis of neurodegenerative disorders like Alzheimer's disease.
The study of geometric isomers in signal transduction has also led to significant advancements in drug design and development. Many pharmaceuticals rely on specific isomeric forms to achieve their therapeutic effects, with the wrong isomer potentially leading to reduced efficacy or unwanted side effects. This understanding has driven the development of stereospecific synthesis methods and chiral separation techniques in the pharmaceutical industry.
As we delve deeper into the complexities of cellular signaling, the importance of geometric isomerism continues to grow. It represents a fundamental principle in molecular biology, bridging the gap between chemical structure and biological function. Understanding how geometric isomers alter chemical signal transduction pathways is not only crucial for basic science but also holds immense potential for medical and biotechnological applications.
The concept of geometric isomerism dates back to the late 19th century, with early observations in organic chemistry. However, its importance in biological systems was not fully appreciated until the mid-20th century. The discovery of cis-trans isomerism in retinal, a key component in the visual cycle, marked a turning point in understanding how subtle molecular changes can profoundly impact physiological processes.
In the context of signal transduction, geometric isomers often serve as molecular switches, capable of triggering or inhibiting specific cellular responses. This property is particularly evident in hormone signaling, neurotransmitter function, and photoreceptor mechanisms. For instance, the light-induced isomerization of 11-cis-retinal to all-trans-retinal in rhodopsin is the primary event in visual phototransduction.
The field of signal transduction has evolved significantly over the past few decades, with geometric isomerism emerging as a critical factor in understanding the intricacies of cellular communication. Advances in structural biology, particularly X-ray crystallography and NMR spectroscopy, have provided detailed insights into how isomeric changes affect protein-ligand interactions and subsequent signaling cascades.
Recent research has highlighted the role of geometric isomers in various physiological processes, including circadian rhythm regulation, plant growth responses, and even in the mechanisms of certain diseases. For example, the cis-trans isomerization of proline residues in proteins has been implicated in the pathogenesis of neurodegenerative disorders like Alzheimer's disease.
The study of geometric isomers in signal transduction has also led to significant advancements in drug design and development. Many pharmaceuticals rely on specific isomeric forms to achieve their therapeutic effects, with the wrong isomer potentially leading to reduced efficacy or unwanted side effects. This understanding has driven the development of stereospecific synthesis methods and chiral separation techniques in the pharmaceutical industry.
As we delve deeper into the complexities of cellular signaling, the importance of geometric isomerism continues to grow. It represents a fundamental principle in molecular biology, bridging the gap between chemical structure and biological function. Understanding how geometric isomers alter chemical signal transduction pathways is not only crucial for basic science but also holds immense potential for medical and biotechnological applications.
Market Analysis: Isomer-Based Therapeutics
The market for isomer-based therapeutics has been experiencing significant growth in recent years, driven by advancements in understanding how geometric isomers alter chemical signal transduction pathways. This emerging field offers promising opportunities for developing more targeted and effective drugs with reduced side effects.
The global market for isomer-based therapeutics is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing traditional pharmaceutical sectors. This growth is fueled by increasing demand for personalized medicine and the need for more precise drug delivery systems.
Key market segments for isomer-based therapeutics include oncology, neurology, and cardiovascular diseases. In oncology, isomer-specific drugs have shown potential in targeting cancer cells more effectively while minimizing damage to healthy tissues. Neurological applications focus on developing treatments for disorders such as Alzheimer's and Parkinson's disease, where specific isomers can enhance drug efficacy and reduce side effects.
The cardiovascular segment is also witnessing increased interest, with isomer-based therapies showing promise in treating hypertension and heart failure. These applications leverage the unique properties of geometric isomers to modulate signal transduction pathways more precisely, leading to improved patient outcomes.
Geographically, North America and Europe currently dominate the market for isomer-based therapeutics, owing to their advanced healthcare infrastructure and significant investments in research and development. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing healthcare expenditure and rising awareness of personalized medicine approaches.
Several factors are contributing to the market's growth potential. Firstly, advancements in analytical techniques and computational modeling have enhanced our ability to design and synthesize specific isomers with desired pharmacological properties. Secondly, regulatory bodies are increasingly recognizing the importance of isomeric purity in drug development, leading to more stringent guidelines that favor isomer-specific therapies.
Despite the promising outlook, challenges remain in the isomer-based therapeutics market. These include high development costs, complex manufacturing processes, and the need for extensive clinical trials to demonstrate the superiority of isomer-specific drugs over existing treatments. Additionally, patent expirations for some pioneering isomer-based drugs may impact market dynamics in the coming years.
Looking ahead, the market for isomer-based therapeutics is expected to continue its growth trajectory. Emerging trends such as combination therapies involving multiple isomers and the development of isomer-specific drug delivery systems are likely to drive innovation and expand market opportunities. As our understanding of how geometric isomers alter chemical signal transduction pathways deepens, the potential for breakthrough treatments across various therapeutic areas remains high.
The global market for isomer-based therapeutics is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing traditional pharmaceutical sectors. This growth is fueled by increasing demand for personalized medicine and the need for more precise drug delivery systems.
Key market segments for isomer-based therapeutics include oncology, neurology, and cardiovascular diseases. In oncology, isomer-specific drugs have shown potential in targeting cancer cells more effectively while minimizing damage to healthy tissues. Neurological applications focus on developing treatments for disorders such as Alzheimer's and Parkinson's disease, where specific isomers can enhance drug efficacy and reduce side effects.
The cardiovascular segment is also witnessing increased interest, with isomer-based therapies showing promise in treating hypertension and heart failure. These applications leverage the unique properties of geometric isomers to modulate signal transduction pathways more precisely, leading to improved patient outcomes.
Geographically, North America and Europe currently dominate the market for isomer-based therapeutics, owing to their advanced healthcare infrastructure and significant investments in research and development. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing healthcare expenditure and rising awareness of personalized medicine approaches.
Several factors are contributing to the market's growth potential. Firstly, advancements in analytical techniques and computational modeling have enhanced our ability to design and synthesize specific isomers with desired pharmacological properties. Secondly, regulatory bodies are increasingly recognizing the importance of isomeric purity in drug development, leading to more stringent guidelines that favor isomer-specific therapies.
Despite the promising outlook, challenges remain in the isomer-based therapeutics market. These include high development costs, complex manufacturing processes, and the need for extensive clinical trials to demonstrate the superiority of isomer-specific drugs over existing treatments. Additionally, patent expirations for some pioneering isomer-based drugs may impact market dynamics in the coming years.
Looking ahead, the market for isomer-based therapeutics is expected to continue its growth trajectory. Emerging trends such as combination therapies involving multiple isomers and the development of isomer-specific drug delivery systems are likely to drive innovation and expand market opportunities. As our understanding of how geometric isomers alter chemical signal transduction pathways deepens, the potential for breakthrough treatments across various therapeutic areas remains high.
Current Challenges in Isomer Signaling Research
The field of geometric isomer signaling research faces several significant challenges that hinder our comprehensive understanding of how these molecules influence chemical signal transduction pathways. One of the primary obstacles is the complexity of isomeric structures and their interactions with biological systems. Geometric isomers, despite having the same molecular formula, can exhibit vastly different biological activities due to their spatial arrangements. This structural diversity complicates the prediction and interpretation of their effects on signaling cascades.
Another major challenge lies in the development of sensitive and specific analytical techniques capable of distinguishing between geometric isomers in complex biological matrices. Current methods often struggle to accurately quantify and differentiate isomers, especially when present at low concentrations or in the presence of structurally similar compounds. This limitation hampers our ability to fully elucidate the roles of specific isomers in signaling processes.
The dynamic nature of isomerization in biological systems presents an additional layer of complexity. Many geometric isomers can interconvert under physiological conditions, making it difficult to attribute observed effects to a single isomeric form. This interconversion can be influenced by various factors such as pH, temperature, and the presence of enzymes, further complicating the study of isomer-specific signaling mechanisms.
Furthermore, the context-dependent nature of isomer signaling poses a significant challenge. The same geometric isomer may elicit different responses depending on the cellular environment, tissue type, or physiological state. This variability necessitates extensive studies across diverse biological systems to fully understand the scope and specificity of isomer-mediated signaling.
The integration of isomer signaling with other cellular processes and signaling pathways remains a complex task. Geometric isomers often interact with multiple targets and can modulate various signaling cascades simultaneously. Unraveling these intricate networks and determining the relative contributions of different isomers to overall cellular responses requires sophisticated experimental designs and advanced computational modeling approaches.
Lastly, translating findings from in vitro studies to in vivo systems presents ongoing challenges. The behavior of geometric isomers in controlled laboratory conditions may not accurately reflect their activities in complex living organisms. Factors such as metabolism, distribution, and interactions with other biomolecules can significantly alter the signaling properties of isomers in vivo, necessitating the development of more physiologically relevant models and experimental paradigms.
Another major challenge lies in the development of sensitive and specific analytical techniques capable of distinguishing between geometric isomers in complex biological matrices. Current methods often struggle to accurately quantify and differentiate isomers, especially when present at low concentrations or in the presence of structurally similar compounds. This limitation hampers our ability to fully elucidate the roles of specific isomers in signaling processes.
The dynamic nature of isomerization in biological systems presents an additional layer of complexity. Many geometric isomers can interconvert under physiological conditions, making it difficult to attribute observed effects to a single isomeric form. This interconversion can be influenced by various factors such as pH, temperature, and the presence of enzymes, further complicating the study of isomer-specific signaling mechanisms.
Furthermore, the context-dependent nature of isomer signaling poses a significant challenge. The same geometric isomer may elicit different responses depending on the cellular environment, tissue type, or physiological state. This variability necessitates extensive studies across diverse biological systems to fully understand the scope and specificity of isomer-mediated signaling.
The integration of isomer signaling with other cellular processes and signaling pathways remains a complex task. Geometric isomers often interact with multiple targets and can modulate various signaling cascades simultaneously. Unraveling these intricate networks and determining the relative contributions of different isomers to overall cellular responses requires sophisticated experimental designs and advanced computational modeling approaches.
Lastly, translating findings from in vitro studies to in vivo systems presents ongoing challenges. The behavior of geometric isomers in controlled laboratory conditions may not accurately reflect their activities in complex living organisms. Factors such as metabolism, distribution, and interactions with other biomolecules can significantly alter the signaling properties of isomers in vivo, necessitating the development of more physiologically relevant models and experimental paradigms.
Existing Models of Isomer-Induced Pathway Alterations
01 Geometric isomers in signal transduction pathways
Geometric isomers play a crucial role in chemical signal transduction pathways. These isomers can affect the binding affinity and specificity of signaling molecules to their receptors, potentially altering the downstream signaling cascade. Understanding the impact of geometric isomerization on signal transduction can lead to the development of more effective drugs and therapeutic strategies.- Geometric isomers in signal transduction pathways: Geometric isomers play a crucial role in chemical signal transduction pathways. These isomers can affect the binding affinity and specificity of signaling molecules to their receptors, potentially altering the downstream signaling cascade. Understanding the impact of geometric isomerization on signal transduction can lead to the development of more effective drugs and therapeutic strategies.
- Methods for detecting and analyzing geometric isomers: Various analytical techniques have been developed to detect and characterize geometric isomers involved in signal transduction pathways. These methods include spectroscopic techniques, chromatography, and advanced imaging technologies. Improved detection and analysis of geometric isomers can enhance our understanding of their role in cellular signaling and facilitate the design of targeted interventions.
- Computational modeling of geometric isomers in signaling: Computational approaches are increasingly used to model and predict the behavior of geometric isomers in chemical signal transduction pathways. These models can simulate the interactions between isomers and their target molecules, helping researchers to understand the structural basis of signaling processes and to design novel therapeutic compounds that target specific isomeric forms.
- Regulation of signal transduction by isomerization: The interconversion between geometric isomers can serve as a regulatory mechanism in signal transduction pathways. Enzymes that catalyze isomerization reactions can modulate the activity of signaling molecules, providing an additional layer of control over cellular responses. Understanding these regulatory mechanisms can lead to new strategies for manipulating signaling pathways in various biological contexts.
- Therapeutic applications targeting geometric isomers: The knowledge of geometric isomers in chemical signal transduction pathways has led to the development of novel therapeutic approaches. By designing drugs that selectively target specific isomeric forms of signaling molecules or their receptors, researchers aim to achieve more precise control over cellular signaling processes. This approach has potential applications in treating various diseases, including cancer and neurological disorders.
02 Detection and analysis of geometric isomers in biological systems
Methods and techniques for detecting and analyzing geometric isomers in biological systems are essential for studying their roles in signal transduction pathways. These may include spectroscopic techniques, chromatography, and advanced imaging methods. Improved detection and analysis can provide insights into the dynamics of isomerization and its effects on cellular signaling.Expand Specific Solutions03 Computational modeling of geometric isomers in signaling pathways
Computational approaches are employed to model and predict the behavior of geometric isomers in chemical signal transduction pathways. These models can simulate the interactions between isomers and cellular components, helping researchers understand the complex dynamics of signaling networks and potentially identify novel therapeutic targets.Expand Specific Solutions04 Regulation of signal transduction by geometric isomerization
Geometric isomerization can serve as a regulatory mechanism in signal transduction pathways. The interconversion between different isomeric forms of signaling molecules can modulate their activity, providing a means of fine-tuning cellular responses. Understanding this regulation can lead to new strategies for controlling cellular processes and developing targeted therapies.Expand Specific Solutions05 Therapeutic applications targeting geometric isomers in signaling pathways
Targeting geometric isomers involved in chemical signal transduction pathways presents opportunities for therapeutic interventions. By developing drugs that can selectively interact with specific isomeric forms or modulate isomerization processes, it may be possible to control signaling pathways implicated in various diseases, leading to novel treatment approaches.Expand Specific Solutions
Key Players in Isomer-Signaling Research
The field of geometric isomers and chemical signal transduction pathways is in a growth phase, with increasing market size and technological advancements. Major players like AbbVie, Janssen Pharmaceutica, and Abbott Laboratories are investing heavily in research and development. The technology is maturing, with companies like Foghorn Therapeutics and Novartis AG making significant strides in understanding how geometric isomers affect signal transduction. Academic institutions such as King Abdullah University of Science & Technology and Imperial College London are contributing valuable research, fostering collaborations between industry and academia. As the field progresses, we can expect more targeted therapies and innovative approaches to drug development leveraging insights from geometric isomer studies.
AbbVie, Inc.
Technical Solution: AbbVie, Inc. has developed a proprietary platform called GeomSig for investigating the effects of geometric isomers on chemical signal transduction pathways. This platform integrates advanced molecular dynamics simulations with experimental validation using state-of-the-art biochemical and biophysical techniques[2]. GeomSig employs machine learning algorithms to predict the conformational changes induced by different geometric isomers and their subsequent impact on protein-protein interactions within signaling cascades[4]. AbbVie's approach also includes the development of novel synthetic methodologies to generate libraries of geometric isomers with precise control over stereochemistry. This allows for the systematic exploration of structure-activity relationships in the context of signal transduction pathways[6].
Strengths: Integration of computational and experimental approaches; Precise control over isomer generation. Weaknesses: Potential limitations in predicting complex in vivo effects; High computational demands for large-scale simulations.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica NV has developed the IsoSignal platform to investigate the impact of geometric isomers on chemical signal transduction pathways. This platform combines high-resolution structural biology techniques, such as X-ray crystallography and NMR spectroscopy, with advanced cell-based assays to elucidate the molecular mechanisms by which geometric isomers modulate signaling processes[7]. Janssen's approach includes the use of photoswitch-able geometric isomers, allowing for real-time manipulation of isomer conformation in living cells and precise control over signaling events[9]. The company has also implemented CRISPR-Cas9 gene editing techniques to create cellular models with modified signaling components, enabling the study of isomer effects in various genetic backgrounds[11].
Strengths: Real-time manipulation of isomer conformation in living cells; Integration of structural biology with functional assays. Weaknesses: Complexity in translating in vitro findings to in vivo systems; Potential off-target effects of photoswitch-able compounds.
Core Innovations in Isomer-Pathway Interactions
Erbb/BTK inhibitors
PatentPendingEP4356975A2
Innovation
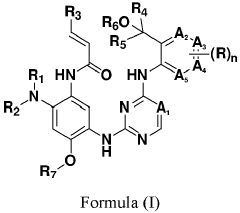
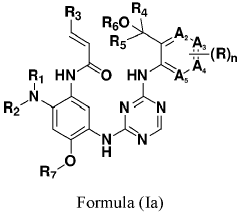
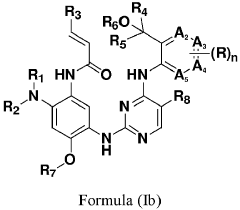
- Development of compounds represented by Formula (I) and its pharmaceutically acceptable salts, esters, hydrates, and stereoisomers, which are used in pharmaceutical compositions to inhibit ErbB family kinases and BTK, particularly targeting mutant forms to enhance therapeutic efficacy.
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Regulatory Landscape for Isomer-Based Drugs
The regulatory landscape for isomer-based drugs is complex and evolving, reflecting the growing understanding of how geometric isomers can significantly impact chemical signal transduction pathways. Regulatory bodies worldwide, including the FDA in the United States and the EMA in Europe, have established specific guidelines for the development and approval of drugs containing geometric isomers.
These regulations primarily focus on ensuring the safety and efficacy of isomer-based drugs, given that different isomers can exhibit varying pharmacological properties. Manufacturers are required to provide comprehensive data on the individual isomers and their mixtures, including detailed information on their physical and chemical properties, as well as their biological activities.
One key aspect of the regulatory framework is the requirement for chiral separation and characterization. Drug developers must demonstrate their ability to isolate and identify individual isomers, as well as control their ratios in the final drug product. This often involves sophisticated analytical techniques such as chiral chromatography and spectroscopy.
Safety assessments for isomer-based drugs are particularly rigorous. Regulatory agencies mandate extensive toxicological studies to evaluate the potential adverse effects of each isomer independently and in combination. This includes assessing the impact on various signal transduction pathways, which can be differentially affected by geometric isomers.
Efficacy evaluations also play a crucial role in the regulatory process. Developers must provide evidence of the therapeutic benefits of specific isomeric forms or ratios, often through comparative clinical trials. This requirement stems from the recognition that geometric isomers can have distinct pharmacodynamic and pharmacokinetic profiles.
The regulatory landscape also addresses manufacturing and quality control aspects. Good Manufacturing Practice (GMP) guidelines have been adapted to include specific provisions for isomer-based drugs, ensuring consistent production of the desired isomeric composition. This includes stringent controls on raw materials, synthesis processes, and final product specifications.
Labeling requirements for isomer-based drugs are another critical regulatory consideration. Product labels must clearly indicate the isomeric composition and any known differences in the biological activities of the isomers. This transparency is essential for healthcare providers and patients to make informed decisions about drug use.
As research continues to uncover the intricate ways in which geometric isomers alter chemical signal transduction pathways, regulatory frameworks are likely to evolve. Future regulations may incorporate more sophisticated approaches to assessing isomer-specific effects on cellular signaling, potentially leading to more personalized and effective therapeutic strategies.
These regulations primarily focus on ensuring the safety and efficacy of isomer-based drugs, given that different isomers can exhibit varying pharmacological properties. Manufacturers are required to provide comprehensive data on the individual isomers and their mixtures, including detailed information on their physical and chemical properties, as well as their biological activities.
One key aspect of the regulatory framework is the requirement for chiral separation and characterization. Drug developers must demonstrate their ability to isolate and identify individual isomers, as well as control their ratios in the final drug product. This often involves sophisticated analytical techniques such as chiral chromatography and spectroscopy.
Safety assessments for isomer-based drugs are particularly rigorous. Regulatory agencies mandate extensive toxicological studies to evaluate the potential adverse effects of each isomer independently and in combination. This includes assessing the impact on various signal transduction pathways, which can be differentially affected by geometric isomers.
Efficacy evaluations also play a crucial role in the regulatory process. Developers must provide evidence of the therapeutic benefits of specific isomeric forms or ratios, often through comparative clinical trials. This requirement stems from the recognition that geometric isomers can have distinct pharmacodynamic and pharmacokinetic profiles.
The regulatory landscape also addresses manufacturing and quality control aspects. Good Manufacturing Practice (GMP) guidelines have been adapted to include specific provisions for isomer-based drugs, ensuring consistent production of the desired isomeric composition. This includes stringent controls on raw materials, synthesis processes, and final product specifications.
Labeling requirements for isomer-based drugs are another critical regulatory consideration. Product labels must clearly indicate the isomeric composition and any known differences in the biological activities of the isomers. This transparency is essential for healthcare providers and patients to make informed decisions about drug use.
As research continues to uncover the intricate ways in which geometric isomers alter chemical signal transduction pathways, regulatory frameworks are likely to evolve. Future regulations may incorporate more sophisticated approaches to assessing isomer-specific effects on cellular signaling, potentially leading to more personalized and effective therapeutic strategies.
Computational Approaches in Isomer-Pathway Modeling
Computational approaches have become increasingly vital in understanding and predicting the complex interactions between geometric isomers and chemical signal transduction pathways. These methods offer powerful tools for modeling and simulating the intricate molecular processes involved in isomer-induced pathway alterations.
One of the primary computational techniques employed in this field is molecular dynamics (MD) simulations. MD simulations allow researchers to observe the dynamic behavior of isomers and their interactions with signaling proteins at an atomic level. By incorporating force fields that accurately represent the structural and energetic properties of different isomers, these simulations can reveal how subtle changes in molecular geometry influence binding affinities and conformational changes in receptor proteins.
Quantum mechanical (QM) calculations provide another essential computational approach for studying isomer-pathway interactions. These methods enable the accurate prediction of electronic properties and reactivity of isomers, which are crucial for understanding their effects on signal transduction. Hybrid QM/MM (quantum mechanics/molecular mechanics) methods have proven particularly useful in modeling the active sites of enzymes involved in signaling cascades, allowing for the investigation of isomer-induced changes in catalytic activity.
Machine learning (ML) algorithms have emerged as powerful tools for analyzing large datasets generated from experimental studies and computational simulations. These algorithms can identify patterns and correlations between isomer structures and their effects on signaling pathways, potentially uncovering novel mechanisms and predicting the outcomes of previously untested isomer-pathway interactions.
Systems biology approaches, such as pathway modeling and network analysis, provide a holistic view of how isomers influence entire signaling networks. By integrating experimental data with computational models, researchers can simulate the propagation of isomer-induced changes through complex signaling cascades, predicting downstream effects and potential feedback mechanisms.
Docking studies and virtual screening techniques offer efficient methods for exploring the binding interactions between isomers and their target proteins. These approaches can rapidly assess the potential of different isomers to modulate specific signaling pathways, guiding experimental efforts and drug discovery initiatives.
As computational power continues to increase, more sophisticated modeling approaches are being developed. These include multiscale modeling techniques that bridge the gap between atomic-level simulations and cellular-level effects, providing a more comprehensive understanding of how geometric isomers alter chemical signal transduction pathways across different biological scales.
One of the primary computational techniques employed in this field is molecular dynamics (MD) simulations. MD simulations allow researchers to observe the dynamic behavior of isomers and their interactions with signaling proteins at an atomic level. By incorporating force fields that accurately represent the structural and energetic properties of different isomers, these simulations can reveal how subtle changes in molecular geometry influence binding affinities and conformational changes in receptor proteins.
Quantum mechanical (QM) calculations provide another essential computational approach for studying isomer-pathway interactions. These methods enable the accurate prediction of electronic properties and reactivity of isomers, which are crucial for understanding their effects on signal transduction. Hybrid QM/MM (quantum mechanics/molecular mechanics) methods have proven particularly useful in modeling the active sites of enzymes involved in signaling cascades, allowing for the investigation of isomer-induced changes in catalytic activity.
Machine learning (ML) algorithms have emerged as powerful tools for analyzing large datasets generated from experimental studies and computational simulations. These algorithms can identify patterns and correlations between isomer structures and their effects on signaling pathways, potentially uncovering novel mechanisms and predicting the outcomes of previously untested isomer-pathway interactions.
Systems biology approaches, such as pathway modeling and network analysis, provide a holistic view of how isomers influence entire signaling networks. By integrating experimental data with computational models, researchers can simulate the propagation of isomer-induced changes through complex signaling cascades, predicting downstream effects and potential feedback mechanisms.
Docking studies and virtual screening techniques offer efficient methods for exploring the binding interactions between isomers and their target proteins. These approaches can rapidly assess the potential of different isomers to modulate specific signaling pathways, guiding experimental efforts and drug discovery initiatives.
As computational power continues to increase, more sophisticated modeling approaches are being developed. These include multiscale modeling techniques that bridge the gap between atomic-level simulations and cellular-level effects, providing a more comprehensive understanding of how geometric isomers alter chemical signal transduction pathways across different biological scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!