How Geometric Isomers Influence Solubility in Different Solvents
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomerism and Solubility: Background and Objectives
Geometric isomerism, a fundamental concept in organic chemistry, plays a crucial role in determining the physical and chemical properties of molecules. This phenomenon occurs when compounds have the same molecular formula but different spatial arrangements of atoms, leading to distinct isomers. The study of how geometric isomers influence solubility in various solvents has gained significant attention in recent years due to its wide-ranging implications in fields such as pharmaceuticals, materials science, and environmental chemistry.
The historical development of geometric isomerism can be traced back to the 19th century, with key contributions from scientists like Johannes Wislicenus and Jacobus Henricus van 't Hoff. Their work laid the foundation for understanding the three-dimensional structure of molecules and its impact on chemical behavior. As research progressed, the relationship between molecular geometry and solubility emerged as a critical area of investigation.
The primary objective of exploring geometric isomerism's influence on solubility is to gain a deeper understanding of the molecular-level interactions between solutes and solvents. This knowledge is essential for predicting and controlling the behavior of chemical compounds in solution, which has far-reaching applications in drug development, chemical processing, and environmental remediation.
One of the key aspects of this research is the examination of how subtle changes in molecular geometry can lead to significant differences in solubility. For instance, cis and trans isomers of the same compound often exhibit markedly different solubilities in polar and non-polar solvents. This phenomenon is attributed to variations in intermolecular forces, dipole moments, and the ability to form hydrogen bonds.
The technological evolution in this field has been driven by advancements in analytical techniques, computational modeling, and experimental methodologies. High-resolution spectroscopy, X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy have enabled researchers to precisely determine molecular structures and study isomerization processes. Concurrently, the development of sophisticated computational tools has allowed for accurate prediction of solubility based on molecular geometry.
As we look towards the future, the study of geometric isomerism and solubility continues to evolve. Emerging trends include the investigation of more complex systems, such as biomolecules and nanomaterials, where geometric isomerism can have profound effects on function and behavior. Additionally, there is growing interest in leveraging this knowledge for the design of smart materials with tunable solubility properties.
The ultimate goal of this research is to establish a comprehensive framework that allows for the precise prediction and control of solubility based on molecular geometry. This would not only enhance our fundamental understanding of chemical interactions but also enable the development of more effective and efficient processes in various industries, from drug delivery systems to environmental cleanup technologies.
The historical development of geometric isomerism can be traced back to the 19th century, with key contributions from scientists like Johannes Wislicenus and Jacobus Henricus van 't Hoff. Their work laid the foundation for understanding the three-dimensional structure of molecules and its impact on chemical behavior. As research progressed, the relationship between molecular geometry and solubility emerged as a critical area of investigation.
The primary objective of exploring geometric isomerism's influence on solubility is to gain a deeper understanding of the molecular-level interactions between solutes and solvents. This knowledge is essential for predicting and controlling the behavior of chemical compounds in solution, which has far-reaching applications in drug development, chemical processing, and environmental remediation.
One of the key aspects of this research is the examination of how subtle changes in molecular geometry can lead to significant differences in solubility. For instance, cis and trans isomers of the same compound often exhibit markedly different solubilities in polar and non-polar solvents. This phenomenon is attributed to variations in intermolecular forces, dipole moments, and the ability to form hydrogen bonds.
The technological evolution in this field has been driven by advancements in analytical techniques, computational modeling, and experimental methodologies. High-resolution spectroscopy, X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy have enabled researchers to precisely determine molecular structures and study isomerization processes. Concurrently, the development of sophisticated computational tools has allowed for accurate prediction of solubility based on molecular geometry.
As we look towards the future, the study of geometric isomerism and solubility continues to evolve. Emerging trends include the investigation of more complex systems, such as biomolecules and nanomaterials, where geometric isomerism can have profound effects on function and behavior. Additionally, there is growing interest in leveraging this knowledge for the design of smart materials with tunable solubility properties.
The ultimate goal of this research is to establish a comprehensive framework that allows for the precise prediction and control of solubility based on molecular geometry. This would not only enhance our fundamental understanding of chemical interactions but also enable the development of more effective and efficient processes in various industries, from drug delivery systems to environmental cleanup technologies.
Market Analysis of Isomer-Specific Solvents
The market for isomer-specific solvents has been experiencing significant growth in recent years, driven by the increasing demand for high-purity chemicals in various industries. The global market for these specialized solvents is expected to reach several billion dollars by 2025, with a compound annual growth rate (CAGR) of over 5% during the forecast period.
One of the key factors contributing to this market growth is the rising adoption of isomer-specific solvents in the pharmaceutical industry. As drug development processes become more complex, the need for solvents that can selectively dissolve specific isomers has become crucial. This trend is particularly evident in the production of chiral drugs, where the separation of enantiomers is essential for achieving desired therapeutic effects.
The electronics industry is another major consumer of isomer-specific solvents. With the increasing miniaturization of electronic components and the development of advanced semiconductor materials, there is a growing demand for solvents that can precisely control the dissolution and deposition of materials at the molecular level. This has led to the development of specialized solvents tailored for specific isomeric configurations.
In the field of materials science, isomer-specific solvents are gaining traction in the production of advanced polymers and composites. The ability to selectively dissolve and manipulate geometric isomers allows for the creation of materials with unique properties, such as enhanced strength, flexibility, or thermal resistance.
The market for isomer-specific solvents is characterized by a high degree of product differentiation and customization. Manufacturers are increasingly focusing on developing solvents that cater to specific applications and industries, leading to a diverse range of products in the market. This trend is expected to continue as end-users demand more specialized solutions for their processes.
Geographically, North America and Europe currently dominate the isomer-specific solvents market, owing to their well-established pharmaceutical and chemical industries. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by the rapid industrialization and increasing investments in research and development activities in countries like China, Japan, and South Korea.
Despite the positive outlook, the market faces challenges such as stringent environmental regulations and the high cost of production for specialized solvents. Manufacturers are investing in research and development to develop more environmentally friendly and cost-effective solutions to address these concerns and maintain their competitive edge in the market.
One of the key factors contributing to this market growth is the rising adoption of isomer-specific solvents in the pharmaceutical industry. As drug development processes become more complex, the need for solvents that can selectively dissolve specific isomers has become crucial. This trend is particularly evident in the production of chiral drugs, where the separation of enantiomers is essential for achieving desired therapeutic effects.
The electronics industry is another major consumer of isomer-specific solvents. With the increasing miniaturization of electronic components and the development of advanced semiconductor materials, there is a growing demand for solvents that can precisely control the dissolution and deposition of materials at the molecular level. This has led to the development of specialized solvents tailored for specific isomeric configurations.
In the field of materials science, isomer-specific solvents are gaining traction in the production of advanced polymers and composites. The ability to selectively dissolve and manipulate geometric isomers allows for the creation of materials with unique properties, such as enhanced strength, flexibility, or thermal resistance.
The market for isomer-specific solvents is characterized by a high degree of product differentiation and customization. Manufacturers are increasingly focusing on developing solvents that cater to specific applications and industries, leading to a diverse range of products in the market. This trend is expected to continue as end-users demand more specialized solutions for their processes.
Geographically, North America and Europe currently dominate the isomer-specific solvents market, owing to their well-established pharmaceutical and chemical industries. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by the rapid industrialization and increasing investments in research and development activities in countries like China, Japan, and South Korea.
Despite the positive outlook, the market faces challenges such as stringent environmental regulations and the high cost of production for specialized solvents. Manufacturers are investing in research and development to develop more environmentally friendly and cost-effective solutions to address these concerns and maintain their competitive edge in the market.
Current Challenges in Isomer Solubility Prediction
Predicting the solubility of geometric isomers in various solvents remains a significant challenge in the field of physical chemistry and pharmaceutical sciences. Despite advancements in computational methods and experimental techniques, several obstacles hinder accurate solubility predictions for these structurally similar yet distinct compounds.
One of the primary challenges is the complexity of intermolecular interactions between isomers and solvents. Geometric isomers, while sharing the same molecular formula, exhibit different spatial arrangements of atoms, leading to variations in their electronic distribution and molecular shape. These subtle differences can significantly impact their interactions with solvent molecules, making it difficult to develop universal models for solubility prediction.
The influence of conformational flexibility on solubility adds another layer of complexity. Geometric isomers often possess multiple conformations that can interconvert in solution, each with its own solubility profile. Current predictive models struggle to account for this dynamic behavior, leading to discrepancies between predicted and observed solubility values.
Environmental factors, such as temperature, pressure, and pH, further complicate solubility predictions. These variables can affect the stability of different isomeric forms and alter their interactions with solvents. Developing models that accurately incorporate these environmental influences remains a significant hurdle in the field.
The lack of comprehensive and reliable experimental data for many geometric isomer-solvent systems poses a substantial challenge. High-quality solubility data is essential for developing and validating predictive models. However, obtaining such data is often time-consuming and resource-intensive, particularly for newly synthesized compounds or uncommon solvents.
Current computational methods, while powerful, still face limitations in accurately representing the complex interplay between isomers and solvents at the molecular level. Quantum mechanical calculations, while theoretically capable of capturing these interactions, are computationally expensive and often impractical for large-scale solubility predictions.
The development of machine learning and artificial intelligence approaches for solubility prediction shows promise but faces challenges in generalizability. These models often struggle to extrapolate beyond their training data, particularly when dealing with novel isomeric structures or solvent systems.
Addressing these challenges requires a multidisciplinary approach, combining advances in experimental techniques, computational methods, and theoretical understanding of isomer-solvent interactions. Improved experimental methodologies for high-throughput solubility measurements, coupled with more sophisticated computational models that can account for conformational flexibility and environmental factors, are needed to enhance the accuracy and reliability of isomer solubility predictions.
One of the primary challenges is the complexity of intermolecular interactions between isomers and solvents. Geometric isomers, while sharing the same molecular formula, exhibit different spatial arrangements of atoms, leading to variations in their electronic distribution and molecular shape. These subtle differences can significantly impact their interactions with solvent molecules, making it difficult to develop universal models for solubility prediction.
The influence of conformational flexibility on solubility adds another layer of complexity. Geometric isomers often possess multiple conformations that can interconvert in solution, each with its own solubility profile. Current predictive models struggle to account for this dynamic behavior, leading to discrepancies between predicted and observed solubility values.
Environmental factors, such as temperature, pressure, and pH, further complicate solubility predictions. These variables can affect the stability of different isomeric forms and alter their interactions with solvents. Developing models that accurately incorporate these environmental influences remains a significant hurdle in the field.
The lack of comprehensive and reliable experimental data for many geometric isomer-solvent systems poses a substantial challenge. High-quality solubility data is essential for developing and validating predictive models. However, obtaining such data is often time-consuming and resource-intensive, particularly for newly synthesized compounds or uncommon solvents.
Current computational methods, while powerful, still face limitations in accurately representing the complex interplay between isomers and solvents at the molecular level. Quantum mechanical calculations, while theoretically capable of capturing these interactions, are computationally expensive and often impractical for large-scale solubility predictions.
The development of machine learning and artificial intelligence approaches for solubility prediction shows promise but faces challenges in generalizability. These models often struggle to extrapolate beyond their training data, particularly when dealing with novel isomeric structures or solvent systems.
Addressing these challenges requires a multidisciplinary approach, combining advances in experimental techniques, computational methods, and theoretical understanding of isomer-solvent interactions. Improved experimental methodologies for high-throughput solubility measurements, coupled with more sophisticated computational models that can account for conformational flexibility and environmental factors, are needed to enhance the accuracy and reliability of isomer solubility predictions.
Existing Methods for Isomer Solubility Determination
01 Solubility differences between geometric isomers
Geometric isomers often exhibit different solubility properties due to their spatial arrangements. These differences can be attributed to variations in intermolecular forces, polarity, and hydrogen bonding capabilities. Understanding these solubility differences is crucial for applications in pharmaceuticals, chemical synthesis, and material science.- Solubility differences between geometric isomers: Geometric isomers often exhibit different solubility properties due to their spatial arrangements. This difference can be exploited in various chemical processes, including separation and purification techniques. The solubility disparities are particularly significant in polar solvents, where the more polar isomer tends to have higher solubility.
- Effect of temperature on geometric isomer solubility: Temperature plays a crucial role in the solubility of geometric isomers. As temperature increases, the solubility of both isomers generally increases, but often at different rates. This temperature-dependent solubility difference can be utilized in selective crystallization processes for isomer separation.
- Solvent selection for geometric isomer separation: The choice of solvent significantly impacts the solubility of geometric isomers. Solvents with different polarities can selectively dissolve one isomer over another, facilitating their separation. This principle is often applied in liquid-liquid extraction and recrystallization techniques for isomer purification.
- pH influence on geometric isomer solubility: The pH of the solution can dramatically affect the solubility of geometric isomers, especially for compounds with ionizable groups. By adjusting the pH, it's possible to alter the ionization state of the isomers, leading to changes in their solubility profiles. This property is often exploited in pharmaceutical formulations and analytical separations.
- Computational methods for predicting isomer solubility: Advanced computational techniques are increasingly used to predict the solubility of geometric isomers. These methods, including molecular dynamics simulations and quantum mechanical calculations, can provide insights into solubility differences without extensive experimental work. Such predictions are valuable in drug design, formulation development, and process optimization.
02 Techniques for enhancing solubility of specific geometric isomers
Various methods can be employed to improve the solubility of certain geometric isomers. These may include the use of co-solvents, surfactants, or complexing agents. Additionally, pH adjustment, temperature control, and particle size reduction can be utilized to enhance the solubility of specific isomers in different solvents.Expand Specific Solutions03 Separation and purification based on solubility differences
The solubility differences between geometric isomers can be exploited for separation and purification purposes. Techniques such as fractional crystallization, selective extraction, and chromatography can be used to isolate specific isomers based on their unique solubility properties in various solvents or under different conditions.Expand Specific Solutions04 Impact of geometric isomerism on drug solubility and bioavailability
Geometric isomerism can significantly affect the solubility and bioavailability of pharmaceutical compounds. Different isomers may have varying solubilities in physiological fluids, leading to differences in absorption, distribution, and overall efficacy. Understanding these effects is crucial for drug development and formulation strategies.Expand Specific Solutions05 Computational methods for predicting geometric isomer solubility
Advanced computational techniques and modeling approaches are being developed to predict the solubility of geometric isomers. These methods may involve quantum mechanical calculations, molecular dynamics simulations, or machine learning algorithms to estimate solubility parameters and behavior of different isomers in various solvents.Expand Specific Solutions
Key Players in Isomer Solubility Research
The field of geometric isomer solubility in different solvents is in a mature stage of development, with established principles and ongoing research. The market for this technology is significant, spanning pharmaceutical, chemical, and materials industries. The technology's maturity is evident in the involvement of major players like Novartis AG and BASF Corp., which have extensive research capabilities and market presence. Academic institutions such as MIT and the University of Nebraska contribute to fundamental research, while specialized companies like Sentinel Oncology Ltd. and SCYNEXIS, Inc. focus on applications in drug discovery. The competitive landscape is diverse, with both large corporations and smaller, specialized firms contributing to advancements in understanding and applying geometric isomer solubility principles.
Novartis AG
Technical Solution: Novartis AG has developed a comprehensive approach to studying geometric isomers and their solubility in various solvents. Their method involves using advanced computational modeling and high-throughput screening techniques to predict and analyze the solubility of different geometric isomers. They employ molecular dynamics simulations to understand the interactions between isomers and solvent molecules at the atomic level[1]. Additionally, Novartis has implemented a machine learning algorithm that can predict solubility based on molecular descriptors and isomeric configurations[3]. This approach allows for rapid assessment of solubility profiles for a wide range of geometric isomers in different solvents, significantly accelerating drug discovery and formulation processes[5].
Strengths: Comprehensive approach combining computational and experimental methods, high-throughput capabilities, and machine learning integration. Weaknesses: May require significant computational resources and expertise to implement and interpret results.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach to studying the influence of geometric isomers on solubility using advanced spectroscopic techniques and theoretical modeling. Their method combines time-resolved laser spectroscopy with quantum mechanical calculations to probe the molecular-level interactions between geometric isomers and solvent molecules[2]. They have also developed a unique microfluidic platform that allows for real-time monitoring of isomerization processes and their effects on solubility[4]. This platform enables researchers to study the dynamic behavior of geometric isomers in various solvents under controlled conditions. Furthermore, MIT has pioneered the use of machine learning algorithms to predict solubility trends based on isomeric structures and solvent properties, significantly enhancing the speed and accuracy of solubility predictions[6].
Strengths: Cutting-edge spectroscopic techniques, innovative microfluidic platform, and integration of machine learning for predictive modeling. Weaknesses: Highly specialized equipment and expertise required, which may limit widespread adoption.
Innovative Approaches in Isomer-Solvent Interactions
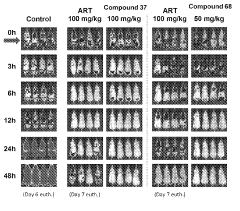
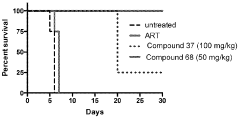
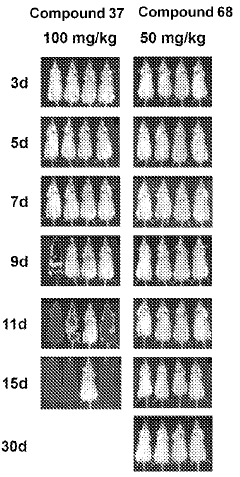
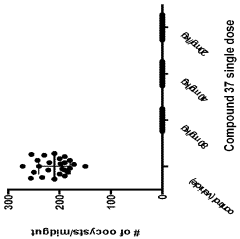
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Low cost and high yield method of making large quantity and homogenous metal nanoparticles and controlling their solubility
PatentInactiveUS20110218304A1
Innovation
- A method involving the use of both hydrophilic and hydrophobic surfactants to control the solubility of metal nanoparticles in water, water-miscible, and non-polar solvents, achieved by dissolving reducible metal precursors with surfactants and a reducing agent under vigorous agitation, allowing for scalable production of nanoparticles with controlled size and solubility.
Environmental Impact of Isomer-Specific Solvents
The environmental impact of isomer-specific solvents is a critical consideration in the context of geometric isomers and their solubility. Different geometric isomers can exhibit varying solubility profiles in different solvents, which can lead to significant environmental consequences. This is particularly relevant in industrial processes, waste management, and environmental remediation efforts.
One of the primary environmental concerns related to isomer-specific solvents is their potential for persistence in ecosystems. Certain geometric isomers may be more resistant to degradation, leading to longer-lasting environmental contamination. This persistence can result in bioaccumulation in various organisms, potentially disrupting food chains and ecosystems.
The solubility differences between geometric isomers can also affect their mobility in the environment. More soluble isomers may be more easily transported through water systems, potentially contaminating groundwater and surface water sources. Conversely, less soluble isomers may accumulate in sediments, creating long-term environmental hazards.
Industrial applications that involve isomer-specific solvents can contribute to air pollution through volatile organic compound (VOC) emissions. The release of these compounds can lead to the formation of ground-level ozone and other air quality issues, impacting both human health and the environment.
The choice of solvents in chemical processes can significantly influence waste generation and disposal. Isomers with higher solubility may require more energy-intensive separation processes, leading to increased energy consumption and associated environmental impacts. Additionally, the disposal of solvent waste containing different geometric isomers may require specialized treatment methods to prevent environmental contamination.
In the context of environmental remediation, understanding the solubility behavior of geometric isomers is crucial for developing effective cleanup strategies. Isomers with different solubilities may require tailored approaches for removal from contaminated sites, potentially increasing the complexity and cost of remediation efforts.
The environmental fate of isomer-specific solvents also depends on their interaction with soil and sediment particles. Adsorption and desorption processes can be influenced by the geometric configuration of isomers, affecting their distribution and persistence in terrestrial environments.
To mitigate the environmental impact of isomer-specific solvents, there is a growing emphasis on green chemistry principles. This includes the development of more environmentally friendly solvents and processes that minimize the use of harmful isomers or exploit the beneficial properties of specific geometric configurations.
One of the primary environmental concerns related to isomer-specific solvents is their potential for persistence in ecosystems. Certain geometric isomers may be more resistant to degradation, leading to longer-lasting environmental contamination. This persistence can result in bioaccumulation in various organisms, potentially disrupting food chains and ecosystems.
The solubility differences between geometric isomers can also affect their mobility in the environment. More soluble isomers may be more easily transported through water systems, potentially contaminating groundwater and surface water sources. Conversely, less soluble isomers may accumulate in sediments, creating long-term environmental hazards.
Industrial applications that involve isomer-specific solvents can contribute to air pollution through volatile organic compound (VOC) emissions. The release of these compounds can lead to the formation of ground-level ozone and other air quality issues, impacting both human health and the environment.
The choice of solvents in chemical processes can significantly influence waste generation and disposal. Isomers with higher solubility may require more energy-intensive separation processes, leading to increased energy consumption and associated environmental impacts. Additionally, the disposal of solvent waste containing different geometric isomers may require specialized treatment methods to prevent environmental contamination.
In the context of environmental remediation, understanding the solubility behavior of geometric isomers is crucial for developing effective cleanup strategies. Isomers with different solubilities may require tailored approaches for removal from contaminated sites, potentially increasing the complexity and cost of remediation efforts.
The environmental fate of isomer-specific solvents also depends on their interaction with soil and sediment particles. Adsorption and desorption processes can be influenced by the geometric configuration of isomers, affecting their distribution and persistence in terrestrial environments.
To mitigate the environmental impact of isomer-specific solvents, there is a growing emphasis on green chemistry principles. This includes the development of more environmentally friendly solvents and processes that minimize the use of harmful isomers or exploit the beneficial properties of specific geometric configurations.
Computational Modeling of Isomer-Solvent Systems
Computational modeling of isomer-solvent systems has become an indispensable tool in understanding the complex interactions between geometric isomers and various solvents. These models provide valuable insights into the molecular-level processes that govern solubility, offering a cost-effective and efficient approach to predicting and analyzing isomer behavior in different solvent environments.
One of the primary computational methods employed in this field is molecular dynamics (MD) simulations. MD simulations allow researchers to observe the time-dependent behavior of molecules, providing a detailed view of how geometric isomers interact with solvent molecules. These simulations can reveal important factors such as hydrogen bonding patterns, electrostatic interactions, and conformational changes that influence solubility.
Quantum mechanical calculations, particularly density functional theory (DFT), are also widely used to model isomer-solvent systems. DFT calculations can provide accurate information about electronic structures, energetics, and molecular geometries. This approach is particularly useful for understanding how the electronic properties of geometric isomers affect their interactions with different solvents.
Monte Carlo simulations offer another powerful computational technique for studying isomer-solvent systems. These simulations are especially effective in exploring the configurational space of complex molecular systems, allowing researchers to predict thermodynamic properties and phase behavior of isomers in various solvents.
Continuum solvation models, such as the polarizable continuum model (PCM) and the conductor-like screening model (COSMO), provide a computationally efficient way to account for solvent effects. These models treat the solvent as a continuous medium, allowing for rapid calculations of solvation energies and other properties for different isomer-solvent combinations.
Machine learning approaches are increasingly being applied to the study of isomer-solvent systems. By leveraging large datasets of experimental and computational results, machine learning algorithms can identify patterns and make predictions about solubility behavior, potentially accelerating the discovery of new solvent systems for specific applications.
The integration of these computational methods with experimental data has led to the development of predictive models that can accurately estimate the solubility of geometric isomers in a wide range of solvents. These models not only enhance our understanding of fundamental solubility mechanisms but also have practical applications in fields such as drug discovery, materials science, and chemical engineering.
One of the primary computational methods employed in this field is molecular dynamics (MD) simulations. MD simulations allow researchers to observe the time-dependent behavior of molecules, providing a detailed view of how geometric isomers interact with solvent molecules. These simulations can reveal important factors such as hydrogen bonding patterns, electrostatic interactions, and conformational changes that influence solubility.
Quantum mechanical calculations, particularly density functional theory (DFT), are also widely used to model isomer-solvent systems. DFT calculations can provide accurate information about electronic structures, energetics, and molecular geometries. This approach is particularly useful for understanding how the electronic properties of geometric isomers affect their interactions with different solvents.
Monte Carlo simulations offer another powerful computational technique for studying isomer-solvent systems. These simulations are especially effective in exploring the configurational space of complex molecular systems, allowing researchers to predict thermodynamic properties and phase behavior of isomers in various solvents.
Continuum solvation models, such as the polarizable continuum model (PCM) and the conductor-like screening model (COSMO), provide a computationally efficient way to account for solvent effects. These models treat the solvent as a continuous medium, allowing for rapid calculations of solvation energies and other properties for different isomer-solvent combinations.
Machine learning approaches are increasingly being applied to the study of isomer-solvent systems. By leveraging large datasets of experimental and computational results, machine learning algorithms can identify patterns and make predictions about solubility behavior, potentially accelerating the discovery of new solvent systems for specific applications.
The integration of these computational methods with experimental data has led to the development of predictive models that can accurately estimate the solubility of geometric isomers in a wide range of solvents. These models not only enhance our understanding of fundamental solubility mechanisms but also have practical applications in fields such as drug discovery, materials science, and chemical engineering.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!