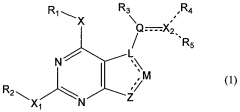
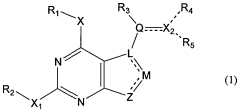
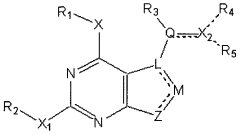
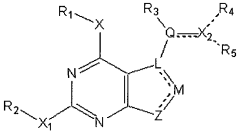
How Geometric Isomers Influence Toxicological Profiles of Compounds
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomerism in Toxicology: Background and Objectives
Geometric isomerism, a fundamental concept in organic chemistry, plays a crucial role in determining the toxicological profiles of compounds. This phenomenon occurs when molecules have the same molecular formula and bonding sequence but differ in the spatial arrangement of their atoms. The study of how geometric isomers influence toxicological profiles has gained significant attention in recent years due to its profound implications for drug development, environmental safety, and regulatory policies.
The historical context of this field dates back to the early 20th century when researchers first recognized the importance of molecular structure in biological activity. However, it was not until the 1960s that scientists began to systematically investigate the relationship between geometric isomerism and toxicity. This shift was largely driven by advances in analytical techniques, such as X-ray crystallography and nuclear magnetic resonance spectroscopy, which allowed for more precise determination of molecular structures.
Over the past few decades, the field has witnessed remarkable progress, with researchers uncovering numerous examples of geometric isomers exhibiting vastly different toxicological properties. These findings have had far-reaching consequences, particularly in the pharmaceutical industry, where understanding the toxicological implications of different isomers has become a critical aspect of drug design and safety assessment.
The primary objective of studying geometric isomerism in toxicology is to elucidate the mechanisms by which subtle changes in molecular geometry can lead to significant alterations in biological activity and toxicity. This knowledge is essential for developing safer and more effective drugs, as well as for predicting and mitigating potential environmental hazards associated with chemical compounds.
Furthermore, this research aims to establish structure-activity relationships that can guide the rational design of molecules with desired toxicological profiles. By understanding how geometric isomerism influences toxicity, scientists can potentially manipulate molecular structures to enhance therapeutic efficacy while minimizing adverse effects.
Another key goal is to develop more accurate predictive models for toxicity assessment. As regulatory agencies increasingly emphasize the importance of in silico methods for toxicity prediction, incorporating geometric isomerism into these models becomes crucial for improving their accuracy and reliability.
In the broader context of technological advancement, the study of geometric isomerism in toxicology aligns with the growing trend towards precision medicine and personalized risk assessment. By considering the specific isomeric forms of compounds, researchers can potentially tailor treatments and safety measures to individual genetic profiles, leading to more effective and safer interventions.
The historical context of this field dates back to the early 20th century when researchers first recognized the importance of molecular structure in biological activity. However, it was not until the 1960s that scientists began to systematically investigate the relationship between geometric isomerism and toxicity. This shift was largely driven by advances in analytical techniques, such as X-ray crystallography and nuclear magnetic resonance spectroscopy, which allowed for more precise determination of molecular structures.
Over the past few decades, the field has witnessed remarkable progress, with researchers uncovering numerous examples of geometric isomers exhibiting vastly different toxicological properties. These findings have had far-reaching consequences, particularly in the pharmaceutical industry, where understanding the toxicological implications of different isomers has become a critical aspect of drug design and safety assessment.
The primary objective of studying geometric isomerism in toxicology is to elucidate the mechanisms by which subtle changes in molecular geometry can lead to significant alterations in biological activity and toxicity. This knowledge is essential for developing safer and more effective drugs, as well as for predicting and mitigating potential environmental hazards associated with chemical compounds.
Furthermore, this research aims to establish structure-activity relationships that can guide the rational design of molecules with desired toxicological profiles. By understanding how geometric isomerism influences toxicity, scientists can potentially manipulate molecular structures to enhance therapeutic efficacy while minimizing adverse effects.
Another key goal is to develop more accurate predictive models for toxicity assessment. As regulatory agencies increasingly emphasize the importance of in silico methods for toxicity prediction, incorporating geometric isomerism into these models becomes crucial for improving their accuracy and reliability.
In the broader context of technological advancement, the study of geometric isomerism in toxicology aligns with the growing trend towards precision medicine and personalized risk assessment. By considering the specific isomeric forms of compounds, researchers can potentially tailor treatments and safety measures to individual genetic profiles, leading to more effective and safer interventions.
Market Demand for Isomer-Specific Toxicity Assessment
The market demand for isomer-specific toxicity assessment has been steadily growing in recent years, driven by increasing awareness of the significant impact geometric isomers can have on compound toxicity. This demand spans across various industries, including pharmaceuticals, agrochemicals, and environmental protection.
In the pharmaceutical sector, there is a pressing need for more accurate toxicity profiling of drug candidates. Geometric isomers often exhibit different pharmacological and toxicological properties, which can greatly influence drug efficacy and safety. As regulatory bodies tighten their requirements for drug approval, pharmaceutical companies are investing heavily in isomer-specific toxicity assessment to minimize the risk of late-stage failures and potential post-market withdrawals.
The agrochemical industry also faces growing pressure to develop safer and more environmentally friendly products. With increasing public concern over pesticide residues in food and their environmental impact, there is a strong market demand for tools that can accurately predict the toxicity of different geometric isomers of agrochemicals. This allows for the development of more targeted and less harmful crop protection solutions.
Environmental protection agencies and regulatory bodies are another significant driver of market demand. As our understanding of environmental toxicology advances, there is an increasing recognition of the need to assess the specific toxicity profiles of geometric isomers in environmental contaminants. This is particularly important for persistent organic pollutants, where small structural differences can lead to significant variations in bioaccumulation and toxicity.
The cosmetics and personal care products industry is also showing growing interest in isomer-specific toxicity assessment. With consumers becoming more conscious of product safety and ingredient transparency, companies are seeking more detailed toxicological data to ensure product safety and comply with stringent regulatory standards.
Furthermore, the emerging field of green chemistry is creating new market opportunities for isomer-specific toxicity assessment. As companies strive to develop more sustainable and less toxic chemical processes, there is a growing need for tools that can accurately predict the toxicological profiles of different geometric isomers early in the product development cycle.
Overall, the market demand for isomer-specific toxicity assessment is expected to continue its upward trajectory. This is driven by a combination of regulatory pressures, consumer awareness, and industry initiatives towards safer and more sustainable products across multiple sectors.
In the pharmaceutical sector, there is a pressing need for more accurate toxicity profiling of drug candidates. Geometric isomers often exhibit different pharmacological and toxicological properties, which can greatly influence drug efficacy and safety. As regulatory bodies tighten their requirements for drug approval, pharmaceutical companies are investing heavily in isomer-specific toxicity assessment to minimize the risk of late-stage failures and potential post-market withdrawals.
The agrochemical industry also faces growing pressure to develop safer and more environmentally friendly products. With increasing public concern over pesticide residues in food and their environmental impact, there is a strong market demand for tools that can accurately predict the toxicity of different geometric isomers of agrochemicals. This allows for the development of more targeted and less harmful crop protection solutions.
Environmental protection agencies and regulatory bodies are another significant driver of market demand. As our understanding of environmental toxicology advances, there is an increasing recognition of the need to assess the specific toxicity profiles of geometric isomers in environmental contaminants. This is particularly important for persistent organic pollutants, where small structural differences can lead to significant variations in bioaccumulation and toxicity.
The cosmetics and personal care products industry is also showing growing interest in isomer-specific toxicity assessment. With consumers becoming more conscious of product safety and ingredient transparency, companies are seeking more detailed toxicological data to ensure product safety and comply with stringent regulatory standards.
Furthermore, the emerging field of green chemistry is creating new market opportunities for isomer-specific toxicity assessment. As companies strive to develop more sustainable and less toxic chemical processes, there is a growing need for tools that can accurately predict the toxicological profiles of different geometric isomers early in the product development cycle.
Overall, the market demand for isomer-specific toxicity assessment is expected to continue its upward trajectory. This is driven by a combination of regulatory pressures, consumer awareness, and industry initiatives towards safer and more sustainable products across multiple sectors.
Current Challenges in Geometric Isomer Toxicology
The field of geometric isomer toxicology faces several significant challenges that hinder our comprehensive understanding of how these compounds affect biological systems. One of the primary obstacles is the complexity of isomer-specific toxicity mechanisms. Geometric isomers, despite having the same molecular formula, can exhibit vastly different toxicological profiles due to their spatial arrangements. This variability makes it difficult to predict and generalize the toxic effects of compounds based solely on their chemical structure.
Another major challenge lies in the development of accurate and efficient analytical methods for distinguishing between geometric isomers. Current techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, while effective, often require sophisticated equipment and expertise. This limitation hampers the widespread implementation of isomer-specific toxicity testing in routine safety assessments.
The lack of standardized protocols for evaluating the toxicological impact of geometric isomers presents a significant hurdle. Without consistent methodologies, it becomes challenging to compare results across different studies and draw meaningful conclusions. This inconsistency can lead to conflicting data and interpretations, complicating regulatory decision-making processes.
Furthermore, the biological fate of geometric isomers within living systems poses a complex challenge. The metabolic pathways and biotransformation processes of these compounds can differ significantly, leading to the formation of various metabolites with potentially diverse toxicological profiles. Tracking these metabolic changes and understanding their implications for toxicity is a formidable task that requires advanced analytical and computational approaches.
The environmental persistence and transformation of geometric isomers add another layer of complexity to toxicological assessments. Factors such as UV radiation, pH changes, and microbial activity can induce isomerization or degradation, altering the toxicological properties of these compounds in the environment. This dynamic nature makes it challenging to predict long-term ecological impacts and design appropriate risk assessment strategies.
Lastly, the integration of geometric isomer toxicology into regulatory frameworks remains a significant challenge. Current guidelines often do not adequately address the specific considerations required for assessing the safety of geometric isomers. This gap in regulatory approaches can lead to inconsistencies in safety evaluations and potentially overlook important toxicological differences between isomers.
Another major challenge lies in the development of accurate and efficient analytical methods for distinguishing between geometric isomers. Current techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, while effective, often require sophisticated equipment and expertise. This limitation hampers the widespread implementation of isomer-specific toxicity testing in routine safety assessments.
The lack of standardized protocols for evaluating the toxicological impact of geometric isomers presents a significant hurdle. Without consistent methodologies, it becomes challenging to compare results across different studies and draw meaningful conclusions. This inconsistency can lead to conflicting data and interpretations, complicating regulatory decision-making processes.
Furthermore, the biological fate of geometric isomers within living systems poses a complex challenge. The metabolic pathways and biotransformation processes of these compounds can differ significantly, leading to the formation of various metabolites with potentially diverse toxicological profiles. Tracking these metabolic changes and understanding their implications for toxicity is a formidable task that requires advanced analytical and computational approaches.
The environmental persistence and transformation of geometric isomers add another layer of complexity to toxicological assessments. Factors such as UV radiation, pH changes, and microbial activity can induce isomerization or degradation, altering the toxicological properties of these compounds in the environment. This dynamic nature makes it challenging to predict long-term ecological impacts and design appropriate risk assessment strategies.
Lastly, the integration of geometric isomer toxicology into regulatory frameworks remains a significant challenge. Current guidelines often do not adequately address the specific considerations required for assessing the safety of geometric isomers. This gap in regulatory approaches can lead to inconsistencies in safety evaluations and potentially overlook important toxicological differences between isomers.
Existing Approaches for Isomer Toxicity Differentiation
01 Toxicological profiles of geometric isomers in pharmaceuticals
Geometric isomers of pharmaceutical compounds can exhibit different toxicological profiles. The spatial arrangement of atoms in these isomers can affect their interactions with biological systems, potentially leading to variations in toxicity, efficacy, and side effects. Understanding these differences is crucial for drug development and safety assessment.- Toxicological profiles of geometric isomers in pharmaceuticals: Geometric isomers of pharmaceutical compounds can exhibit different toxicological profiles. These differences are crucial in drug development and safety assessment. Researchers study the toxicity, metabolism, and biological effects of various geometric isomers to determine their suitability for therapeutic use and potential side effects.
- Environmental impact of geometric isomers: Geometric isomers of chemical compounds can have varying effects on the environment. Their toxicological profiles are important in assessing ecological risks, biodegradation, and bioaccumulation potential. Understanding these differences is crucial for environmental protection and regulatory compliance in chemical manufacturing and waste management.
- Analytical methods for distinguishing geometric isomers: Developing and improving analytical techniques to distinguish between geometric isomers is essential for accurate toxicological profiling. These methods may include spectroscopic techniques, chromatography, and advanced imaging technologies. Precise identification and quantification of geometric isomers are crucial for toxicity assessments and quality control in various industries.
- Geometric isomers in pesticides and their toxicological implications: Pesticides often contain geometric isomers with different toxicological profiles. Understanding these differences is crucial for developing safer and more effective pest control products. Researchers investigate the toxicity, environmental fate, and potential health impacts of various geometric isomers in pesticide formulations to optimize their use and minimize risks.
- Toxicological considerations in the synthesis of geometric isomers: The synthesis of geometric isomers requires careful consideration of potential toxicological impacts. Researchers develop methods to selectively produce desired isomers while minimizing the formation of toxic byproducts. This approach is crucial in the pharmaceutical, agrochemical, and fine chemical industries to ensure product safety and regulatory compliance.
02 Environmental impact of geometric isomers in pesticides
Geometric isomers of pesticides can have varying toxicological profiles, affecting their environmental persistence, bioaccumulation, and impact on non-target organisms. The differences in spatial arrangement can influence their interaction with soil, water, and biological systems, leading to distinct ecological consequences.Expand Specific Solutions03 Analytical methods for distinguishing geometric isomers
Developing and implementing analytical techniques to distinguish between geometric isomers is essential for accurate toxicological profiling. These methods may include spectroscopic techniques, chromatography, and advanced imaging technologies, enabling researchers to identify and quantify different isomers in complex mixtures.Expand Specific Solutions04 Regulatory considerations for geometric isomers
Regulatory agencies may require separate toxicological assessments for geometric isomers of chemical substances. This approach recognizes that isomers can have distinct biological activities and safety profiles, necessitating individual evaluation for risk assessment and regulatory approval processes.Expand Specific Solutions05 Structure-activity relationships in geometric isomers
Investigating the structure-activity relationships of geometric isomers can provide insights into their toxicological profiles. Understanding how subtle changes in spatial arrangement affect biological interactions can aid in predicting toxicity, designing safer chemicals, and developing more effective risk assessment strategies.Expand Specific Solutions
Key Players in Isomeric Compound Research and Testing
The competitive landscape for understanding how geometric isomers influence toxicological profiles of compounds is in a growth phase, with increasing market size and technological advancements. The field is attracting attention from both pharmaceutical giants and specialized biotech firms, indicating its strategic importance. Companies like AbbVie, Pfizer, and Astex Therapeutics are leveraging their extensive R&D capabilities to explore this area, while innovative startups such as Foghorn Therapeutics and Sentinel Oncology are bringing fresh approaches. The technology's maturity is evolving, with academic institutions like Harvard and EPFL contributing fundamental research, and companies like Fochon Pharmaceuticals and Dizal Pharmaceutical advancing practical applications, suggesting a collaborative ecosystem driving progress in this critical area of drug development.
President & Fellows of Harvard College
Technical Solution: Harvard's research on geometric isomers and toxicology focuses on understanding the molecular mechanisms underlying isomer-specific toxicity. Their approach combines advanced computational chemistry with innovative experimental techniques. Harvard researchers have developed machine learning algorithms that can predict toxicological profiles based on the geometric properties of molecules[7]. These models incorporate data from large-scale toxicogenomic studies, allowing for the identification of subtle structure-toxicity relationships. Additionally, Harvard has pioneered the use of single-cell sequencing technologies to study how different geometric isomers affect gene expression patterns in various cell types, providing unprecedented resolution in toxicity profiling[8]. Their work has also extended to investigating how environmental factors and epigenetic modifications can influence the toxicological impact of geometric isomers, offering a more comprehensive understanding of real-world toxicity scenarios.
Strengths: Cutting-edge computational and experimental techniques, holistic approach incorporating environmental and epigenetic factors. Weaknesses: Potential challenges in scaling up to high-throughput industrial applications, complexity in translating multi-factorial findings to practical guidelines.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has developed a multi-omics approach to study the influence of geometric isomers on toxicological profiles. Their strategy integrates genomics, proteomics, and metabolomics data to create comprehensive toxicity signatures for different geometric isomers. The institute has established a large-scale screening platform that can rapidly assess the toxicological impact of numerous geometric isomers across multiple cell types and model organisms[9]. This high-throughput system is coupled with advanced bioinformatics tools that can identify subtle patterns in toxicity profiles, allowing for the prediction of potential adverse effects early in the drug discovery process. The Broad Institute has also pioneered the use of CRISPR-based genetic screens to identify cellular pathways that mediate isomer-specific toxicity, providing valuable insights into the mechanisms of toxicological differences between geometric isomers[10]. Their research has led to the development of novel biomarkers for isomer-specific toxicity, enhancing the ability to predict and mitigate adverse effects in drug development.
Strengths: Comprehensive multi-omics approach, high-throughput screening capabilities, advanced bioinformatics for pattern recognition. Weaknesses: Potential overreliance on in vitro systems, challenges in translating complex data sets into actionable insights for drug development.
Innovative Techniques in Geometric Isomer Toxicology
Methods of preparation and resolution of e/z isomers of vinylfuro[2,3-d] pyrimidine and their biological activities
PatentWO2007109661A2
Innovation
- Development of methods for the preparation, resolution, and isolation of individual E- and Z-isomers of 2,4-substituted-5-vinylfuro[2,3-d]pyrimidine compounds using optimized reaction conditions, catalysis, chromatography, and crystallization to separate and purify these isomers, allowing for the production of pharmaceutically acceptable salts and prodrugs, which can act as receptor tyrosine kinase inhibitors and anti-angiogenic agents.
Erbb/BTK inhibitors
PatentPendingEP4356975A2
Innovation
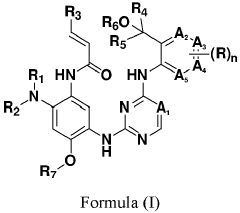
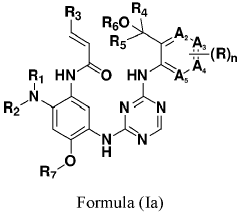
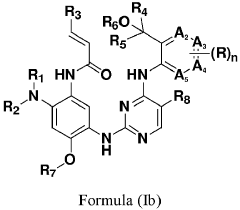
- Development of compounds represented by Formula (I) and its pharmaceutically acceptable salts, esters, hydrates, and stereoisomers, which are used in pharmaceutical compositions to inhibit ErbB family kinases and BTK, particularly targeting mutant forms to enhance therapeutic efficacy.
Regulatory Framework for Isomeric Compound Safety
The regulatory framework for isomeric compound safety is a critical aspect of pharmaceutical and chemical industry oversight. Regulatory bodies worldwide have established guidelines and protocols to assess the safety of geometric isomers, recognizing their potential to exhibit different toxicological profiles despite having the same molecular formula.
In the United States, the Food and Drug Administration (FDA) has implemented specific requirements for the evaluation of geometric isomers in drug development. The agency mandates that pharmaceutical companies provide comprehensive data on all relevant isomers, including their individual toxicological profiles and potential interactions. This approach ensures a thorough understanding of the safety implications associated with different geometric configurations.
Similarly, the European Medicines Agency (EMA) has developed guidelines for the assessment of isomeric compounds. These guidelines emphasize the importance of characterizing each isomer separately and evaluating their potential impact on drug efficacy and safety. The EMA requires manufacturers to provide detailed information on the isomeric composition of drug substances and products, as well as any potential differences in their pharmacological and toxicological properties.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has also addressed the issue of isomeric compounds in its guidelines. The ICH Q3A(R2) guideline on impurities in new drug substances specifically mentions the need to consider geometric isomers as potential impurities and evaluate their safety implications.
Regulatory frameworks often require manufacturers to develop analytical methods capable of distinguishing between different geometric isomers. This is crucial for quality control and ensuring that the desired isomer is present in the correct proportion. Techniques such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy are commonly employed for this purpose.
In the field of environmental regulation, agencies such as the Environmental Protection Agency (EPA) in the United States have established protocols for assessing the ecological impact of isomeric compounds. These protocols take into account the potential differences in environmental fate and toxicity between geometric isomers, particularly in the context of pesticides and industrial chemicals.
The regulatory landscape for isomeric compound safety continues to evolve as new scientific evidence emerges. Regulatory bodies are increasingly adopting a risk-based approach, focusing on the specific toxicological profiles of individual isomers rather than treating them as a single entity. This shift reflects a growing recognition of the complex relationship between molecular structure and biological activity, and the need for more nuanced safety assessments in the development and regulation of chemical compounds.
In the United States, the Food and Drug Administration (FDA) has implemented specific requirements for the evaluation of geometric isomers in drug development. The agency mandates that pharmaceutical companies provide comprehensive data on all relevant isomers, including their individual toxicological profiles and potential interactions. This approach ensures a thorough understanding of the safety implications associated with different geometric configurations.
Similarly, the European Medicines Agency (EMA) has developed guidelines for the assessment of isomeric compounds. These guidelines emphasize the importance of characterizing each isomer separately and evaluating their potential impact on drug efficacy and safety. The EMA requires manufacturers to provide detailed information on the isomeric composition of drug substances and products, as well as any potential differences in their pharmacological and toxicological properties.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has also addressed the issue of isomeric compounds in its guidelines. The ICH Q3A(R2) guideline on impurities in new drug substances specifically mentions the need to consider geometric isomers as potential impurities and evaluate their safety implications.
Regulatory frameworks often require manufacturers to develop analytical methods capable of distinguishing between different geometric isomers. This is crucial for quality control and ensuring that the desired isomer is present in the correct proportion. Techniques such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy are commonly employed for this purpose.
In the field of environmental regulation, agencies such as the Environmental Protection Agency (EPA) in the United States have established protocols for assessing the ecological impact of isomeric compounds. These protocols take into account the potential differences in environmental fate and toxicity between geometric isomers, particularly in the context of pesticides and industrial chemicals.
The regulatory landscape for isomeric compound safety continues to evolve as new scientific evidence emerges. Regulatory bodies are increasingly adopting a risk-based approach, focusing on the specific toxicological profiles of individual isomers rather than treating them as a single entity. This shift reflects a growing recognition of the complex relationship between molecular structure and biological activity, and the need for more nuanced safety assessments in the development and regulation of chemical compounds.
Computational Methods in Isomer Toxicity Prediction
Computational methods have become increasingly important in predicting the toxicological profiles of geometric isomers. These methods leverage advanced algorithms and machine learning techniques to analyze the structural differences between isomers and their potential impact on toxicity.
One of the primary computational approaches is quantitative structure-activity relationship (QSAR) modeling. QSAR models use mathematical equations to correlate the chemical structure of isomers with their biological activities, including toxicity. These models can be trained on large datasets of known isomers and their toxicological profiles, allowing for the prediction of toxicity for novel compounds.
Molecular docking simulations are another valuable tool in isomer toxicity prediction. These simulations model the interaction between isomers and biological targets, such as proteins or enzymes, to assess potential toxic effects. By comparing the binding affinities and interaction patterns of different geometric isomers, researchers can gain insights into their relative toxicities.
Machine learning algorithms, particularly deep learning neural networks, have shown promise in predicting isomer toxicity. These algorithms can process complex structural data and identify subtle patterns that may influence toxicity. Convolutional neural networks (CNNs) and graph neural networks (GNNs) are particularly well-suited for analyzing the spatial arrangements of atoms in geometric isomers.
Molecular dynamics simulations provide a dynamic view of isomer behavior in biological systems. These simulations can reveal how the three-dimensional structure of isomers affects their interactions with cellular components, potentially leading to toxic effects. By comparing the behavior of different geometric isomers over time, researchers can identify key factors contributing to toxicity.
Quantum mechanical calculations offer insights into the electronic properties of isomers, which can influence their reactivity and toxicity. Density functional theory (DFT) calculations, for example, can provide information on molecular orbitals, charge distributions, and reactivity indices, all of which may correlate with toxicological profiles.
Integrative approaches that combine multiple computational methods are becoming increasingly popular. These approaches leverage the strengths of different techniques to provide a more comprehensive prediction of isomer toxicity. For instance, combining QSAR modeling with molecular dynamics simulations and quantum mechanical calculations can offer a multi-scale perspective on toxicity mechanisms.
As computational power continues to increase and algorithms become more sophisticated, these methods are likely to play an even more significant role in predicting and understanding the toxicological profiles of geometric isomers. This will contribute to more efficient and ethical drug development processes, as well as improved risk assessment for environmental contaminants and industrial chemicals.
One of the primary computational approaches is quantitative structure-activity relationship (QSAR) modeling. QSAR models use mathematical equations to correlate the chemical structure of isomers with their biological activities, including toxicity. These models can be trained on large datasets of known isomers and their toxicological profiles, allowing for the prediction of toxicity for novel compounds.
Molecular docking simulations are another valuable tool in isomer toxicity prediction. These simulations model the interaction between isomers and biological targets, such as proteins or enzymes, to assess potential toxic effects. By comparing the binding affinities and interaction patterns of different geometric isomers, researchers can gain insights into their relative toxicities.
Machine learning algorithms, particularly deep learning neural networks, have shown promise in predicting isomer toxicity. These algorithms can process complex structural data and identify subtle patterns that may influence toxicity. Convolutional neural networks (CNNs) and graph neural networks (GNNs) are particularly well-suited for analyzing the spatial arrangements of atoms in geometric isomers.
Molecular dynamics simulations provide a dynamic view of isomer behavior in biological systems. These simulations can reveal how the three-dimensional structure of isomers affects their interactions with cellular components, potentially leading to toxic effects. By comparing the behavior of different geometric isomers over time, researchers can identify key factors contributing to toxicity.
Quantum mechanical calculations offer insights into the electronic properties of isomers, which can influence their reactivity and toxicity. Density functional theory (DFT) calculations, for example, can provide information on molecular orbitals, charge distributions, and reactivity indices, all of which may correlate with toxicological profiles.
Integrative approaches that combine multiple computational methods are becoming increasingly popular. These approaches leverage the strengths of different techniques to provide a more comprehensive prediction of isomer toxicity. For instance, combining QSAR modeling with molecular dynamics simulations and quantum mechanical calculations can offer a multi-scale perspective on toxicity mechanisms.
As computational power continues to increase and algorithms become more sophisticated, these methods are likely to play an even more significant role in predicting and understanding the toxicological profiles of geometric isomers. This will contribute to more efficient and ethical drug development processes, as well as improved risk assessment for environmental contaminants and industrial chemicals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!