How Glycerol-Based Polymers Contribute to Medical Device Innovation
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycerol-Based Polymers in Medical Devices: Overview and Objectives
Glycerol-based polymers have emerged as a promising class of materials in the field of medical device innovation. These polymers, derived from the renewable resource glycerol, offer unique properties that make them particularly suitable for various biomedical applications. The development of glycerol-based polymers represents a significant advancement in biomaterials science, addressing the growing demand for sustainable and biocompatible materials in healthcare.
The primary objective of utilizing glycerol-based polymers in medical devices is to enhance biocompatibility, improve functionality, and reduce environmental impact. These polymers can be tailored to exhibit a wide range of physical and chemical properties, making them versatile candidates for diverse medical applications. From drug delivery systems to tissue engineering scaffolds, glycerol-based polymers are being explored for their potential to revolutionize medical device design and performance.
One of the key advantages of glycerol-based polymers is their inherent biocompatibility. Glycerol, a natural component of lipids, is well-tolerated by the human body, minimizing the risk of adverse reactions when used in medical devices. This characteristic makes glycerol-based polymers particularly attractive for implantable devices and tissue-contacting applications, where long-term compatibility is crucial.
The versatility of glycerol-based polymers allows for the development of materials with tunable properties. By adjusting the polymer composition and synthesis methods, researchers can create materials with specific mechanical strengths, degradation rates, and surface properties. This flexibility enables the design of medical devices that can better mimic natural tissues or provide targeted functionalities for specific medical applications.
Furthermore, the use of glycerol-based polymers aligns with the growing emphasis on sustainable and eco-friendly materials in the medical industry. As a renewable resource, glycerol offers a more environmentally responsible alternative to petroleum-based polymers. This aspect is particularly relevant in the context of increasing environmental awareness and regulatory pressures to reduce the carbon footprint of medical products.
The evolution of glycerol-based polymers in medical devices is driven by ongoing research and technological advancements. Scientists and engineers are continually exploring new synthesis methods, polymer architectures, and functionalization strategies to expand the capabilities of these materials. This research aims to overcome current limitations and unlock new possibilities for medical device innovation.
As the field progresses, glycerol-based polymers are expected to play a crucial role in addressing unmet needs in healthcare. From improving the performance of existing medical devices to enabling entirely new therapeutic approaches, these materials hold significant promise. The continued development and application of glycerol-based polymers in medical devices represent a convergence of sustainability, biocompatibility, and advanced functionality, paving the way for next-generation healthcare solutions.
The primary objective of utilizing glycerol-based polymers in medical devices is to enhance biocompatibility, improve functionality, and reduce environmental impact. These polymers can be tailored to exhibit a wide range of physical and chemical properties, making them versatile candidates for diverse medical applications. From drug delivery systems to tissue engineering scaffolds, glycerol-based polymers are being explored for their potential to revolutionize medical device design and performance.
One of the key advantages of glycerol-based polymers is their inherent biocompatibility. Glycerol, a natural component of lipids, is well-tolerated by the human body, minimizing the risk of adverse reactions when used in medical devices. This characteristic makes glycerol-based polymers particularly attractive for implantable devices and tissue-contacting applications, where long-term compatibility is crucial.
The versatility of glycerol-based polymers allows for the development of materials with tunable properties. By adjusting the polymer composition and synthesis methods, researchers can create materials with specific mechanical strengths, degradation rates, and surface properties. This flexibility enables the design of medical devices that can better mimic natural tissues or provide targeted functionalities for specific medical applications.
Furthermore, the use of glycerol-based polymers aligns with the growing emphasis on sustainable and eco-friendly materials in the medical industry. As a renewable resource, glycerol offers a more environmentally responsible alternative to petroleum-based polymers. This aspect is particularly relevant in the context of increasing environmental awareness and regulatory pressures to reduce the carbon footprint of medical products.
The evolution of glycerol-based polymers in medical devices is driven by ongoing research and technological advancements. Scientists and engineers are continually exploring new synthesis methods, polymer architectures, and functionalization strategies to expand the capabilities of these materials. This research aims to overcome current limitations and unlock new possibilities for medical device innovation.
As the field progresses, glycerol-based polymers are expected to play a crucial role in addressing unmet needs in healthcare. From improving the performance of existing medical devices to enabling entirely new therapeutic approaches, these materials hold significant promise. The continued development and application of glycerol-based polymers in medical devices represent a convergence of sustainability, biocompatibility, and advanced functionality, paving the way for next-generation healthcare solutions.
Market Analysis for Glycerol-Based Medical Polymers
The market for glycerol-based medical polymers has experienced significant growth in recent years, driven by the increasing demand for biocompatible and sustainable materials in the healthcare industry. These polymers offer unique properties that make them particularly suitable for medical device applications, including biodegradability, biocompatibility, and versatility in terms of mechanical and physical characteristics.
The global market for glycerol-based medical polymers is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to exceed that of traditional petroleum-based polymers in the medical sector. This growth is primarily attributed to the rising awareness of environmental concerns and the push for more sustainable healthcare solutions.
Key market segments for glycerol-based medical polymers include wound care, drug delivery systems, tissue engineering, and implantable devices. The wound care segment, in particular, has shown robust growth due to the increasing prevalence of chronic wounds and the need for advanced wound healing solutions. Glycerol-based polymers offer excellent moisture retention properties and can be engineered to release therapeutic agents, making them ideal for advanced wound dressings.
In the drug delivery systems market, glycerol-based polymers are gaining traction due to their ability to encapsulate and release drugs in a controlled manner. This characteristic is particularly valuable in the development of long-acting injectable formulations and targeted drug delivery systems, which are becoming increasingly important in the treatment of chronic diseases and cancer.
The tissue engineering segment represents a promising growth area for glycerol-based polymers. These materials can be tailored to mimic the extracellular matrix, providing an ideal scaffold for cell growth and tissue regeneration. As regenerative medicine advances, the demand for biocompatible and biodegradable scaffolds is expected to surge, further driving the market for glycerol-based polymers.
Geographically, North America and Europe currently dominate the market for glycerol-based medical polymers, owing to their advanced healthcare infrastructure and strong focus on innovation. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, fueled by increasing healthcare expenditure, growing awareness of sustainable materials, and the rapid expansion of the medical device industry in countries like China and India.
Despite the positive outlook, the market faces challenges such as the higher cost of glycerol-based polymers compared to traditional materials and the need for extensive regulatory approvals. However, ongoing research and development efforts are expected to address these challenges, potentially leading to more cost-effective production methods and streamlined regulatory pathways.
The global market for glycerol-based medical polymers is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to exceed that of traditional petroleum-based polymers in the medical sector. This growth is primarily attributed to the rising awareness of environmental concerns and the push for more sustainable healthcare solutions.
Key market segments for glycerol-based medical polymers include wound care, drug delivery systems, tissue engineering, and implantable devices. The wound care segment, in particular, has shown robust growth due to the increasing prevalence of chronic wounds and the need for advanced wound healing solutions. Glycerol-based polymers offer excellent moisture retention properties and can be engineered to release therapeutic agents, making them ideal for advanced wound dressings.
In the drug delivery systems market, glycerol-based polymers are gaining traction due to their ability to encapsulate and release drugs in a controlled manner. This characteristic is particularly valuable in the development of long-acting injectable formulations and targeted drug delivery systems, which are becoming increasingly important in the treatment of chronic diseases and cancer.
The tissue engineering segment represents a promising growth area for glycerol-based polymers. These materials can be tailored to mimic the extracellular matrix, providing an ideal scaffold for cell growth and tissue regeneration. As regenerative medicine advances, the demand for biocompatible and biodegradable scaffolds is expected to surge, further driving the market for glycerol-based polymers.
Geographically, North America and Europe currently dominate the market for glycerol-based medical polymers, owing to their advanced healthcare infrastructure and strong focus on innovation. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, fueled by increasing healthcare expenditure, growing awareness of sustainable materials, and the rapid expansion of the medical device industry in countries like China and India.
Despite the positive outlook, the market faces challenges such as the higher cost of glycerol-based polymers compared to traditional materials and the need for extensive regulatory approvals. However, ongoing research and development efforts are expected to address these challenges, potentially leading to more cost-effective production methods and streamlined regulatory pathways.
Current Challenges in Glycerol-Based Polymer Development
Despite the promising potential of glycerol-based polymers in medical device innovation, several significant challenges persist in their development and application. One of the primary obstacles is achieving consistent and reproducible mechanical properties. The inherent variability in glycerol-based polymer structures can lead to inconsistencies in strength, flexibility, and durability, which are critical factors in medical device performance and reliability.
Another major challenge lies in the biocompatibility and long-term stability of these polymers within the human body. While glycerol-based polymers generally exhibit good initial biocompatibility, ensuring their sustained performance and resistance to degradation over extended periods remains a complex issue. This is particularly crucial for implantable devices or those intended for long-term use.
The scalability of production processes for glycerol-based polymers presents another significant hurdle. Current synthesis methods often yield limited quantities or require complex procedures, making large-scale manufacturing challenging and potentially cost-prohibitive. This limitation hampers the widespread adoption of these materials in medical device manufacturing.
Furthermore, regulatory compliance poses a substantial challenge. The novel nature of glycerol-based polymers means that regulatory frameworks may not be fully established for their use in medical devices. Manufacturers face the task of navigating complex approval processes and demonstrating the safety and efficacy of these materials in various medical applications.
The optimization of surface properties is another area of ongoing challenge. Tailoring the surface characteristics of glycerol-based polymers to enhance their interaction with biological tissues or to prevent unwanted reactions (such as protein adsorption or bacterial adhesion) requires sophisticated engineering approaches that are still being developed.
Lastly, the integration of glycerol-based polymers with other materials and components in medical devices presents technical difficulties. Ensuring strong and durable bonds between these polymers and other device materials, as well as maintaining the integrity of the polymer during sterilization processes, are areas that demand continued research and innovation.
Addressing these challenges requires a multidisciplinary approach, combining expertise in polymer chemistry, materials science, bioengineering, and medical device design. As research progresses, overcoming these hurdles will be crucial in fully realizing the potential of glycerol-based polymers in advancing medical device technology.
Another major challenge lies in the biocompatibility and long-term stability of these polymers within the human body. While glycerol-based polymers generally exhibit good initial biocompatibility, ensuring their sustained performance and resistance to degradation over extended periods remains a complex issue. This is particularly crucial for implantable devices or those intended for long-term use.
The scalability of production processes for glycerol-based polymers presents another significant hurdle. Current synthesis methods often yield limited quantities or require complex procedures, making large-scale manufacturing challenging and potentially cost-prohibitive. This limitation hampers the widespread adoption of these materials in medical device manufacturing.
Furthermore, regulatory compliance poses a substantial challenge. The novel nature of glycerol-based polymers means that regulatory frameworks may not be fully established for their use in medical devices. Manufacturers face the task of navigating complex approval processes and demonstrating the safety and efficacy of these materials in various medical applications.
The optimization of surface properties is another area of ongoing challenge. Tailoring the surface characteristics of glycerol-based polymers to enhance their interaction with biological tissues or to prevent unwanted reactions (such as protein adsorption or bacterial adhesion) requires sophisticated engineering approaches that are still being developed.
Lastly, the integration of glycerol-based polymers with other materials and components in medical devices presents technical difficulties. Ensuring strong and durable bonds between these polymers and other device materials, as well as maintaining the integrity of the polymer during sterilization processes, are areas that demand continued research and innovation.
Addressing these challenges requires a multidisciplinary approach, combining expertise in polymer chemistry, materials science, bioengineering, and medical device design. As research progresses, overcoming these hurdles will be crucial in fully realizing the potential of glycerol-based polymers in advancing medical device technology.
Existing Applications of Glycerol-Based Polymers in Medical Devices
01 Synthesis of glycerol-based polymers
Various methods for synthesizing glycerol-based polymers are described, including polymerization techniques and the use of different catalysts. These processes aim to create polymers with specific properties for various applications, such as biodegradable materials or specialty chemicals.- Synthesis of glycerol-based polymers: Various methods for synthesizing glycerol-based polymers are described, including polymerization techniques and the use of different catalysts. These processes aim to create polymers with specific properties for various applications, such as biodegradable materials or specialty chemicals.
- Applications of glycerol-based polymers in biomedicine: Glycerol-based polymers are utilized in biomedical applications due to their biocompatibility and biodegradability. These polymers can be used in drug delivery systems, tissue engineering scaffolds, and other medical devices, offering potential advantages over traditional synthetic polymers.
- Glycerol-based polymers for environmental applications: The use of glycerol-based polymers in environmental applications is explored, including their potential for biodegradable plastics, water treatment, and as alternatives to petroleum-based materials. These polymers offer eco-friendly solutions to various environmental challenges.
- Modification and functionalization of glycerol-based polymers: Techniques for modifying and functionalizing glycerol-based polymers are described, including the incorporation of various functional groups or the creation of copolymers. These modifications aim to enhance the properties and expand the potential applications of the polymers.
- Characterization and analysis of glycerol-based polymers: Methods for characterizing and analyzing glycerol-based polymers are discussed, including techniques for determining molecular weight, structure, and thermal properties. These analytical approaches are crucial for understanding and optimizing the performance of glycerol-based polymers in various applications.
02 Applications of glycerol-based polymers in biomedicine
Glycerol-based polymers are utilized in biomedical applications due to their biocompatibility and biodegradability. These polymers can be used in drug delivery systems, tissue engineering scaffolds, and other medical devices, offering potential advantages over traditional synthetic polymers.Expand Specific Solutions03 Glycerol-based polymers for environmental applications
The use of glycerol-based polymers in environmental applications is explored, including their potential for biodegradable plastics, water treatment, and soil remediation. These polymers offer eco-friendly alternatives to conventional petroleum-based materials.Expand Specific Solutions04 Modification of glycerol-based polymers
Techniques for modifying glycerol-based polymers to enhance their properties or functionalities are described. These modifications can include chemical alterations, blending with other materials, or the incorporation of additives to improve characteristics such as mechanical strength, thermal stability, or specific reactivity.Expand Specific Solutions05 Industrial production of glycerol-based polymers
Methods and processes for the large-scale industrial production of glycerol-based polymers are outlined. This includes reactor designs, process optimization, and quality control measures to ensure consistent and efficient manufacturing of these polymers for commercial applications.Expand Specific Solutions
Key Players in Medical-Grade Glycerol Polymer Industry
The glycerol-based polymers market for medical device innovation is in a growth phase, driven by increasing demand for biocompatible materials in healthcare. The market size is expanding, with projections indicating significant growth potential in the coming years. Technologically, glycerol-based polymers are advancing rapidly, with companies like Ethicon, Boston Scientific, and Medtronic leading innovation. These firms are developing novel applications in areas such as wound healing, drug delivery, and tissue engineering. The technology's maturity varies across applications, with some products already commercialized while others are still in research and development stages. Universities like Zurich and Boston are contributing to fundamental research, while companies focus on practical applications and product development.
Ethicon, Inc.
Technical Solution: Ethicon, a Johnson & Johnson company, has developed innovative glycerol-based polymers for medical devices, particularly in surgical applications. Their technology focuses on creating absorbable sutures and surgical meshes using glycerol-based polymers. These materials offer improved biocompatibility and controlled degradation rates, enhancing patient outcomes. Ethicon's approach involves copolymerizing glycerol with other monomers to create tailored polymers with specific mechanical properties and degradation profiles[1]. This allows for the development of sutures that maintain tensile strength for an appropriate duration before being safely absorbed by the body[2]. Additionally, Ethicon has explored the use of glycerol-based polymers in drug-eluting devices, where the polymer matrix can be designed to release therapeutic agents at controlled rates[3].
Strengths: Excellent biocompatibility, controllable degradation rates, and versatility in surgical applications. Weaknesses: Potential for higher production costs compared to traditional materials and limited long-term clinical data for some applications.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular has pioneered the use of glycerol-based polymers in cardiovascular devices, particularly in drug-eluting stents and balloon catheters. Their technology incorporates glycerol-based polymers as coatings on metal stents or as the primary material in biodegradable stents. These polymers are engineered to provide controlled release of anti-restenosis drugs while gradually degrading over time[4]. Medtronic's approach involves optimizing the polymer composition to achieve the desired drug release kinetics and mechanical properties. They have developed proprietary polymer blends that incorporate glycerol to enhance the hydrophilicity and biocompatibility of the coating[5]. This technology has shown promise in reducing the risk of late stent thrombosis and improving long-term outcomes for patients with coronary artery disease[6].
Strengths: Advanced drug delivery capabilities, reduced risk of long-term complications, and improved healing response. Weaknesses: Complex manufacturing process and potential for higher initial costs compared to bare-metal stents.
Innovative Glycerol Polymer Formulations and Properties
Implantable Polymeric Films
PatentActiveUS20120082712A1
Innovation
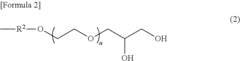
- The development of implantable medical devices featuring a polymeric film layer made from a mixture of glycerol and a biopolymer, with a weight ratio ranging from 3:1 to 1:3, which includes a therapeutic agent, ensuring the film's strength and flexibility for effective drug delivery.
Polymer for medical device, medical device material and medical device prepared from the material, and monomer composition for polymer for use in production of medical device
PatentActiveUS20170157304A1
Innovation
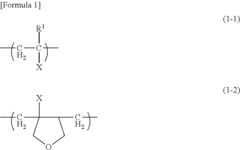
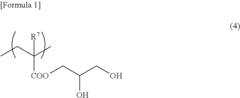
- Development of polymers with structural units derived from glycerol group-containing monomers and unsaturated monomers with organic groups bound to ethylenically unsaturated groups, exhibiting glass-transition temperatures of 10°C or less at saturated water content, which enhance antithrombogenicity and suppress platelet adhesion.
Regulatory Considerations for Glycerol-Based Medical Polymers
The regulatory landscape for glycerol-based medical polymers is complex and multifaceted, requiring careful consideration throughout the development and commercialization process. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the safety and efficacy of medical devices incorporating these polymers. The regulatory pathway for such devices typically depends on their intended use and risk classification.
For Class I devices, which pose minimal risk, manufacturers may often proceed with a 510(k) premarket notification, demonstrating substantial equivalence to a predicate device. However, more complex devices utilizing glycerol-based polymers may fall into Class II or III categories, necessitating more rigorous premarket approval (PMA) processes.
The FDA's guidance on biocompatibility testing is particularly relevant for glycerol-based polymers. Manufacturers must conduct comprehensive tests to ensure these materials do not elicit adverse biological responses when in contact with human tissues. This includes cytotoxicity, sensitization, and irritation studies, as well as more specific tests depending on the device's intended use and duration of contact.
In the European Union, the Medical Device Regulation (MDR) governs the approval process for devices incorporating glycerol-based polymers. The MDR places a strong emphasis on clinical evidence and post-market surveillance, requiring manufacturers to demonstrate ongoing safety and performance throughout the product lifecycle.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of medical devices. For glycerol-based polymers, this translates to a growing need for manufacturers to consider biodegradability and sustainable sourcing in their product development strategies. This trend is likely to influence future regulatory requirements and guidelines.
As nanotechnology advances, the use of glycerol-based nanocomposites in medical devices is gaining traction. This presents new regulatory challenges, as agencies grapple with assessing the unique properties and potential risks associated with nanomaterials. Manufacturers working with these advanced materials should anticipate evolving regulatory frameworks and proactively engage with authorities to navigate this emerging landscape.
Harmonization efforts, such as those led by the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions. For glycerol-based polymer manufacturers, staying abreast of these harmonization initiatives can facilitate smoother market access and reduce regulatory burdens associated with multi-country submissions.
For Class I devices, which pose minimal risk, manufacturers may often proceed with a 510(k) premarket notification, demonstrating substantial equivalence to a predicate device. However, more complex devices utilizing glycerol-based polymers may fall into Class II or III categories, necessitating more rigorous premarket approval (PMA) processes.
The FDA's guidance on biocompatibility testing is particularly relevant for glycerol-based polymers. Manufacturers must conduct comprehensive tests to ensure these materials do not elicit adverse biological responses when in contact with human tissues. This includes cytotoxicity, sensitization, and irritation studies, as well as more specific tests depending on the device's intended use and duration of contact.
In the European Union, the Medical Device Regulation (MDR) governs the approval process for devices incorporating glycerol-based polymers. The MDR places a strong emphasis on clinical evidence and post-market surveillance, requiring manufacturers to demonstrate ongoing safety and performance throughout the product lifecycle.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of medical devices. For glycerol-based polymers, this translates to a growing need for manufacturers to consider biodegradability and sustainable sourcing in their product development strategies. This trend is likely to influence future regulatory requirements and guidelines.
As nanotechnology advances, the use of glycerol-based nanocomposites in medical devices is gaining traction. This presents new regulatory challenges, as agencies grapple with assessing the unique properties and potential risks associated with nanomaterials. Manufacturers working with these advanced materials should anticipate evolving regulatory frameworks and proactively engage with authorities to navigate this emerging landscape.
Harmonization efforts, such as those led by the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes across different regions. For glycerol-based polymer manufacturers, staying abreast of these harmonization initiatives can facilitate smoother market access and reduce regulatory burdens associated with multi-country submissions.
Biocompatibility and Safety Assessment of Glycerol Polymers
The biocompatibility and safety assessment of glycerol polymers is a critical aspect in their application for medical device innovation. These assessments ensure that the materials used in medical devices do not cause adverse effects when in contact with biological systems, particularly the human body.
Glycerol-based polymers have shown promising results in terms of biocompatibility due to their structural similarity to natural biological molecules. The presence of hydroxyl groups in glycerol polymers allows for enhanced hydrophilicity, which can reduce protein adsorption and subsequent inflammatory responses when used in medical devices.
Safety assessments of glycerol polymers typically involve a series of in vitro and in vivo tests. These tests evaluate cytotoxicity, genotoxicity, carcinogenicity, and potential for allergic reactions. In vitro studies often include cell culture experiments to assess the material's effect on cell viability, proliferation, and function. More advanced in vitro models, such as 3D cell cultures or organ-on-a-chip systems, can provide insights into tissue-level responses.
In vivo studies are crucial for understanding the long-term effects of glycerol polymers in a physiological environment. Animal models are used to evaluate local tissue responses, systemic toxicity, and potential degradation products. These studies also help in assessing the polymer's performance under physiological conditions and its interaction with various biological systems.
The degradation profile of glycerol-based polymers is a key factor in their safety assessment. Understanding the rate and products of degradation is essential to predict potential long-term effects. Glycerol, being a natural metabolite, is generally considered safe. However, the safety of other degradation products must be thoroughly evaluated.
Regulatory bodies, such as the FDA and EMA, have established guidelines for the biocompatibility testing of medical devices. These guidelines, including ISO 10993 standards, provide a framework for comprehensive safety evaluations. Adherence to these standards is crucial for the successful development and approval of medical devices incorporating glycerol polymers.
Recent advancements in analytical techniques have enhanced the ability to assess the safety of glycerol polymers. High-resolution imaging methods, such as atomic force microscopy and confocal microscopy, allow for detailed examination of material-tissue interfaces. Advanced spectroscopic techniques enable the detection of minute quantities of leachables or degradation products.
The biocompatibility and safety profile of glycerol polymers can be further improved through surface modifications or the incorporation of bioactive molecules. These strategies can enhance cell adhesion, promote tissue integration, or impart antimicrobial properties, thereby expanding the potential applications in medical devices.
Glycerol-based polymers have shown promising results in terms of biocompatibility due to their structural similarity to natural biological molecules. The presence of hydroxyl groups in glycerol polymers allows for enhanced hydrophilicity, which can reduce protein adsorption and subsequent inflammatory responses when used in medical devices.
Safety assessments of glycerol polymers typically involve a series of in vitro and in vivo tests. These tests evaluate cytotoxicity, genotoxicity, carcinogenicity, and potential for allergic reactions. In vitro studies often include cell culture experiments to assess the material's effect on cell viability, proliferation, and function. More advanced in vitro models, such as 3D cell cultures or organ-on-a-chip systems, can provide insights into tissue-level responses.
In vivo studies are crucial for understanding the long-term effects of glycerol polymers in a physiological environment. Animal models are used to evaluate local tissue responses, systemic toxicity, and potential degradation products. These studies also help in assessing the polymer's performance under physiological conditions and its interaction with various biological systems.
The degradation profile of glycerol-based polymers is a key factor in their safety assessment. Understanding the rate and products of degradation is essential to predict potential long-term effects. Glycerol, being a natural metabolite, is generally considered safe. However, the safety of other degradation products must be thoroughly evaluated.
Regulatory bodies, such as the FDA and EMA, have established guidelines for the biocompatibility testing of medical devices. These guidelines, including ISO 10993 standards, provide a framework for comprehensive safety evaluations. Adherence to these standards is crucial for the successful development and approval of medical devices incorporating glycerol polymers.
Recent advancements in analytical techniques have enhanced the ability to assess the safety of glycerol polymers. High-resolution imaging methods, such as atomic force microscopy and confocal microscopy, allow for detailed examination of material-tissue interfaces. Advanced spectroscopic techniques enable the detection of minute quantities of leachables or degradation products.
The biocompatibility and safety profile of glycerol polymers can be further improved through surface modifications or the incorporation of bioactive molecules. These strategies can enhance cell adhesion, promote tissue integration, or impart antimicrobial properties, thereby expanding the potential applications in medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!