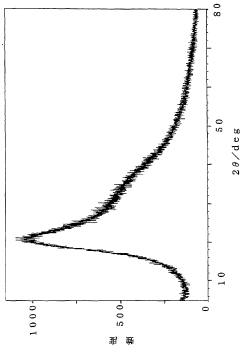
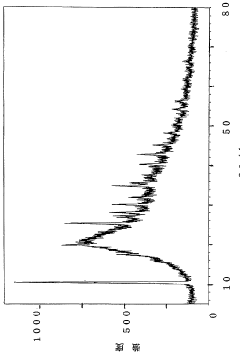
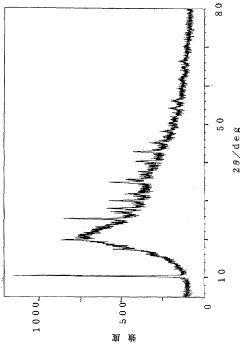
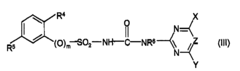
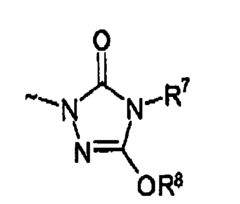
How Glycerol Improves Suspension Stability in Pharmaceuticals
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Glycerol in Pharmaceuticals: Background and Objectives
Glycerol, also known as glycerin or glycerine, has been a staple ingredient in pharmaceutical formulations for decades. Its history in medicine dates back to the late 19th century when it was first recognized for its therapeutic properties. As a versatile excipient, glycerol has played a crucial role in enhancing the stability, solubility, and efficacy of various pharmaceutical preparations.
The evolution of glycerol's use in pharmaceuticals has been closely tied to advancements in drug delivery systems and formulation technologies. Initially utilized primarily as a sweetening agent and preservative, glycerol's potential as a suspension stabilizer became increasingly apparent in the mid-20th century. This realization led to extensive research into its physicochemical properties and their impact on pharmaceutical suspensions.
In recent years, the pharmaceutical industry has witnessed a growing demand for more stable and effective suspension formulations. This trend is driven by the need to improve patient compliance, enhance drug bioavailability, and extend product shelf life. As a result, the role of glycerol in suspension stability has gained renewed attention from researchers and formulators alike.
The primary objective of investigating glycerol's impact on suspension stability is to develop more robust and reliable pharmaceutical products. By understanding the mechanisms through which glycerol improves suspension characteristics, formulators can optimize drug formulations for enhanced stability, uniformity, and therapeutic efficacy. This knowledge is particularly valuable for drugs with poor aqueous solubility or those prone to sedimentation and aggregation in suspension form.
Furthermore, the exploration of glycerol's stabilizing properties aligns with the industry's broader goals of developing patient-centric dosage forms and reducing the environmental impact of pharmaceutical manufacturing. Glycerol, being a natural and biodegradable compound, offers a sustainable alternative to synthetic stabilizers, addressing growing concerns about the ecological footprint of pharmaceutical products.
As we delve deeper into the role of glycerol in pharmaceutical suspensions, it is essential to consider the multifaceted nature of suspension stability. This includes factors such as particle size distribution, rheological properties, and the interactions between suspended particles and the dispersion medium. By elucidating how glycerol influences these parameters, we can pave the way for innovative formulation strategies and potentially expand the range of drugs that can be effectively delivered via suspension formulations.
The evolution of glycerol's use in pharmaceuticals has been closely tied to advancements in drug delivery systems and formulation technologies. Initially utilized primarily as a sweetening agent and preservative, glycerol's potential as a suspension stabilizer became increasingly apparent in the mid-20th century. This realization led to extensive research into its physicochemical properties and their impact on pharmaceutical suspensions.
In recent years, the pharmaceutical industry has witnessed a growing demand for more stable and effective suspension formulations. This trend is driven by the need to improve patient compliance, enhance drug bioavailability, and extend product shelf life. As a result, the role of glycerol in suspension stability has gained renewed attention from researchers and formulators alike.
The primary objective of investigating glycerol's impact on suspension stability is to develop more robust and reliable pharmaceutical products. By understanding the mechanisms through which glycerol improves suspension characteristics, formulators can optimize drug formulations for enhanced stability, uniformity, and therapeutic efficacy. This knowledge is particularly valuable for drugs with poor aqueous solubility or those prone to sedimentation and aggregation in suspension form.
Furthermore, the exploration of glycerol's stabilizing properties aligns with the industry's broader goals of developing patient-centric dosage forms and reducing the environmental impact of pharmaceutical manufacturing. Glycerol, being a natural and biodegradable compound, offers a sustainable alternative to synthetic stabilizers, addressing growing concerns about the ecological footprint of pharmaceutical products.
As we delve deeper into the role of glycerol in pharmaceutical suspensions, it is essential to consider the multifaceted nature of suspension stability. This includes factors such as particle size distribution, rheological properties, and the interactions between suspended particles and the dispersion medium. By elucidating how glycerol influences these parameters, we can pave the way for innovative formulation strategies and potentially expand the range of drugs that can be effectively delivered via suspension formulations.
Market Analysis of Stable Pharmaceutical Suspensions
The global market for stable pharmaceutical suspensions has been experiencing significant growth, driven by the increasing demand for liquid dosage forms and the need for improved drug delivery systems. This market segment is particularly important for pediatric and geriatric populations, who often have difficulty swallowing solid dosage forms. The use of glycerol as a stabilizing agent in these formulations has gained traction due to its effectiveness in improving suspension stability and enhancing overall product quality.
Market research indicates that the pharmaceutical suspension market is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is attributed to several factors, including the rising prevalence of chronic diseases, the expansion of the biopharmaceutical industry, and the increasing focus on patient-centric drug delivery systems.
The demand for stable pharmaceutical suspensions is particularly strong in emerging markets, where there is a growing need for cost-effective and easily administrable medications. Countries in Asia-Pacific and Latin America are showing rapid market expansion, driven by improving healthcare infrastructure and increasing healthcare expenditure. In contrast, mature markets such as North America and Europe are experiencing steady growth, with a focus on innovative formulations and enhanced stability profiles.
Within the pharmaceutical suspension market, there is a notable trend towards the development of long-acting injectable suspensions. These formulations offer advantages such as reduced dosing frequency and improved patient compliance. The use of glycerol in these advanced suspension systems is being explored to further enhance their stability and efficacy.
The competitive landscape of the stable pharmaceutical suspension market is characterized by the presence of both large pharmaceutical companies and specialized formulation development firms. Key players are investing heavily in research and development to improve suspension stability techniques, with glycerol-based formulations gaining prominence. Patent filings related to glycerol-enhanced suspension stability have seen an uptick in recent years, indicating growing interest and innovation in this area.
Regulatory considerations play a crucial role in shaping the market for stable pharmaceutical suspensions. Stringent quality standards and the need for bioequivalence studies for generic suspensions are influencing product development strategies. The use of glycerol as a stabilizing agent is generally well-received by regulatory bodies due to its long history of safe use in pharmaceutical formulations.
In conclusion, the market analysis reveals a robust and growing demand for stable pharmaceutical suspensions, with glycerol playing an increasingly important role in formulation development. As the industry continues to innovate and address unmet medical needs, the market for glycerol-enhanced suspension formulations is poised for sustained growth and technological advancements.
Market research indicates that the pharmaceutical suspension market is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years. This growth is attributed to several factors, including the rising prevalence of chronic diseases, the expansion of the biopharmaceutical industry, and the increasing focus on patient-centric drug delivery systems.
The demand for stable pharmaceutical suspensions is particularly strong in emerging markets, where there is a growing need for cost-effective and easily administrable medications. Countries in Asia-Pacific and Latin America are showing rapid market expansion, driven by improving healthcare infrastructure and increasing healthcare expenditure. In contrast, mature markets such as North America and Europe are experiencing steady growth, with a focus on innovative formulations and enhanced stability profiles.
Within the pharmaceutical suspension market, there is a notable trend towards the development of long-acting injectable suspensions. These formulations offer advantages such as reduced dosing frequency and improved patient compliance. The use of glycerol in these advanced suspension systems is being explored to further enhance their stability and efficacy.
The competitive landscape of the stable pharmaceutical suspension market is characterized by the presence of both large pharmaceutical companies and specialized formulation development firms. Key players are investing heavily in research and development to improve suspension stability techniques, with glycerol-based formulations gaining prominence. Patent filings related to glycerol-enhanced suspension stability have seen an uptick in recent years, indicating growing interest and innovation in this area.
Regulatory considerations play a crucial role in shaping the market for stable pharmaceutical suspensions. Stringent quality standards and the need for bioequivalence studies for generic suspensions are influencing product development strategies. The use of glycerol as a stabilizing agent is generally well-received by regulatory bodies due to its long history of safe use in pharmaceutical formulations.
In conclusion, the market analysis reveals a robust and growing demand for stable pharmaceutical suspensions, with glycerol playing an increasingly important role in formulation development. As the industry continues to innovate and address unmet medical needs, the market for glycerol-enhanced suspension formulations is poised for sustained growth and technological advancements.
Current Challenges in Suspension Stability
Suspension stability remains a critical challenge in pharmaceutical formulations, particularly for oral and injectable medications. One of the primary issues is particle sedimentation, which can lead to non-uniform dosing and reduced efficacy. This problem is exacerbated by the varying particle sizes and densities commonly found in pharmaceutical suspensions, making it difficult to maintain a homogeneous distribution over time.
Another significant challenge is the tendency for suspended particles to aggregate or flocculate. This phenomenon can alter the suspension's rheological properties, potentially affecting its flow characteristics and ease of administration. Moreover, particle aggregation can lead to cake formation at the bottom of the container, which may be difficult to redisperse, compromising the product's quality and patient safety.
The stability of suspensions is also highly sensitive to environmental factors such as temperature fluctuations and mechanical stress during transportation and storage. These external influences can trigger physical and chemical changes in the suspension, potentially leading to phase separation or degradation of active pharmaceutical ingredients.
Maintaining the chemical stability of suspended drugs presents another hurdle. The large surface area of suspended particles can increase their reactivity, potentially leading to accelerated degradation or unwanted interactions with other components in the formulation. This is particularly problematic for drugs with limited aqueous solubility, where the suspension form is often the only viable option for liquid administration.
The choice of suspending agents and stabilizers is crucial yet challenging. While these additives are necessary to improve suspension stability, they must be carefully selected to avoid adverse effects on bioavailability, therapeutic efficacy, or patient acceptability. Finding the right balance between stability enhancement and maintaining other critical quality attributes of the pharmaceutical product is a complex task.
Regulatory considerations add another layer of complexity to suspension stability challenges. Demonstrating long-term stability and consistent quality throughout the product's shelf life is essential for regulatory approval. This requires extensive stability testing under various conditions, which can be time-consuming and resource-intensive.
Addressing these challenges often requires a multifaceted approach, combining innovative formulation strategies, advanced characterization techniques, and a deep understanding of the physicochemical properties of both the drug and the suspension system. The use of novel excipients, such as glycerol, offers promising avenues for improving suspension stability, but their integration into pharmaceutical formulations must be carefully evaluated to ensure safety, efficacy, and regulatory compliance.
Another significant challenge is the tendency for suspended particles to aggregate or flocculate. This phenomenon can alter the suspension's rheological properties, potentially affecting its flow characteristics and ease of administration. Moreover, particle aggregation can lead to cake formation at the bottom of the container, which may be difficult to redisperse, compromising the product's quality and patient safety.
The stability of suspensions is also highly sensitive to environmental factors such as temperature fluctuations and mechanical stress during transportation and storage. These external influences can trigger physical and chemical changes in the suspension, potentially leading to phase separation or degradation of active pharmaceutical ingredients.
Maintaining the chemical stability of suspended drugs presents another hurdle. The large surface area of suspended particles can increase their reactivity, potentially leading to accelerated degradation or unwanted interactions with other components in the formulation. This is particularly problematic for drugs with limited aqueous solubility, where the suspension form is often the only viable option for liquid administration.
The choice of suspending agents and stabilizers is crucial yet challenging. While these additives are necessary to improve suspension stability, they must be carefully selected to avoid adverse effects on bioavailability, therapeutic efficacy, or patient acceptability. Finding the right balance between stability enhancement and maintaining other critical quality attributes of the pharmaceutical product is a complex task.
Regulatory considerations add another layer of complexity to suspension stability challenges. Demonstrating long-term stability and consistent quality throughout the product's shelf life is essential for regulatory approval. This requires extensive stability testing under various conditions, which can be time-consuming and resource-intensive.
Addressing these challenges often requires a multifaceted approach, combining innovative formulation strategies, advanced characterization techniques, and a deep understanding of the physicochemical properties of both the drug and the suspension system. The use of novel excipients, such as glycerol, offers promising avenues for improving suspension stability, but their integration into pharmaceutical formulations must be carefully evaluated to ensure safety, efficacy, and regulatory compliance.
Glycerol-based Suspension Stabilization Methods
01 Stabilization of glycerol suspensions using additives
Various additives can be used to enhance the stability of glycerol suspensions. These may include surfactants, emulsifiers, or other stabilizing agents that help prevent particle aggregation and sedimentation. The choice of additives depends on the specific components of the suspension and the desired properties.- Stabilization of glycerol suspensions using additives: Various additives can be used to enhance the stability of glycerol suspensions. These may include surfactants, emulsifiers, or other stabilizing agents that help prevent particle aggregation and sedimentation. The choice of additives depends on the specific components of the suspension and the desired properties of the final product.
- Temperature control for glycerol suspension stability: Maintaining appropriate temperature conditions is crucial for the stability of glycerol suspensions. Temperature fluctuations can affect viscosity, solubility, and particle interactions. Implementing precise temperature control systems during production, storage, and transportation can significantly improve the long-term stability of glycerol-based suspensions.
- Particle size optimization in glycerol suspensions: The size and distribution of particles in a glycerol suspension play a critical role in its stability. Optimizing particle size through various techniques such as milling, homogenization, or controlled crystallization can enhance suspension stability by reducing sedimentation and improving overall uniformity.
- pH adjustment for glycerol suspension stability: Controlling the pH of glycerol suspensions can significantly impact their stability. Adjusting the pH to an optimal range can help prevent chemical degradation, minimize particle aggregation, and maintain the desired rheological properties of the suspension over time.
- Rheological modifiers for glycerol suspension stability: Incorporating rheological modifiers into glycerol suspensions can enhance their stability by altering flow properties and preventing particle sedimentation. These modifiers can include thickeners, gelling agents, or other viscosity-enhancing compounds that help maintain a uniform dispersion of particles throughout the glycerol medium.
02 Temperature control for glycerol suspension stability
Maintaining appropriate temperature conditions is crucial for the stability of glycerol suspensions. Temperature fluctuations can affect viscosity, solubility, and particle interactions. Implementing temperature control measures during production, storage, and transportation can significantly improve suspension stability.Expand Specific Solutions03 Particle size optimization in glycerol suspensions
The size and distribution of particles in glycerol suspensions play a vital role in their stability. Optimizing particle size through various techniques such as milling, homogenization, or controlled crystallization can enhance suspension stability by reducing sedimentation and improving overall uniformity.Expand Specific Solutions04 pH adjustment for glycerol suspension stability
Controlling the pH of glycerol suspensions can significantly impact their stability. Adjusting the pH to an optimal range can help prevent particle agglomeration, reduce chemical degradation, and maintain the desired properties of the suspended components. This may involve the use of buffering agents or pH modifiers.Expand Specific Solutions05 Rheological modification of glycerol suspensions
Modifying the rheological properties of glycerol suspensions can enhance their stability. This may involve the addition of thickening agents, gelling agents, or other rheology modifiers to adjust viscosity and flow characteristics. Proper rheological control can help prevent sedimentation and maintain uniform particle distribution.Expand Specific Solutions
Key Players in Pharmaceutical Excipient Industry
The glycerol-based suspension stability in pharmaceuticals market is in a growth phase, driven by increasing demand for stable drug formulations. The global market size is estimated to be in the hundreds of millions, with steady expansion projected. Technologically, the field is moderately mature, with ongoing innovations. Key players like Merck, Novartis, and Amgen are investing in R&D to enhance suspension stability using glycerol. Universities such as the University of Michigan are contributing through academic research. Smaller specialized firms like Corcept Therapeutics and Zealand Pharma are also making advancements in this area, focusing on niche applications and novel formulation techniques.
Novartis AG
Technical Solution: Novartis AG has developed a novel approach to improve suspension stability in pharmaceuticals using glycerol. Their method involves creating a glycerol-based suspension medium that enhances the dispersibility and stability of active pharmaceutical ingredients (APIs). The company has optimized the glycerol concentration to achieve a balance between viscosity and particle suspension, typically using 10-30% w/w glycerol [1]. This formulation significantly increases the shelf life of suspensions by preventing sedimentation and aggregation of particles. Novartis has also incorporated additional excipients such as surfactants and polymers to further enhance the stabilizing effect of glycerol, creating a synergistic system that maintains uniform drug distribution throughout the product's lifespan [3].
Strengths: Improved long-term stability, enhanced bioavailability of APIs, and versatility across various drug formulations. Weaknesses: Potential increase in production costs and possible limitations in high-temperature storage conditions.
Merck & Co., Inc.
Technical Solution: Merck & Co., Inc. has pioneered a glycerol-based stabilization technology for pharmaceutical suspensions. Their approach utilizes a proprietary blend of glycerol and other polyols to create a multi-component stabilizing system. This system works by modulating the viscosity of the suspension medium and altering the surface properties of suspended particles. Merck's research has shown that glycerol concentrations between 15-25% w/w, combined with carefully selected co-solvents, can significantly improve suspension stability for up to 24 months [2]. The company has also developed a novel process for incorporating glycerol into nanosuspensions, which has shown promise in enhancing the bioavailability of poorly soluble drugs [4].
Strengths: Extended stability period, improved dissolution rates of APIs, and applicability to a wide range of drug classes. Weaknesses: Complex formulation process and potential for increased viscosity affecting syringeability.
Innovations in Glycerol Utilization for Stability
Suspension of ascorbic acid in glycerin and process for production thereof
PatentWO2008050676A1
Innovation
- A glycerin suspension of ascorbic acid is created by dissolving part of the ascorbic acid in glycerin or diglycerin at 8-12% concentration, with the remaining ascorbic acid precipitating as fine crystals of 25 μm or less, and a method involving mixing with ethyl alcohol and aging at 20-40°C for stability and usability improvement.
Active agent suspensions in glycerine
PatentInactiveEP2005824A1
Innovation
- The development of active ingredient suspensions containing at least 60% glycerin, combined with wetting agents, dispersants, antifoams, and other formulation aids, which provide a stable external phase for agrochemical active ingredients, enhancing their storage stability and biological effectiveness.
Regulatory Considerations for Excipients
The regulatory landscape for excipients in pharmaceutical formulations is complex and constantly evolving. Glycerol, as an excipient used to improve suspension stability, is subject to various regulatory considerations. Regulatory bodies such as the FDA, EMA, and other national health authorities have established guidelines and requirements for the use of excipients in pharmaceutical products.
One of the primary regulatory considerations for glycerol as an excipient is its inclusion in pharmacopoeias. The United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) all have monographs for glycerol, specifying quality standards and testing methods. Manufacturers must ensure that the glycerol used in their formulations meets these pharmacopoeial standards.
Safety assessment is another crucial regulatory aspect. While glycerol is generally recognized as safe (GRAS) by the FDA for use in food products, its use in pharmaceuticals requires additional scrutiny. Manufacturers must provide safety data and risk assessments to regulatory authorities, demonstrating that the use of glycerol in their specific formulation does not pose any unacceptable risks to patients.
The concentration of glycerol used in pharmaceutical suspensions is also subject to regulatory oversight. Regulatory bodies may set limits on the maximum allowable concentration of glycerol in different types of formulations, considering factors such as route of administration and patient population. Manufacturers must justify the chosen concentration based on its effectiveness in improving suspension stability while maintaining an acceptable safety profile.
Quality control and good manufacturing practices (GMP) are essential regulatory requirements for excipients. Manufacturers must implement robust quality management systems to ensure the consistent production of glycerol that meets specified quality standards. This includes maintaining detailed documentation of the manufacturing process, conducting regular quality testing, and implementing appropriate storage and handling procedures.
Regulatory authorities also require manufacturers to assess the potential impact of glycerol on the stability and efficacy of the active pharmaceutical ingredients (APIs) in the suspension. Compatibility studies and stability testing must be conducted to demonstrate that the presence of glycerol does not adversely affect the API or other components of the formulation over the product's shelf life.
Labeling and packaging requirements are another important regulatory consideration. The presence of glycerol in the formulation must be clearly indicated on the product label, along with any relevant warnings or precautions. Regulatory bodies may also require specific storage instructions or expiration dating based on the stability profile of the glycerol-containing suspension.
In conclusion, manufacturers using glycerol to improve suspension stability in pharmaceuticals must navigate a complex regulatory landscape. Compliance with pharmacopoeial standards, safety assessments, concentration limits, quality control measures, stability testing, and labeling requirements are all critical aspects of ensuring regulatory approval and maintaining product safety and efficacy.
One of the primary regulatory considerations for glycerol as an excipient is its inclusion in pharmacopoeias. The United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) all have monographs for glycerol, specifying quality standards and testing methods. Manufacturers must ensure that the glycerol used in their formulations meets these pharmacopoeial standards.
Safety assessment is another crucial regulatory aspect. While glycerol is generally recognized as safe (GRAS) by the FDA for use in food products, its use in pharmaceuticals requires additional scrutiny. Manufacturers must provide safety data and risk assessments to regulatory authorities, demonstrating that the use of glycerol in their specific formulation does not pose any unacceptable risks to patients.
The concentration of glycerol used in pharmaceutical suspensions is also subject to regulatory oversight. Regulatory bodies may set limits on the maximum allowable concentration of glycerol in different types of formulations, considering factors such as route of administration and patient population. Manufacturers must justify the chosen concentration based on its effectiveness in improving suspension stability while maintaining an acceptable safety profile.
Quality control and good manufacturing practices (GMP) are essential regulatory requirements for excipients. Manufacturers must implement robust quality management systems to ensure the consistent production of glycerol that meets specified quality standards. This includes maintaining detailed documentation of the manufacturing process, conducting regular quality testing, and implementing appropriate storage and handling procedures.
Regulatory authorities also require manufacturers to assess the potential impact of glycerol on the stability and efficacy of the active pharmaceutical ingredients (APIs) in the suspension. Compatibility studies and stability testing must be conducted to demonstrate that the presence of glycerol does not adversely affect the API or other components of the formulation over the product's shelf life.
Labeling and packaging requirements are another important regulatory consideration. The presence of glycerol in the formulation must be clearly indicated on the product label, along with any relevant warnings or precautions. Regulatory bodies may also require specific storage instructions or expiration dating based on the stability profile of the glycerol-containing suspension.
In conclusion, manufacturers using glycerol to improve suspension stability in pharmaceuticals must navigate a complex regulatory landscape. Compliance with pharmacopoeial standards, safety assessments, concentration limits, quality control measures, stability testing, and labeling requirements are all critical aspects of ensuring regulatory approval and maintaining product safety and efficacy.
Environmental Impact of Glycerol in Pharmaceuticals
The use of glycerol in pharmaceutical suspensions has significant environmental implications that warrant careful consideration. Glycerol, being a biodegradable and non-toxic compound, generally poses minimal direct environmental risks. However, its production and disposal processes can have broader ecological impacts.
The production of glycerol, particularly when derived from petrochemical sources, contributes to carbon emissions and energy consumption. However, the increasing use of bio-based glycerol, obtained as a byproduct of biodiesel production, offers a more sustainable alternative. This shift towards renewable sources helps reduce the overall carbon footprint associated with glycerol use in pharmaceuticals.
Water systems are a key concern when considering the environmental impact of glycerol. While glycerol is highly soluble in water and biodegradable, its release in large quantities can potentially lead to oxygen depletion in aquatic environments. This is due to the rapid biodegradation process consuming dissolved oxygen. However, the quantities used in pharmaceutical applications are typically not significant enough to pose a major threat to aquatic ecosystems.
In terms of waste management, glycerol presents both challenges and opportunities. Unused or expired pharmaceutical products containing glycerol require proper disposal to prevent environmental contamination. On the positive side, glycerol's biodegradability makes it less persistent in the environment compared to many synthetic stabilizers.
The pharmaceutical industry's increasing focus on green chemistry principles has led to greater scrutiny of excipients like glycerol. Its low toxicity and natural origin align well with efforts to develop more environmentally friendly formulations. Moreover, glycerol's versatility allows it to replace multiple synthetic additives in some formulations, potentially reducing the overall environmental impact of pharmaceutical products.
Regulatory bodies are increasingly considering the environmental impact of pharmaceutical ingredients, including excipients like glycerol. While glycerol itself is generally regarded as safe, manufacturers are encouraged to assess and minimize the environmental footprint of their entire production and supply chain processes involving glycerol.
In conclusion, while glycerol offers many benefits in pharmaceutical suspensions, its environmental impact, though relatively low, should not be overlooked. The industry's ongoing shift towards more sustainable sourcing and production methods for glycerol, coupled with responsible use and disposal practices, can further mitigate its environmental impact, aligning with broader sustainability goals in pharmaceutical manufacturing.
The production of glycerol, particularly when derived from petrochemical sources, contributes to carbon emissions and energy consumption. However, the increasing use of bio-based glycerol, obtained as a byproduct of biodiesel production, offers a more sustainable alternative. This shift towards renewable sources helps reduce the overall carbon footprint associated with glycerol use in pharmaceuticals.
Water systems are a key concern when considering the environmental impact of glycerol. While glycerol is highly soluble in water and biodegradable, its release in large quantities can potentially lead to oxygen depletion in aquatic environments. This is due to the rapid biodegradation process consuming dissolved oxygen. However, the quantities used in pharmaceutical applications are typically not significant enough to pose a major threat to aquatic ecosystems.
In terms of waste management, glycerol presents both challenges and opportunities. Unused or expired pharmaceutical products containing glycerol require proper disposal to prevent environmental contamination. On the positive side, glycerol's biodegradability makes it less persistent in the environment compared to many synthetic stabilizers.
The pharmaceutical industry's increasing focus on green chemistry principles has led to greater scrutiny of excipients like glycerol. Its low toxicity and natural origin align well with efforts to develop more environmentally friendly formulations. Moreover, glycerol's versatility allows it to replace multiple synthetic additives in some formulations, potentially reducing the overall environmental impact of pharmaceutical products.
Regulatory bodies are increasingly considering the environmental impact of pharmaceutical ingredients, including excipients like glycerol. While glycerol itself is generally regarded as safe, manufacturers are encouraged to assess and minimize the environmental footprint of their entire production and supply chain processes involving glycerol.
In conclusion, while glycerol offers many benefits in pharmaceutical suspensions, its environmental impact, though relatively low, should not be overlooked. The industry's ongoing shift towards more sustainable sourcing and production methods for glycerol, coupled with responsible use and disposal practices, can further mitigate its environmental impact, aligning with broader sustainability goals in pharmaceutical manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!