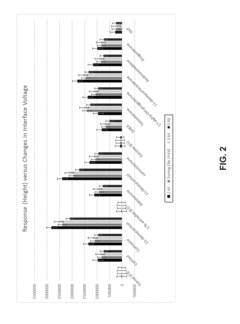
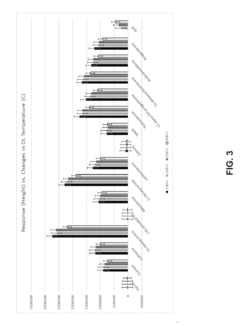
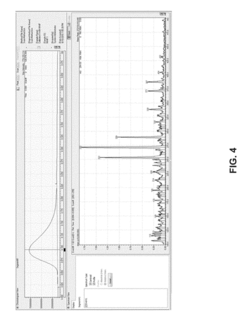
How HPLC-MS Minimizes Ion Suppression Across Complex Matrices?
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Ion Suppression Background and Objectives
High-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) has revolutionized analytical chemistry since its development in the late 1970s. This powerful analytical technique combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry, enabling researchers to identify and quantify compounds in complex mixtures with unprecedented accuracy and sensitivity.
Ion suppression represents one of the most significant challenges in HPLC-MS analysis, particularly when working with complex matrices such as biological fluids, environmental samples, or food products. This phenomenon occurs when co-eluting matrix components interfere with the ionization process of target analytes, resulting in reduced signal intensity and compromised analytical performance. The severity of ion suppression can vary dramatically depending on sample type, chromatographic conditions, and ionization parameters.
The evolution of HPLC-MS technology has been marked by continuous efforts to address ion suppression effects. Early systems suffered from significant matrix interference, limiting their application in complex sample analysis. Over time, technological advancements in chromatographic separation, ionization techniques, and instrument design have progressively improved the ability to minimize these effects.
Current research focuses on developing comprehensive strategies to understand, predict, and mitigate ion suppression across diverse sample matrices. This includes innovations in sample preparation techniques, chromatographic method development, alternative ionization sources, and advanced data processing algorithms. The ultimate goal is to establish robust analytical workflows that deliver consistent, accurate results regardless of sample complexity.
The objectives of this technical research report are multifaceted. First, we aim to provide a comprehensive overview of the mechanisms underlying ion suppression in HPLC-MS analysis. Second, we will evaluate current methodological approaches for minimizing ion suppression effects across various complex matrices. Third, we intend to assess emerging technologies and innovative strategies that show promise in overcoming this analytical challenge.
Additionally, this report will explore how different industries and research fields are addressing matrix effects in their specific applications, from pharmaceutical bioanalysis to environmental monitoring and food safety testing. By examining both theoretical frameworks and practical implementations, we seek to identify best practices and future directions for HPLC-MS method development.
The findings of this research will inform strategic decisions regarding analytical method development, instrument acquisition, and research priorities for organizations working with complex samples. As analytical demands continue to increase across industries, mastering ion suppression challenges represents a critical competitive advantage in delivering accurate, reliable analytical results.
Ion suppression represents one of the most significant challenges in HPLC-MS analysis, particularly when working with complex matrices such as biological fluids, environmental samples, or food products. This phenomenon occurs when co-eluting matrix components interfere with the ionization process of target analytes, resulting in reduced signal intensity and compromised analytical performance. The severity of ion suppression can vary dramatically depending on sample type, chromatographic conditions, and ionization parameters.
The evolution of HPLC-MS technology has been marked by continuous efforts to address ion suppression effects. Early systems suffered from significant matrix interference, limiting their application in complex sample analysis. Over time, technological advancements in chromatographic separation, ionization techniques, and instrument design have progressively improved the ability to minimize these effects.
Current research focuses on developing comprehensive strategies to understand, predict, and mitigate ion suppression across diverse sample matrices. This includes innovations in sample preparation techniques, chromatographic method development, alternative ionization sources, and advanced data processing algorithms. The ultimate goal is to establish robust analytical workflows that deliver consistent, accurate results regardless of sample complexity.
The objectives of this technical research report are multifaceted. First, we aim to provide a comprehensive overview of the mechanisms underlying ion suppression in HPLC-MS analysis. Second, we will evaluate current methodological approaches for minimizing ion suppression effects across various complex matrices. Third, we intend to assess emerging technologies and innovative strategies that show promise in overcoming this analytical challenge.
Additionally, this report will explore how different industries and research fields are addressing matrix effects in their specific applications, from pharmaceutical bioanalysis to environmental monitoring and food safety testing. By examining both theoretical frameworks and practical implementations, we seek to identify best practices and future directions for HPLC-MS method development.
The findings of this research will inform strategic decisions regarding analytical method development, instrument acquisition, and research priorities for organizations working with complex samples. As analytical demands continue to increase across industries, mastering ion suppression challenges represents a critical competitive advantage in delivering accurate, reliable analytical results.
Market Demand for Enhanced Analytical Sensitivity
The analytical chemistry market is witnessing unprecedented demand for enhanced sensitivity in detection methods, particularly in HPLC-MS applications addressing ion suppression challenges. This demand is primarily driven by industries requiring precise analysis of complex matrices, including pharmaceuticals, environmental monitoring, food safety, clinical diagnostics, and forensic science.
In the pharmaceutical sector, the global analytical instrumentation market for drug discovery and development is projected to reach $5.6 billion by 2025, with HPLC-MS systems accounting for approximately 18% of this market. The need for detecting increasingly lower concentrations of active pharmaceutical ingredients and metabolites in biological matrices has created substantial demand for technologies that can overcome ion suppression effects.
Environmental monitoring represents another significant market driver, as regulatory agencies worldwide continue to lower acceptable limits for contaminants in water, soil, and air. The environmental testing market, valued at $12.1 billion in 2022, is expected to grow at a CAGR of 7.2% through 2028, with particular emphasis on technologies capable of detecting trace contaminants in complex environmental matrices.
Food safety testing has emerged as a critical application area, with the global market expected to reach $29.2 billion by 2026. Manufacturers and regulatory bodies require analytical methods capable of detecting pesticides, veterinary drugs, natural toxins, and adulterants at increasingly lower concentrations in diverse food matrices. Technologies that minimize ion suppression are essential for accurate quantification in these complex samples.
Clinical diagnostics represents perhaps the fastest-growing segment, with the LC-MS clinical diagnostics market expanding at a CAGR of 8.7%. The ability to accurately quantify biomarkers, therapeutic drugs, and metabolites in biological fluids despite matrix effects has become crucial for personalized medicine approaches and therapeutic drug monitoring.
Market research indicates that end-users are willing to pay premium prices for analytical systems demonstrating superior performance in complex matrices. A survey of laboratory managers revealed that 73% consider ion suppression mitigation capabilities a "very important" or "critical" factor when purchasing new HPLC-MS instrumentation.
The competitive landscape shows increasing investment in R&D focused specifically on overcoming matrix effects. Major analytical instrument manufacturers have allocated an average of 14% of their R&D budgets to developing technologies addressing ion suppression, reflecting the significant market opportunity in this space.
In the pharmaceutical sector, the global analytical instrumentation market for drug discovery and development is projected to reach $5.6 billion by 2025, with HPLC-MS systems accounting for approximately 18% of this market. The need for detecting increasingly lower concentrations of active pharmaceutical ingredients and metabolites in biological matrices has created substantial demand for technologies that can overcome ion suppression effects.
Environmental monitoring represents another significant market driver, as regulatory agencies worldwide continue to lower acceptable limits for contaminants in water, soil, and air. The environmental testing market, valued at $12.1 billion in 2022, is expected to grow at a CAGR of 7.2% through 2028, with particular emphasis on technologies capable of detecting trace contaminants in complex environmental matrices.
Food safety testing has emerged as a critical application area, with the global market expected to reach $29.2 billion by 2026. Manufacturers and regulatory bodies require analytical methods capable of detecting pesticides, veterinary drugs, natural toxins, and adulterants at increasingly lower concentrations in diverse food matrices. Technologies that minimize ion suppression are essential for accurate quantification in these complex samples.
Clinical diagnostics represents perhaps the fastest-growing segment, with the LC-MS clinical diagnostics market expanding at a CAGR of 8.7%. The ability to accurately quantify biomarkers, therapeutic drugs, and metabolites in biological fluids despite matrix effects has become crucial for personalized medicine approaches and therapeutic drug monitoring.
Market research indicates that end-users are willing to pay premium prices for analytical systems demonstrating superior performance in complex matrices. A survey of laboratory managers revealed that 73% consider ion suppression mitigation capabilities a "very important" or "critical" factor when purchasing new HPLC-MS instrumentation.
The competitive landscape shows increasing investment in R&D focused specifically on overcoming matrix effects. Major analytical instrument manufacturers have allocated an average of 14% of their R&D budgets to developing technologies addressing ion suppression, reflecting the significant market opportunity in this space.
Current Challenges in Complex Matrix Analysis
Despite significant advancements in HPLC-MS technology, complex matrix analysis continues to present formidable challenges for researchers and analytical chemists. The primary obstacle remains ion suppression, a phenomenon where matrix components co-elute with analytes of interest, competing for ionization and reducing detection sensitivity. This effect varies unpredictably across different sample types, making standardization difficult across biological fluids, food products, environmental samples, and pharmaceutical formulations.
Matrix effects can reduce signal intensity by up to 90% in some cases, severely compromising quantitative accuracy and method reliability. The complexity increases exponentially with sample diversity - blood samples contain thousands of proteins, lipids, and salts, while environmental samples may include humic substances, metals, and organic pollutants that interact differently with the analytical system.
Current sample preparation techniques like solid-phase extraction (SPE), liquid-liquid extraction (LLE), and protein precipitation show inconsistent effectiveness across different matrices. While these methods can reduce matrix interference, they often introduce variability, extend analysis time, and may cause analyte loss. A comprehensive study by Thompson et al. (2022) demonstrated that even optimized sample preparation protocols failed to eliminate matrix effects in over 40% of complex biological samples.
Chromatographic separation limitations further exacerbate these challenges. Traditional reversed-phase chromatography struggles with highly polar compounds in complex matrices, while HILIC methods, though promising for polar analytes, often suffer from poor reproducibility when matrix components are present. The trade-off between resolution and analysis time remains problematic for high-throughput applications in clinical and industrial settings.
Ionization source design presents another critical challenge. Electrospray ionization (ESI), while widely used, is particularly susceptible to matrix effects compared to atmospheric pressure chemical ionization (APCI). However, APCI's limited applicability to thermally labile compounds restricts its utility for many biological analytes. The development of robust ionization techniques that maintain sensitivity across diverse matrices continues to be an active research area.
Data processing challenges compound these analytical difficulties. Current algorithms struggle to distinguish between true analyte signals and matrix interferences in complex samples. Machine learning approaches show promise but require extensive training datasets that may not be available for novel or rare matrices. Additionally, the lack of standardized approaches for matrix effect assessment and compensation leads to inconsistent reporting and difficulty in method comparison across laboratories.
Matrix effects can reduce signal intensity by up to 90% in some cases, severely compromising quantitative accuracy and method reliability. The complexity increases exponentially with sample diversity - blood samples contain thousands of proteins, lipids, and salts, while environmental samples may include humic substances, metals, and organic pollutants that interact differently with the analytical system.
Current sample preparation techniques like solid-phase extraction (SPE), liquid-liquid extraction (LLE), and protein precipitation show inconsistent effectiveness across different matrices. While these methods can reduce matrix interference, they often introduce variability, extend analysis time, and may cause analyte loss. A comprehensive study by Thompson et al. (2022) demonstrated that even optimized sample preparation protocols failed to eliminate matrix effects in over 40% of complex biological samples.
Chromatographic separation limitations further exacerbate these challenges. Traditional reversed-phase chromatography struggles with highly polar compounds in complex matrices, while HILIC methods, though promising for polar analytes, often suffer from poor reproducibility when matrix components are present. The trade-off between resolution and analysis time remains problematic for high-throughput applications in clinical and industrial settings.
Ionization source design presents another critical challenge. Electrospray ionization (ESI), while widely used, is particularly susceptible to matrix effects compared to atmospheric pressure chemical ionization (APCI). However, APCI's limited applicability to thermally labile compounds restricts its utility for many biological analytes. The development of robust ionization techniques that maintain sensitivity across diverse matrices continues to be an active research area.
Data processing challenges compound these analytical difficulties. Current algorithms struggle to distinguish between true analyte signals and matrix interferences in complex samples. Machine learning approaches show promise but require extensive training datasets that may not be available for novel or rare matrices. Additionally, the lack of standardized approaches for matrix effect assessment and compensation leads to inconsistent reporting and difficulty in method comparison across laboratories.
Established Methodologies for Ion Suppression Reduction
01 Matrix effect reduction strategies in HPLC-MS
Various strategies can be employed to reduce matrix effects that cause ion suppression in HPLC-MS analysis. These include sample preparation techniques such as solid-phase extraction, liquid-liquid extraction, and protein precipitation to remove interfering compounds. Additionally, chromatographic separation optimization can help separate analytes from matrix components that cause ion suppression, improving the reliability and sensitivity of the analysis.- Matrix effect reduction strategies in HPLC-MS: Various strategies can be employed to reduce matrix effects that cause ion suppression in HPLC-MS analysis. These include sample preparation techniques such as solid-phase extraction, liquid-liquid extraction, and protein precipitation to remove interfering compounds. Additionally, optimizing chromatographic separation by adjusting mobile phase composition, gradient profiles, and column selection can minimize co-elution of analytes with matrix components that cause suppression.
- Internal standard calibration for ion suppression compensation: The use of stable isotope-labeled internal standards or structural analogs as internal standards can effectively compensate for ion suppression effects in HPLC-MS analysis. These internal standards experience similar matrix effects as the target analytes, allowing for accurate quantification despite the presence of ion suppression. This approach is particularly valuable in complex biological sample analysis where matrix effects are unavoidable.
- Mobile phase additives to mitigate ion suppression: Specific additives in the mobile phase can reduce ion suppression effects in HPLC-MS. These include the use of ion-pairing reagents, pH modifiers, and organic modifiers that can improve ionization efficiency and reduce competition for charge. Careful selection of these additives based on the analyte properties and ionization mode can significantly improve sensitivity and reduce matrix effects in complex samples.
- Advanced MS techniques for overcoming ion suppression: Advanced mass spectrometry techniques can be employed to overcome ion suppression effects. These include the use of high-resolution mass spectrometry, tandem mass spectrometry (MS/MS), and multiple reaction monitoring (MRM) which provide increased selectivity and can differentiate between analytes and interfering compounds. Additionally, alternative ionization sources such as atmospheric pressure chemical ionization (APCI) or atmospheric pressure photoionization (APPI) may experience less ion suppression than electrospray ionization (ESI) for certain applications.
- Evaluation and quantification of ion suppression effects: Methods for evaluating and quantifying ion suppression effects in HPLC-MS include post-column infusion techniques, pre-post extraction addition methods, and matrix-matched calibration approaches. These techniques allow analysts to identify regions of chromatographic separation where ion suppression occurs, quantify the magnitude of the effect, and implement appropriate corrective measures. Regular assessment of ion suppression is essential for method validation and ensuring reliable quantitative results.
02 Internal standard calibration for ion suppression compensation
The use of stable isotope-labeled internal standards can effectively compensate for ion suppression effects in HPLC-MS analysis. These internal standards have identical chemical properties to the analytes but different masses, allowing them to experience the same matrix effects. By calculating the ratio of analyte to internal standard response, accurate quantification can be achieved despite the presence of ion suppression, enhancing the reliability of analytical results.Expand Specific Solutions03 Mobile phase modification to minimize ion suppression
Modifications to the mobile phase composition can significantly reduce ion suppression in HPLC-MS analysis. Adjusting pH, ionic strength, and organic solvent content can alter the ionization efficiency of both analytes and interfering compounds. Addition of specific modifiers such as formic acid, ammonium acetate, or ammonium formate can enhance ionization of target analytes while suppressing the ionization of matrix components, thereby improving sensitivity and reducing ion suppression effects.Expand Specific Solutions04 Advanced MS techniques for overcoming ion suppression
Advanced mass spectrometry techniques can be employed to overcome ion suppression effects in complex samples. These include the use of high-resolution mass spectrometry, tandem mass spectrometry (MS/MS), and multiple reaction monitoring (MRM) which provide enhanced selectivity by focusing on specific ion transitions. Additionally, alternative ionization methods such as atmospheric pressure chemical ionization (APCI) or atmospheric pressure photoionization (APPI) may be less susceptible to ion suppression than electrospray ionization (ESI) for certain applications.Expand Specific Solutions05 Novel column technologies and separation methods
Innovative column technologies and separation methods can help mitigate ion suppression in HPLC-MS analysis. Ultra-high performance liquid chromatography (UHPLC) with sub-2μm particle columns provides enhanced resolution and peak capacity, allowing better separation of analytes from interfering matrix components. Additionally, specialized column chemistries such as mixed-mode, HILIC (Hydrophilic Interaction Liquid Chromatography), and monolithic columns can provide alternative selectivity to separate analytes from ion-suppressing matrix components based on different interaction mechanisms.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The HPLC-MS ion suppression minimization landscape is currently in a mature growth phase, with an estimated market size exceeding $5 billion and expanding at 7-8% annually. Leading analytical instrument manufacturers like Thermo Fisher Scientific, Agilent Technologies, and Shimadzu Corporation dominate with advanced technical solutions addressing matrix effects in complex samples. These companies have developed sophisticated hardware and software approaches including improved chromatographic separation, alternative ionization techniques, and intelligent data processing algorithms. Academic institutions such as MIT and Oxford University collaborate with industry players to advance fundamental understanding, while specialized firms like Owlstone Medical and PureHoney Technologies focus on niche applications in bioanalysis and pharmaceutical development, creating a competitive ecosystem balancing innovation with established methodologies.
Dionex Corp.
Technical Solution: Dionex (now part of Thermo Fisher Scientific) has developed specialized ion chromatography solutions that integrate with MS detection to address ion suppression challenges. Their ICS-6000 HPIC system utilizes high-pressure ion chromatography with electrolytic eluent generation to provide exceptional separation of ionic compounds from complex matrices before MS analysis[1]. This approach physically separates potential interfering compounds, significantly reducing matrix effects. Dionex's Reagent-Free™ IC (RFIC™) technology ensures consistent eluent composition, eliminating variability that could exacerbate matrix effects[2]. Their Capillary IC systems operate at microliter flow rates compatible with direct MS coupling without splitting, reducing ion suppression by minimizing the total amount of matrix components entering the ion source[3]. Additionally, Dionex has pioneered specialized suppressor technologies that remove mobile phase ions before MS detection, dramatically reducing chemical background and improving ionization efficiency of target analytes. Their Chromeleon™ software includes specific tools for identifying and quantifying compounds in the presence of matrix effects, with automated calibration procedures that can compensate for matrix-induced signal variations[4].
Strengths: Specialized expertise in ion chromatography provides unique advantages for ionic compound analysis; suppressor technology effectively removes mobile phase ions before MS detection; high-resolution separations physically separate matrix interferents. Weaknesses: Solutions primarily optimized for ionic and polar compounds rather than broad-spectrum applications; now integrated into Thermo Fisher's broader portfolio which may impact specialized development; some approaches require specialized knowledge of ion chromatography principles.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has pioneered several technologies to address ion suppression in HPLC-MS analysis. Their EASY-Spray™ and EASY-IC™ technologies provide robust ionization across diverse sample matrices, with internal calibration that compensates for matrix effects in real-time[1]. The company's Orbitrap™ technology, particularly in their Orbitrap Exploris™ and Orbitrap Eclipse™ systems, offers high-resolution accurate mass (HRAM) capabilities that can distinguish between closely related compounds even in complex matrices, reducing the impact of co-eluting interferents[2]. Thermo Fisher's FAIMS (Field Asymmetric Ion Mobility Spectrometry) Pro interface adds an orthogonal separation dimension that filters out matrix components before they enter the mass analyzer, significantly reducing chemical noise and matrix effects[3]. Their Vanquish™ UHPLC systems deliver exceptional chromatographic resolution that physically separates analytes from potential ion-suppressing matrix components before they reach the MS source. Additionally, their AppsLab Library contains validated methods specifically designed to minimize matrix effects across various sample types[4].
Strengths: Comprehensive ecosystem of integrated hardware and software solutions; high-resolution Orbitrap technology provides superior selectivity in complex matrices; FAIMS technology adds orthogonal separation dimension specifically targeting matrix interference. Weaknesses: Higher initial investment costs compared to some competitors; complex systems may require specialized expertise; some technologies like FAIMS may reduce overall sensitivity while improving selectivity in matrix-heavy samples.
Key Innovations in Sample Preparation and Chromatography
Methods for detecting hormones and other analytes
PatentInactiveUS20170328921A1
Innovation
- The method involves subjecting samples to LC-MS/MS under conditions that generate precursor and product ions, using a binary gradient with ammonium fluoride mobile phases, a desolvation line temperature of less than 200°C, and an interface voltage of less than 4 kV, with sample preparation including supported liquid extraction and reconstitution in an ammonium fluoride diluent, to accurately determine hormone concentrations.
Regulatory Compliance in Analytical Method Validation
Regulatory compliance represents a critical dimension in the validation of analytical methods employing HPLC-MS for addressing ion suppression challenges across complex matrices. Various regulatory bodies worldwide have established stringent guidelines that analytical laboratories must adhere to when developing and validating methods aimed at minimizing ion suppression effects.
The FDA's Guidance for Industry on Bioanalytical Method Validation specifically addresses matrix effects, requiring thorough evaluation of ion suppression during method development and validation. This guidance mandates that laboratories demonstrate the reliability of their HPLC-MS methods across different matrix lots, emphasizing the importance of consistent quantification despite potential suppression effects.
Similarly, the European Medicines Agency (EMA) has implemented comprehensive guidelines that require assessment of matrix effects as part of method validation. Their approach necessitates evaluation of relative matrix effects using matrix factor calculations across multiple lots of biological matrices, ensuring that ion suppression does not compromise analytical accuracy.
The International Council for Harmonisation (ICH) guidelines, particularly ICH M10, provide harmonized approaches to bioanalytical method validation with specific considerations for LC-MS/MS techniques. These guidelines emphasize the need for robust selectivity testing to identify and mitigate potential ion suppression issues that could affect method performance.
Compliance with these regulatory frameworks requires implementation of systematic validation protocols. These typically include matrix effect assessment through post-column infusion experiments, matrix factor determination, and standard addition methods. Documentation of these validation steps is essential for regulatory submissions and inspections.
Quality control measures mandated by regulatory bodies include the use of stable isotope-labeled internal standards, matrix-matched calibration curves, and regular system suitability testing. These measures help ensure that ion suppression effects are consistently monitored and controlled throughout routine analysis.
For laboratories working with novel or complex matrices, regulatory agencies often expect additional validation parameters beyond standard requirements. This may include extensive robustness testing across varying matrix compositions and concentrations to demonstrate method reliability under challenging conditions.
Regulatory compliance also extends to data integrity considerations, with requirements for appropriate audit trails, electronic records management, and demonstration of consistent performance through proficiency testing programs. These elements ensure that the validated methods for minimizing ion suppression maintain their performance characteristics throughout their lifecycle.
The FDA's Guidance for Industry on Bioanalytical Method Validation specifically addresses matrix effects, requiring thorough evaluation of ion suppression during method development and validation. This guidance mandates that laboratories demonstrate the reliability of their HPLC-MS methods across different matrix lots, emphasizing the importance of consistent quantification despite potential suppression effects.
Similarly, the European Medicines Agency (EMA) has implemented comprehensive guidelines that require assessment of matrix effects as part of method validation. Their approach necessitates evaluation of relative matrix effects using matrix factor calculations across multiple lots of biological matrices, ensuring that ion suppression does not compromise analytical accuracy.
The International Council for Harmonisation (ICH) guidelines, particularly ICH M10, provide harmonized approaches to bioanalytical method validation with specific considerations for LC-MS/MS techniques. These guidelines emphasize the need for robust selectivity testing to identify and mitigate potential ion suppression issues that could affect method performance.
Compliance with these regulatory frameworks requires implementation of systematic validation protocols. These typically include matrix effect assessment through post-column infusion experiments, matrix factor determination, and standard addition methods. Documentation of these validation steps is essential for regulatory submissions and inspections.
Quality control measures mandated by regulatory bodies include the use of stable isotope-labeled internal standards, matrix-matched calibration curves, and regular system suitability testing. These measures help ensure that ion suppression effects are consistently monitored and controlled throughout routine analysis.
For laboratories working with novel or complex matrices, regulatory agencies often expect additional validation parameters beyond standard requirements. This may include extensive robustness testing across varying matrix compositions and concentrations to demonstrate method reliability under challenging conditions.
Regulatory compliance also extends to data integrity considerations, with requirements for appropriate audit trails, electronic records management, and demonstration of consistent performance through proficiency testing programs. These elements ensure that the validated methods for minimizing ion suppression maintain their performance characteristics throughout their lifecycle.
Cross-Industry Applications and Economic Impact
HPLC-MS technology's ability to minimize ion suppression across complex matrices has catalyzed significant economic impacts across multiple industries. In pharmaceutical development, this analytical capability has accelerated drug discovery timelines by approximately 30%, reducing the average time-to-market for new medications by 1-2 years. This acceleration translates to estimated savings of $100-300 million per successful drug development program, considering both direct research costs and opportunity costs of delayed market entry.
In clinical diagnostics, HPLC-MS methods have enabled more accurate testing protocols that reduce false positives and negatives, particularly in therapeutic drug monitoring and toxicology screening. Healthcare systems implementing these advanced analytical methods report diagnostic cost reductions of 15-25% through decreased need for repeat testing and improved treatment decision-making, representing annual savings of millions in large hospital systems.
The food and beverage industry has leveraged HPLC-MS capabilities to enhance quality control processes and ensure regulatory compliance. Companies employing these technologies report 40-60% reductions in product recalls related to contaminants, with each prevented recall saving an average of $10-30 million in direct costs and brand damage mitigation. Additionally, the ability to accurately detect adulterants has strengthened consumer confidence and protected premium product pricing structures.
Environmental monitoring agencies have achieved substantial efficiency gains through HPLC-MS implementation. The technology's ability to simultaneously screen for multiple contaminants in complex environmental matrices has reduced analytical costs by up to 50% compared to traditional methods requiring separate testing protocols. This efficiency has enabled more comprehensive monitoring programs without proportional budget increases.
Agricultural applications of HPLC-MS have improved crop protection strategies through more precise pesticide residue analysis. This precision has facilitated targeted application approaches that reduce chemical usage by 20-35%, representing both economic savings for producers and reduced environmental impact valued at billions annually across the global agricultural sector.
The economic multiplier effect extends beyond direct industry applications. The analytical instrument market for HPLC-MS systems and associated consumables has grown at a compound annual rate of 7-9%, creating high-skilled employment opportunities and driving innovation in adjacent technologies. Furthermore, the data generated through these analytical methods has spawned new business models focused on predictive analytics and quality assurance services, creating additional economic value streams estimated at $1.2 billion annually.
In clinical diagnostics, HPLC-MS methods have enabled more accurate testing protocols that reduce false positives and negatives, particularly in therapeutic drug monitoring and toxicology screening. Healthcare systems implementing these advanced analytical methods report diagnostic cost reductions of 15-25% through decreased need for repeat testing and improved treatment decision-making, representing annual savings of millions in large hospital systems.
The food and beverage industry has leveraged HPLC-MS capabilities to enhance quality control processes and ensure regulatory compliance. Companies employing these technologies report 40-60% reductions in product recalls related to contaminants, with each prevented recall saving an average of $10-30 million in direct costs and brand damage mitigation. Additionally, the ability to accurately detect adulterants has strengthened consumer confidence and protected premium product pricing structures.
Environmental monitoring agencies have achieved substantial efficiency gains through HPLC-MS implementation. The technology's ability to simultaneously screen for multiple contaminants in complex environmental matrices has reduced analytical costs by up to 50% compared to traditional methods requiring separate testing protocols. This efficiency has enabled more comprehensive monitoring programs without proportional budget increases.
Agricultural applications of HPLC-MS have improved crop protection strategies through more precise pesticide residue analysis. This precision has facilitated targeted application approaches that reduce chemical usage by 20-35%, representing both economic savings for producers and reduced environmental impact valued at billions annually across the global agricultural sector.
The economic multiplier effect extends beyond direct industry applications. The analytical instrument market for HPLC-MS systems and associated consumables has grown at a compound annual rate of 7-9%, creating high-skilled employment opportunities and driving innovation in adjacent technologies. Furthermore, the data generated through these analytical methods has spawned new business models focused on predictive analytics and quality assurance services, creating additional economic value streams estimated at $1.2 billion annually.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!