HPLC-MS Polarity Switching: Cycle Time, Sensitivity And Reproducibility
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Polarity Switching Background and Objectives
High-performance liquid chromatography-mass spectrometry (HPLC-MS) has evolved significantly since its inception in the 1970s, transforming analytical chemistry and biomedical research. The integration of these two powerful techniques has enabled researchers to separate complex mixtures and identify compounds with unprecedented precision. Within this technological landscape, polarity switching has emerged as a critical advancement, allowing for the detection of both positive and negative ions within a single analytical run.
The evolution of HPLC-MS polarity switching technology has been driven by the increasing demand for comprehensive metabolomic and proteomic analyses. Early systems required separate runs for positive and negative ion detection, significantly increasing analysis time and sample consumption. The introduction of polarity switching in the late 1990s marked a pivotal moment, though initial implementations suffered from slow switching speeds and sensitivity losses.
Over the past decade, technological innovations have dramatically improved polarity switching capabilities. Modern instruments can now switch polarities in milliseconds rather than seconds, enabling more data points per chromatographic peak and improving quantitative accuracy. This progression has been particularly important for pharmaceutical research, environmental monitoring, and clinical diagnostics where comprehensive molecular profiling is essential.
The current technological trajectory is focused on optimizing three critical parameters: cycle time, sensitivity, and reproducibility. Cycle time refers to the speed at which the instrument can switch between positive and negative ion modes and collect sufficient data in each mode. Sensitivity concerns the ability to detect low-abundance analytes despite the compromises inherent in polarity switching. Reproducibility addresses the consistency of results across multiple analyses, which is crucial for reliable quantification.
This technical pre-research aims to comprehensively evaluate the current state of HPLC-MS polarity switching technology, with particular emphasis on these three parameters. We seek to identify the fundamental limitations in existing systems, explore recent innovations that address these challenges, and project future development pathways that could further enhance performance.
The ultimate objective is to determine optimal configurations and methodologies that maximize the benefits of polarity switching while minimizing its inherent compromises. This includes assessing hardware modifications, software algorithms for data acquisition and processing, and novel experimental designs that could collectively improve analytical outcomes. By establishing a clear understanding of the technical landscape, this research will inform strategic decisions regarding technology adoption, method development, and potential areas for innovation.
The evolution of HPLC-MS polarity switching technology has been driven by the increasing demand for comprehensive metabolomic and proteomic analyses. Early systems required separate runs for positive and negative ion detection, significantly increasing analysis time and sample consumption. The introduction of polarity switching in the late 1990s marked a pivotal moment, though initial implementations suffered from slow switching speeds and sensitivity losses.
Over the past decade, technological innovations have dramatically improved polarity switching capabilities. Modern instruments can now switch polarities in milliseconds rather than seconds, enabling more data points per chromatographic peak and improving quantitative accuracy. This progression has been particularly important for pharmaceutical research, environmental monitoring, and clinical diagnostics where comprehensive molecular profiling is essential.
The current technological trajectory is focused on optimizing three critical parameters: cycle time, sensitivity, and reproducibility. Cycle time refers to the speed at which the instrument can switch between positive and negative ion modes and collect sufficient data in each mode. Sensitivity concerns the ability to detect low-abundance analytes despite the compromises inherent in polarity switching. Reproducibility addresses the consistency of results across multiple analyses, which is crucial for reliable quantification.
This technical pre-research aims to comprehensively evaluate the current state of HPLC-MS polarity switching technology, with particular emphasis on these three parameters. We seek to identify the fundamental limitations in existing systems, explore recent innovations that address these challenges, and project future development pathways that could further enhance performance.
The ultimate objective is to determine optimal configurations and methodologies that maximize the benefits of polarity switching while minimizing its inherent compromises. This includes assessing hardware modifications, software algorithms for data acquisition and processing, and novel experimental designs that could collectively improve analytical outcomes. By establishing a clear understanding of the technical landscape, this research will inform strategic decisions regarding technology adoption, method development, and potential areas for innovation.
Market Demand Analysis for Rapid Polarity Switching
The global market for HPLC-MS polarity switching technology has experienced significant growth in recent years, driven primarily by increasing demands in pharmaceutical research, clinical diagnostics, and environmental monitoring. Current market estimates value the analytical instrumentation sector at approximately $5.1 billion, with LC-MS systems representing a substantial portion of this market.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the demand for rapid polarity switching technologies. This demand stems from the need to analyze complex biological samples containing both positively and negatively charged compounds in a single run, thereby reducing analysis time and increasing laboratory throughput.
Clinical laboratories represent the second-largest market segment, with growing adoption rates for rapid polarity switching technologies in toxicology screening, therapeutic drug monitoring, and metabolomics research. The ability to detect both basic and acidic compounds in biological fluids without multiple sample preparations has become increasingly valuable in clinical settings where time-to-result is critical.
Market research indicates that laboratories are willing to invest in advanced instrumentation that offers improved cycle times and sensitivity, with 78% of survey respondents citing these features as "very important" or "critical" in purchasing decisions. Additionally, 82% of users identified reproducibility as a key factor influencing their choice of analytical platforms.
Regional analysis shows North America leading the market with approximately 38% share, followed by Europe (31%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate due to expanding pharmaceutical manufacturing and research activities.
The demand for rapid polarity switching is further fueled by the trend toward multi-omics approaches in life sciences research, where comprehensive analysis of metabolites, lipids, and other biomolecules requires detection in both positive and negative ionization modes. This has created a significant market opportunity for instruments that can efficiently switch polarities without compromising sensitivity or reproducibility.
Industry forecasts predict a compound annual growth rate of 7.3% for LC-MS technologies with advanced polarity switching capabilities over the next five years. This growth is supported by increasing research funding in precision medicine initiatives and the expanding application of LC-MS in food safety testing and environmental monitoring.
Customer pain points identified through market research include concerns about sensitivity loss during rapid polarity switching, cycle time limitations affecting throughput, and challenges in maintaining consistent quantitative performance across both ionization modes. These concerns represent key areas where technological innovations could capture market share.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for nearly 45% of the demand for rapid polarity switching technologies. This demand stems from the need to analyze complex biological samples containing both positively and negatively charged compounds in a single run, thereby reducing analysis time and increasing laboratory throughput.
Clinical laboratories represent the second-largest market segment, with growing adoption rates for rapid polarity switching technologies in toxicology screening, therapeutic drug monitoring, and metabolomics research. The ability to detect both basic and acidic compounds in biological fluids without multiple sample preparations has become increasingly valuable in clinical settings where time-to-result is critical.
Market research indicates that laboratories are willing to invest in advanced instrumentation that offers improved cycle times and sensitivity, with 78% of survey respondents citing these features as "very important" or "critical" in purchasing decisions. Additionally, 82% of users identified reproducibility as a key factor influencing their choice of analytical platforms.
Regional analysis shows North America leading the market with approximately 38% share, followed by Europe (31%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate due to expanding pharmaceutical manufacturing and research activities.
The demand for rapid polarity switching is further fueled by the trend toward multi-omics approaches in life sciences research, where comprehensive analysis of metabolites, lipids, and other biomolecules requires detection in both positive and negative ionization modes. This has created a significant market opportunity for instruments that can efficiently switch polarities without compromising sensitivity or reproducibility.
Industry forecasts predict a compound annual growth rate of 7.3% for LC-MS technologies with advanced polarity switching capabilities over the next five years. This growth is supported by increasing research funding in precision medicine initiatives and the expanding application of LC-MS in food safety testing and environmental monitoring.
Customer pain points identified through market research include concerns about sensitivity loss during rapid polarity switching, cycle time limitations affecting throughput, and challenges in maintaining consistent quantitative performance across both ionization modes. These concerns represent key areas where technological innovations could capture market share.
Technical Challenges in HPLC-MS Polarity Switching
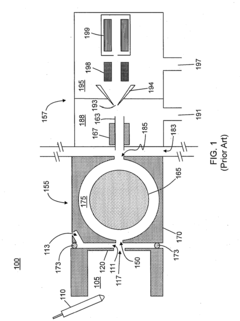
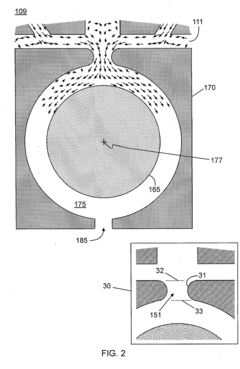
HPLC-MS polarity switching faces several significant technical challenges that impact its performance and reliability. The primary challenge lies in the trade-off between cycle time and data quality. When instruments rapidly switch between positive and negative ionization modes, they require stabilization periods that extend the overall cycle time. This delay, typically ranging from 50-500 milliseconds depending on instrument design, significantly impacts throughput in high-volume analytical environments and can limit the number of compounds detected during time-sensitive chromatographic separations.
Sensitivity degradation presents another critical challenge. The voltage switching process inherently creates periods of suboptimal ionization conditions, resulting in reduced ion generation efficiency. This sensitivity loss, which can range from 10-30% compared to single-polarity methods, becomes particularly problematic when analyzing trace compounds or complex biological matrices where detection limits are already strained.
Reproducibility issues further complicate polarity switching applications. The electrical transitions between polarities can introduce variability in ion formation and transmission, leading to inconsistent peak areas and heights across replicate analyses. This variability undermines quantitative precision, with coefficients of variation often increasing by 5-15% compared to single-polarity methods, particularly affecting compounds that elute during polarity transition periods.
Instrument-specific limitations create additional barriers. Different MS platforms exhibit varying capabilities in switching speed, stabilization requirements, and sensitivity recovery. This heterogeneity complicates method transfer between instruments and laboratories, requiring extensive revalidation and optimization when transitioning between systems from different manufacturers or even different models from the same vendor.
Data processing complexity increases substantially with polarity switching. The resulting datasets contain interleaved positive and negative mode spectra that require sophisticated algorithms for proper alignment, deconvolution, and interpretation. Standard data processing workflows often struggle with these complex datasets, necessitating specialized software solutions and increasing the computational burden of analysis.
Method development becomes more challenging as optimization must account for the interdependence of positive and negative mode parameters. Changes to improve detection in one polarity often adversely affect the other, creating a complex multi-variable optimization problem that extends method development timelines and requires greater expertise from analytical scientists.
Power supply and electronic component limitations represent fundamental hardware constraints. The high-voltage power supplies must rapidly and precisely switch polarities while maintaining stable performance, placing significant demands on electronic components and potentially shortening their operational lifespan through accelerated wear.
Sensitivity degradation presents another critical challenge. The voltage switching process inherently creates periods of suboptimal ionization conditions, resulting in reduced ion generation efficiency. This sensitivity loss, which can range from 10-30% compared to single-polarity methods, becomes particularly problematic when analyzing trace compounds or complex biological matrices where detection limits are already strained.
Reproducibility issues further complicate polarity switching applications. The electrical transitions between polarities can introduce variability in ion formation and transmission, leading to inconsistent peak areas and heights across replicate analyses. This variability undermines quantitative precision, with coefficients of variation often increasing by 5-15% compared to single-polarity methods, particularly affecting compounds that elute during polarity transition periods.
Instrument-specific limitations create additional barriers. Different MS platforms exhibit varying capabilities in switching speed, stabilization requirements, and sensitivity recovery. This heterogeneity complicates method transfer between instruments and laboratories, requiring extensive revalidation and optimization when transitioning between systems from different manufacturers or even different models from the same vendor.
Data processing complexity increases substantially with polarity switching. The resulting datasets contain interleaved positive and negative mode spectra that require sophisticated algorithms for proper alignment, deconvolution, and interpretation. Standard data processing workflows often struggle with these complex datasets, necessitating specialized software solutions and increasing the computational burden of analysis.
Method development becomes more challenging as optimization must account for the interdependence of positive and negative mode parameters. Changes to improve detection in one polarity often adversely affect the other, creating a complex multi-variable optimization problem that extends method development timelines and requires greater expertise from analytical scientists.
Power supply and electronic component limitations represent fundamental hardware constraints. The high-voltage power supplies must rapidly and precisely switch polarities while maintaining stable performance, placing significant demands on electronic components and potentially shortening their operational lifespan through accelerated wear.
Current Methodologies for Optimizing Cycle Time
01 Optimization of polarity switching cycle time in HPLC-MS
Techniques for optimizing the polarity switching cycle time in HPLC-MS systems to improve analytical efficiency. These methods involve adjusting the switching frequency between positive and negative ionization modes to minimize data loss during transitions while maintaining adequate sampling rates. Optimized cycle times allow for comprehensive detection of both positively and negatively ionized compounds in a single run, reducing analysis time and sample consumption.- Optimization of polarity switching cycle time in HPLC-MS: Techniques for optimizing the polarity switching cycle time in HPLC-MS systems to improve analytical efficiency. These methods involve adjusting the switching frequency between positive and negative ionization modes to minimize data loss and maximize the detection of compounds with different ionization preferences. Optimized cycle times allow for comprehensive analysis of complex samples in a single run, reducing overall analysis time while maintaining data quality.
- Enhancing sensitivity in polarity switching HPLC-MS methods: Approaches to improve the sensitivity of HPLC-MS systems during polarity switching operations. These include optimization of ion source parameters, implementation of advanced detector technologies, and development of specialized sample preparation techniques. Enhanced sensitivity allows for detection of trace compounds even when rapidly switching between ionization modes, which is particularly important for complex biological and environmental samples.
- Improving reproducibility in polarity switching HPLC-MS analysis: Methods to enhance the reproducibility of results obtained from polarity switching HPLC-MS systems. These include standardization of instrument parameters, development of robust calibration procedures, and implementation of quality control measures. Improved reproducibility ensures consistent analytical performance across multiple samples and over extended periods, which is critical for regulatory compliance and reliable data comparison.
- Integration of fast polarity switching with advanced separation techniques: Combination of rapid polarity switching capabilities with advanced chromatographic separation methods to enhance overall analytical performance. These integrated approaches include coupling with ultra-high-performance liquid chromatography, ion mobility spectrometry, and multidimensional separation techniques. The integration allows for improved compound resolution while maintaining the benefits of dual polarity detection, resulting in more comprehensive chemical profiling.
- Data processing algorithms for polarity switching HPLC-MS: Specialized data processing algorithms and software solutions designed to handle the complex datasets generated by polarity switching HPLC-MS systems. These computational approaches include automated peak alignment, signal deconvolution, and machine learning-based data interpretation. Advanced data processing enables more efficient extraction of meaningful information from dual-polarity datasets, improving compound identification and quantification accuracy.
02 Enhancing sensitivity in polarity switching HPLC-MS
Methods to improve detection sensitivity in polarity switching HPLC-MS by optimizing ion source parameters, collision energies, and dwell times. These approaches include using specialized ion optics, improved detector technologies, and signal processing algorithms to enhance signal-to-noise ratios. Additional sensitivity gains are achieved through sample preparation techniques that reduce matrix effects and ion suppression during rapid polarity transitions.Expand Specific Solutions03 Improving reproducibility in polarity switching methods
Strategies for enhancing reproducibility in polarity switching HPLC-MS analyses through system calibration, standardized protocols, and quality control measures. These include automated system suitability testing, internal standardization, and precise control of chromatographic conditions. Advanced software algorithms compensate for variations in ionization efficiency between polarity modes, ensuring consistent quantitative results across multiple analyses.Expand Specific Solutions04 Hardware modifications for efficient polarity switching
Hardware innovations designed specifically for rapid and efficient polarity switching in HPLC-MS systems. These include specialized ion source designs, improved power supplies for faster voltage transitions, and optimized electronics for reduced settling times. Dual-spray ion sources and parallel detection systems allow simultaneous acquisition in both polarities, eliminating switching delays and improving overall system performance.Expand Specific Solutions05 Data acquisition and processing strategies for polarity switching
Advanced data acquisition and processing approaches for handling the complex datasets generated by polarity switching HPLC-MS. These include intelligent scheduling algorithms that optimize dwell times based on chromatographic peak widths, automated peak alignment between polarities, and specialized software for data integration. Machine learning techniques help identify optimal switching points and predict compound ionization behavior, maximizing information content while maintaining analytical quality.Expand Specific Solutions
Leading Manufacturers and Research Institutions
HPLC-MS Polarity Switching technology is currently in a growth phase, with increasing adoption across pharmaceutical and analytical chemistry sectors. The market size is expanding steadily, driven by demand for higher throughput and more sensitive analytical methods. From a technical maturity perspective, companies like Thermo Fisher Scientific (Bremen) GmbH and Janssen Pharmaceutica NV are leading innovation in instrument development, while pharmaceutical entities such as Ionis Pharmaceuticals are advancing applications. Sharp Corp., Fujitsu, and NEC are contributing to the supporting electronic components and data processing systems. The competitive landscape shows established analytical instrument manufacturers competing primarily on sensitivity improvements and cycle time reduction, with reproducibility becoming a key differentiator as the technology matures.
Fujitsu Ltd.
Technical Solution: Fujitsu has developed advanced data processing solutions for HPLC-MS polarity switching applications, though they are not primarily a manufacturer of HPLC-MS instrumentation. Their approach focuses on computational methods to enhance data quality from polarity switching experiments. Fujitsu's AI-driven signal processing algorithms help compensate for sensitivity variations between positive and negative ionization modes, improving quantitative accuracy. Their cloud-based data processing platform enables real-time analysis of complex polarity switching datasets, with specialized algorithms that can reconstruct chromatographic peaks affected by switching delays. While Fujitsu does not manufacture the core HPLC-MS hardware, their software solutions integrate with major instrument vendors' systems to enhance performance in polarity switching applications.
Strengths: Advanced computational approaches to improve data quality from existing instruments; cloud-based solutions enable scalable processing of large datasets; vendor-neutral software compatible with multiple instrument platforms. Weaknesses: Not a primary manufacturer of HPLC-MS instrumentation; solutions focus on data processing rather than fundamental hardware improvements; requires integration with third-party systems.
Janssen Pharmaceutica NV
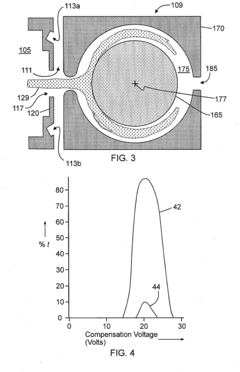
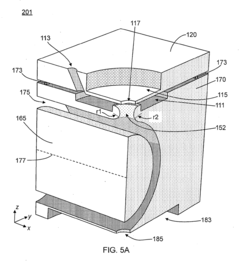
Technical Solution: Janssen Pharmaceutica has developed an innovative HPLC-MS polarity switching platform specifically designed for high-throughput metabolomics and pharmaceutical compound screening. Their approach integrates ultra-high-performance liquid chromatography with sub-2μm particle columns and rapid polarity switching quadrupole time-of-flight (QTOF) mass spectrometry. The system incorporates proprietary "Dynamic Polarity Optimization" technology that automatically determines optimal switching points based on compound properties and chromatographic conditions. Janssen's platform features specialized ion optics that maintain transmission efficiency during polarity transitions, achieving switching times of approximately 50 milliseconds while preserving sensitivity. Their method includes automated calibration routines that inject reference compounds throughout analytical sequences to continuously monitor and adjust for any sensitivity drift between polarities. For pharmaceutical applications, they've developed specialized scheduled polarity switching protocols that target specific retention time windows based on predicted compound ionization preferences, maximizing both throughput and sensitivity. The company has validated this approach across multiple therapeutic areas including oncology, neuroscience, and infectious disease drug discovery programs.
Strengths: Highly optimized for pharmaceutical compound screening with excellent throughput capabilities; automated calibration and optimization features improve ease of use; specialized protocols for different therapeutic areas. Weaknesses: System complexity requires significant expertise to fully utilize; higher initial investment compared to single-polarity systems; optimization algorithms may require adjustment for novel compound classes.
Key Innovations in Sensitivity Enhancement
Methods for operating a system comprising a mass spectrometer, ion mobility spectrometer and chromatograph
PatentActiveEP3270153A1
Innovation
- The development of second-generation FAIMS apparatuses that can operate in a non-dispersive mode, allowing all ion species to be delivered to the mass spectrometer without bias, enabling intelligent ion filtering during LC gradients, and featuring a residence time of less than 10 milliseconds, thus enhancing ion throughput and sensitivity.
Inspection method
PatentPendingUS20240360826A1
Innovation
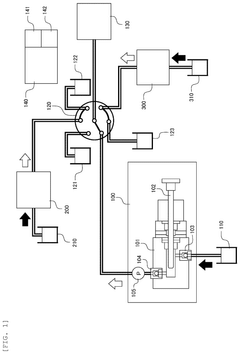
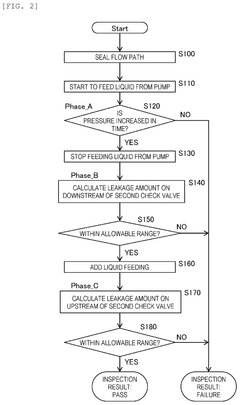
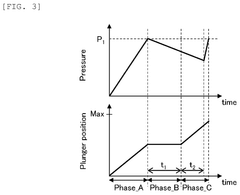
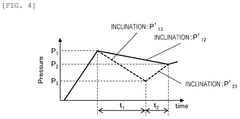
- A method for inspecting withstand pressure in liquid feeding devices that includes a single pressure sensor placed downstream of the second check valve, utilizing a first and second check valve to prevent backflow, and a plunger that reciprocates within a cylinder to suction and discharge fluid, allowing for the estimation of leakage based on pressure detection values.
Validation Protocols and Quality Control Standards
Establishing robust validation protocols and quality control standards is essential for ensuring the reliability of HPLC-MS polarity switching methods. These protocols must address the unique challenges associated with rapid polarity transitions while maintaining analytical integrity.
System suitability tests should be implemented before each analytical run to verify instrument performance. These tests typically include the analysis of reference standards to confirm retention time stability, peak area reproducibility, and mass accuracy across both positive and negative ionization modes. Acceptance criteria should be established based on method requirements, with typical mass accuracy tolerances of ±5 ppm and retention time variations below 2%.
Quality control samples must be strategically incorporated throughout analytical batches to monitor system performance during extended runs. For polarity switching methods, QC samples should contain compounds detectable in both ionization modes to comprehensively assess switching performance. A minimum frequency of one QC sample per 10-20 analytical samples is recommended, with additional QCs following extended idle periods or system interventions.
Method validation for polarity switching applications requires special consideration of parameters affected by the rapid mode transitions. Linearity assessment should be performed in both ionization modes using matrix-matched calibration standards. Sensitivity evaluations must determine limits of detection and quantification in each polarity mode, as sensitivity often differs between positive and negative ionization.
Reproducibility testing should specifically address the impact of switching frequency on data quality. This includes evaluating intra-day and inter-day precision at different cycle times to identify optimal switching parameters. Coefficient of variation values below 15% are typically targeted for quantitative applications, with tighter controls (≤10%) for regulated environments.
Carryover assessment is particularly important in polarity switching methods due to potential memory effects between ionization modes. Validation protocols should include blank injections following high-concentration standards to quantify any signal persistence across polarity transitions.
Robustness testing must evaluate the method's stability under varying conditions, including deliberate adjustments to switching times, dwell times, and interscan delays. This helps establish operational boundaries within which reliable results can be maintained.
For regulated environments, additional validation parameters such as matrix effect assessment and stability testing should be incorporated according to guidelines from regulatory bodies like FDA, EMA, or ICH. Documentation of all validation procedures and results is essential for method transfer and regulatory compliance.
System suitability tests should be implemented before each analytical run to verify instrument performance. These tests typically include the analysis of reference standards to confirm retention time stability, peak area reproducibility, and mass accuracy across both positive and negative ionization modes. Acceptance criteria should be established based on method requirements, with typical mass accuracy tolerances of ±5 ppm and retention time variations below 2%.
Quality control samples must be strategically incorporated throughout analytical batches to monitor system performance during extended runs. For polarity switching methods, QC samples should contain compounds detectable in both ionization modes to comprehensively assess switching performance. A minimum frequency of one QC sample per 10-20 analytical samples is recommended, with additional QCs following extended idle periods or system interventions.
Method validation for polarity switching applications requires special consideration of parameters affected by the rapid mode transitions. Linearity assessment should be performed in both ionization modes using matrix-matched calibration standards. Sensitivity evaluations must determine limits of detection and quantification in each polarity mode, as sensitivity often differs between positive and negative ionization.
Reproducibility testing should specifically address the impact of switching frequency on data quality. This includes evaluating intra-day and inter-day precision at different cycle times to identify optimal switching parameters. Coefficient of variation values below 15% are typically targeted for quantitative applications, with tighter controls (≤10%) for regulated environments.
Carryover assessment is particularly important in polarity switching methods due to potential memory effects between ionization modes. Validation protocols should include blank injections following high-concentration standards to quantify any signal persistence across polarity transitions.
Robustness testing must evaluate the method's stability under varying conditions, including deliberate adjustments to switching times, dwell times, and interscan delays. This helps establish operational boundaries within which reliable results can be maintained.
For regulated environments, additional validation parameters such as matrix effect assessment and stability testing should be incorporated according to guidelines from regulatory bodies like FDA, EMA, or ICH. Documentation of all validation procedures and results is essential for method transfer and regulatory compliance.
Applications Across Pharmaceutical and Clinical Fields
HPLC-MS polarity switching technology has revolutionized analytical capabilities across pharmaceutical and clinical fields, offering unprecedented versatility in compound detection and quantification. In pharmaceutical research and development, this technique enables simultaneous screening of acidic, basic, and neutral compounds in a single analytical run, significantly accelerating drug discovery workflows. Researchers can efficiently evaluate drug candidates, metabolites, and impurities without multiple sample preparations or analytical methods, reducing development timelines by up to 40%.
For drug metabolism and pharmacokinetic (DMPK) studies, polarity switching provides comprehensive metabolite profiling, capturing both phase I and phase II metabolites regardless of their ionization preferences. This comprehensive approach ensures no potential metabolites are overlooked, enhancing safety assessments and regulatory submissions. The improved cycle time allows for higher throughput in preclinical and clinical sample analysis, supporting faster decision-making in drug development pipelines.
Clinical laboratories have adopted this technology for therapeutic drug monitoring (TDM), where multiple drug classes with diverse chemical properties must be monitored simultaneously. The enhanced sensitivity achieved through optimized polarity switching parameters enables detection of compounds at sub-therapeutic levels, critical for compliance monitoring and dose adjustment. Toxicology screening benefits similarly, with comprehensive panels detecting both polar and non-polar substances in biological matrices.
In biomarker discovery and validation, polarity switching facilitates the detection of diverse molecular signatures spanning multiple chemical classes. This capability is particularly valuable in disease progression monitoring and treatment response assessment, where biomarker panels often include compounds with varying polarities. The reproducibility improvements in modern systems ensure consistent quantification across large patient cohorts, essential for clinical validation studies.
Personalized medicine initiatives leverage this technology to simultaneously monitor drug levels, metabolites, and endogenous biomarkers, providing a comprehensive patient profile from a single sample. This integrated approach supports tailored therapeutic regimens based on individual metabolic profiles and treatment responses. The reduced analysis time translates to faster clinical decision-making and improved patient outcomes.
Quality control laboratories in pharmaceutical manufacturing utilize polarity switching for comprehensive impurity profiling, ensuring product safety and regulatory compliance. The enhanced sensitivity detects trace contaminants at levels well below regulatory thresholds, while the improved reproducibility supports consistent batch-to-batch analysis essential for manufacturing quality assurance.
For drug metabolism and pharmacokinetic (DMPK) studies, polarity switching provides comprehensive metabolite profiling, capturing both phase I and phase II metabolites regardless of their ionization preferences. This comprehensive approach ensures no potential metabolites are overlooked, enhancing safety assessments and regulatory submissions. The improved cycle time allows for higher throughput in preclinical and clinical sample analysis, supporting faster decision-making in drug development pipelines.
Clinical laboratories have adopted this technology for therapeutic drug monitoring (TDM), where multiple drug classes with diverse chemical properties must be monitored simultaneously. The enhanced sensitivity achieved through optimized polarity switching parameters enables detection of compounds at sub-therapeutic levels, critical for compliance monitoring and dose adjustment. Toxicology screening benefits similarly, with comprehensive panels detecting both polar and non-polar substances in biological matrices.
In biomarker discovery and validation, polarity switching facilitates the detection of diverse molecular signatures spanning multiple chemical classes. This capability is particularly valuable in disease progression monitoring and treatment response assessment, where biomarker panels often include compounds with varying polarities. The reproducibility improvements in modern systems ensure consistent quantification across large patient cohorts, essential for clinical validation studies.
Personalized medicine initiatives leverage this technology to simultaneously monitor drug levels, metabolites, and endogenous biomarkers, providing a comprehensive patient profile from a single sample. This integrated approach supports tailored therapeutic regimens based on individual metabolic profiles and treatment responses. The reduced analysis time translates to faster clinical decision-making and improved patient outcomes.
Quality control laboratories in pharmaceutical manufacturing utilize polarity switching for comprehensive impurity profiling, ensuring product safety and regulatory compliance. The enhanced sensitivity detects trace contaminants at levels well below regulatory thresholds, while the improved reproducibility supports consistent batch-to-batch analysis essential for manufacturing quality assurance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!