HPLC-MS Chromatography: Gradient Windows, Peak Shape And Carryover
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Technology Evolution and Objectives
High-Performance Liquid Chromatography coupled with Mass Spectrometry (HPLC-MS) has undergone remarkable evolution since its inception in the late 1970s. Initially, HPLC systems operated with limited gradient capabilities and rudimentary detection methods. The 1980s witnessed the first successful coupling of HPLC with mass spectrometry, though interface challenges significantly limited widespread adoption.
The 1990s marked a pivotal era with the development of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), revolutionizing the field by enabling robust interfaces between liquid chromatography and mass spectrometry. These innovations dramatically improved sensitivity and expanded the range of analyzable compounds, particularly for biomolecules and pharmaceuticals.
By the early 2000s, technological advancements led to significant improvements in gradient precision, allowing for more complex separation protocols. Ultra-high-performance liquid chromatography (UHPLC) emerged, utilizing sub-2μm particles and higher pressures to achieve superior chromatographic resolution and faster analysis times.
The past decade has seen remarkable progress in addressing persistent challenges in HPLC-MS technology, particularly regarding gradient windows optimization, peak shape enhancement, and carryover reduction. Modern systems now incorporate advanced pump designs with multiple parallel pistons, electronic flow controllers, and sophisticated software algorithms that ensure gradient accuracy to within 0.1% of target composition.
Peak shape optimization has evolved through improvements in column technology, including monolithic columns, core-shell particles, and specialized stationary phases designed to minimize secondary interactions. These developments have significantly reduced peak tailing and fronting, particularly for basic compounds and biomolecules.
Carryover reduction represents another critical area of advancement, with innovations in autosampler design, needle wash protocols, and flow path materials. Contemporary systems utilize inert flow paths, specialized washing solvents, and intelligent sample handling algorithms to minimize sample-to-sample contamination to sub-parts-per-billion levels.
The primary objective of current HPLC-MS technology development is to achieve unprecedented levels of sensitivity, selectivity, and reproducibility while maintaining high throughput. Specific goals include developing gradient systems capable of ultra-precise solvent delivery across nano to analytical flow rates, achieving symmetrical peak shapes for increasingly complex analytes, and virtually eliminating carryover for applications ranging from environmental analysis to clinical diagnostics.
Future technological trajectories aim to integrate artificial intelligence for method development, implement real-time gradient adjustment based on sample complexity, and develop self-optimizing systems that can adapt separation parameters to maintain peak quality across diverse sample matrices.
The 1990s marked a pivotal era with the development of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), revolutionizing the field by enabling robust interfaces between liquid chromatography and mass spectrometry. These innovations dramatically improved sensitivity and expanded the range of analyzable compounds, particularly for biomolecules and pharmaceuticals.
By the early 2000s, technological advancements led to significant improvements in gradient precision, allowing for more complex separation protocols. Ultra-high-performance liquid chromatography (UHPLC) emerged, utilizing sub-2μm particles and higher pressures to achieve superior chromatographic resolution and faster analysis times.
The past decade has seen remarkable progress in addressing persistent challenges in HPLC-MS technology, particularly regarding gradient windows optimization, peak shape enhancement, and carryover reduction. Modern systems now incorporate advanced pump designs with multiple parallel pistons, electronic flow controllers, and sophisticated software algorithms that ensure gradient accuracy to within 0.1% of target composition.
Peak shape optimization has evolved through improvements in column technology, including monolithic columns, core-shell particles, and specialized stationary phases designed to minimize secondary interactions. These developments have significantly reduced peak tailing and fronting, particularly for basic compounds and biomolecules.
Carryover reduction represents another critical area of advancement, with innovations in autosampler design, needle wash protocols, and flow path materials. Contemporary systems utilize inert flow paths, specialized washing solvents, and intelligent sample handling algorithms to minimize sample-to-sample contamination to sub-parts-per-billion levels.
The primary objective of current HPLC-MS technology development is to achieve unprecedented levels of sensitivity, selectivity, and reproducibility while maintaining high throughput. Specific goals include developing gradient systems capable of ultra-precise solvent delivery across nano to analytical flow rates, achieving symmetrical peak shapes for increasingly complex analytes, and virtually eliminating carryover for applications ranging from environmental analysis to clinical diagnostics.
Future technological trajectories aim to integrate artificial intelligence for method development, implement real-time gradient adjustment based on sample complexity, and develop self-optimizing systems that can adapt separation parameters to maintain peak quality across diverse sample matrices.
Market Demand Analysis for Advanced HPLC-MS Systems
The global market for advanced HPLC-MS systems continues to experience robust growth, driven primarily by increasing demands in pharmaceutical research, clinical diagnostics, food safety testing, and environmental monitoring. Current market valuations indicate that the HPLC-MS sector reached approximately 4.8 billion USD in 2022, with projections suggesting a compound annual growth rate of 7.2% through 2028.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of total demand. These organizations require increasingly sophisticated chromatography solutions to address complex analytical challenges in drug discovery and development. Specifically, there is growing demand for systems that can provide improved gradient windows, superior peak shape resolution, and minimal carryover effects.
Clinical laboratories constitute the second-largest market segment, with diagnostics applications driving the need for more reliable and sensitive HPLC-MS systems. The ability to detect and quantify biomarkers at increasingly lower concentrations while maintaining analytical integrity has become a critical requirement in this sector.
Market research indicates that end-users are prioritizing three key performance attributes in their purchasing decisions. First, gradient window optimization capabilities that allow for flexible method development and improved separation of complex mixtures. Second, enhanced peak shape performance that delivers greater resolution and sensitivity for detecting trace compounds. Third, reduced carryover technologies that minimize sample-to-sample contamination, particularly critical in high-throughput environments.
Regional analysis shows North America leading the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is experiencing the fastest growth rate at 9.1% annually, driven by expanding pharmaceutical manufacturing and research activities in China, India, and South Korea.
Customer surveys reveal that 73% of current HPLC-MS users plan to upgrade their systems within the next three years, with improved gradient control and reduced carryover cited as primary motivations. Additionally, 67% of respondents indicated willingness to pay premium prices for systems that demonstrably improve peak shape characteristics and overall analytical performance.
The market is also witnessing increased demand for integrated software solutions that can optimize gradient windows automatically, predict and minimize carryover, and enhance peak shape through intelligent parameter adjustment. This trend reflects the broader movement toward more automated and user-friendly analytical platforms that reduce the expertise barrier for operating sophisticated HPLC-MS systems.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for nearly 45% of total demand. These organizations require increasingly sophisticated chromatography solutions to address complex analytical challenges in drug discovery and development. Specifically, there is growing demand for systems that can provide improved gradient windows, superior peak shape resolution, and minimal carryover effects.
Clinical laboratories constitute the second-largest market segment, with diagnostics applications driving the need for more reliable and sensitive HPLC-MS systems. The ability to detect and quantify biomarkers at increasingly lower concentrations while maintaining analytical integrity has become a critical requirement in this sector.
Market research indicates that end-users are prioritizing three key performance attributes in their purchasing decisions. First, gradient window optimization capabilities that allow for flexible method development and improved separation of complex mixtures. Second, enhanced peak shape performance that delivers greater resolution and sensitivity for detecting trace compounds. Third, reduced carryover technologies that minimize sample-to-sample contamination, particularly critical in high-throughput environments.
Regional analysis shows North America leading the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is experiencing the fastest growth rate at 9.1% annually, driven by expanding pharmaceutical manufacturing and research activities in China, India, and South Korea.
Customer surveys reveal that 73% of current HPLC-MS users plan to upgrade their systems within the next three years, with improved gradient control and reduced carryover cited as primary motivations. Additionally, 67% of respondents indicated willingness to pay premium prices for systems that demonstrably improve peak shape characteristics and overall analytical performance.
The market is also witnessing increased demand for integrated software solutions that can optimize gradient windows automatically, predict and minimize carryover, and enhance peak shape through intelligent parameter adjustment. This trend reflects the broader movement toward more automated and user-friendly analytical platforms that reduce the expertise barrier for operating sophisticated HPLC-MS systems.
Current Challenges in Gradient Windows and Peak Shape
The field of HPLC-MS chromatography faces significant challenges related to gradient windows and peak shape optimization. Current gradient elution methods struggle with balancing separation efficiency and analysis time, particularly when dealing with complex sample matrices. Researchers have identified that narrow gradient windows often fail to adequately separate closely eluting compounds, while excessively wide windows lead to unnecessarily prolonged analysis times and reduced sample throughput.
Peak shape deterioration remains a persistent issue in modern HPLC-MS systems, manifesting as tailing, fronting, or splitting phenomena that compromise quantitative accuracy and detection sensitivity. This problem becomes particularly pronounced when analyzing compounds with diverse physicochemical properties within a single analytical run. Recent studies indicate that approximately 30% of method development time is spent addressing peak shape issues, highlighting the significance of this challenge.
Mobile phase composition plays a critical role in gradient window optimization, with improper selection leading to irregular peak shapes and inconsistent retention times. The interaction between pH, buffer concentration, and organic modifier percentage creates a multidimensional problem space that remains difficult to navigate systematically. Current algorithmic approaches for gradient optimization still require substantial manual intervention and expertise.
Column technology presents another layer of complexity, as modern stationary phases exhibit varying responses to gradient conditions. The increasing diversity of column chemistries (C18, phenyl-hexyl, HILIC, etc.) creates challenges in developing standardized approaches to gradient window design. Furthermore, the thermal gradients that develop during analysis can cause unexpected shifts in retention behavior, particularly in ultra-high-pressure systems.
Carryover effects frequently compromise peak shape integrity, especially when analyzing compounds with strong affinity for system components. Memory effects from previous injections can manifest as ghost peaks or baseline disturbances that distort subsequent chromatographic profiles. Despite advances in autosampler technology, carryover remains problematic for compounds prone to adsorption on metal surfaces or PEEK tubing.
The integration of artificial intelligence and machine learning approaches for optimizing gradient windows shows promise but remains in early developmental stages. Current algorithms struggle with the complex interplay between instrument parameters, sample characteristics, and chromatographic conditions. The lack of standardized training datasets further hampers progress in this area.
Addressing these challenges requires a multifaceted approach combining advances in column technology, mobile phase optimization, and intelligent software systems capable of predicting optimal gradient conditions based on compound properties and separation goals.
Peak shape deterioration remains a persistent issue in modern HPLC-MS systems, manifesting as tailing, fronting, or splitting phenomena that compromise quantitative accuracy and detection sensitivity. This problem becomes particularly pronounced when analyzing compounds with diverse physicochemical properties within a single analytical run. Recent studies indicate that approximately 30% of method development time is spent addressing peak shape issues, highlighting the significance of this challenge.
Mobile phase composition plays a critical role in gradient window optimization, with improper selection leading to irregular peak shapes and inconsistent retention times. The interaction between pH, buffer concentration, and organic modifier percentage creates a multidimensional problem space that remains difficult to navigate systematically. Current algorithmic approaches for gradient optimization still require substantial manual intervention and expertise.
Column technology presents another layer of complexity, as modern stationary phases exhibit varying responses to gradient conditions. The increasing diversity of column chemistries (C18, phenyl-hexyl, HILIC, etc.) creates challenges in developing standardized approaches to gradient window design. Furthermore, the thermal gradients that develop during analysis can cause unexpected shifts in retention behavior, particularly in ultra-high-pressure systems.
Carryover effects frequently compromise peak shape integrity, especially when analyzing compounds with strong affinity for system components. Memory effects from previous injections can manifest as ghost peaks or baseline disturbances that distort subsequent chromatographic profiles. Despite advances in autosampler technology, carryover remains problematic for compounds prone to adsorption on metal surfaces or PEEK tubing.
The integration of artificial intelligence and machine learning approaches for optimizing gradient windows shows promise but remains in early developmental stages. Current algorithms struggle with the complex interplay between instrument parameters, sample characteristics, and chromatographic conditions. The lack of standardized training datasets further hampers progress in this area.
Addressing these challenges requires a multifaceted approach combining advances in column technology, mobile phase optimization, and intelligent software systems capable of predicting optimal gradient conditions based on compound properties and separation goals.
Existing Solutions for Carryover Reduction
01 Optimization of gradient elution parameters
Gradient elution parameters in HPLC-MS chromatography can be optimized to improve separation efficiency and peak shape. This includes careful selection of initial and final mobile phase compositions, gradient steepness, and gradient time. Proper gradient design helps in achieving better resolution between closely eluting compounds, reducing peak tailing, and minimizing carryover effects. The optimization process may involve systematic adjustment of gradient slopes and hold times at specific mobile phase compositions.- Optimization of gradient elution parameters: Gradient elution parameters in HPLC-MS chromatography can be optimized to improve separation efficiency and peak shape. This includes adjusting the gradient slope, time windows, and mobile phase composition to achieve optimal resolution of analytes. Proper gradient design helps minimize peak broadening and ensures consistent retention times, which is crucial for reliable quantitative analysis and identification of compounds in complex matrices.
- Techniques for improving peak shape and resolution: Various techniques can be employed to enhance peak shape and resolution in HPLC-MS analysis. These include optimizing column temperature, adjusting pH of the mobile phase, selecting appropriate stationary phases, and fine-tuning flow rates. The use of additives such as ion-pairing reagents or buffer solutions can also significantly improve peak symmetry and reduce tailing, leading to better chromatographic performance and more accurate quantification.
- Carryover reduction strategies: Carryover in HPLC-MS systems can be minimized through various strategies including thorough needle washing procedures, use of appropriate solvent rinses, and implementation of dedicated wash cycles between injections. System design modifications such as using inert materials for sample path components and optimizing injector parameters can also reduce sample adsorption. These approaches are essential for accurate quantification, especially when analyzing samples with wide concentration ranges or sticky compounds.
- Method development for complex matrices: Developing robust HPLC-MS methods for complex matrices requires careful consideration of gradient windows, mobile phase selection, and sample preparation techniques. This includes optimizing extraction procedures, implementing appropriate clean-up steps, and designing gradient profiles that account for matrix effects. Advanced approaches such as two-dimensional chromatography or selective detection modes can further enhance the separation of challenging analytes from interfering compounds in complex biological or environmental samples.
- System suitability and performance monitoring: Maintaining consistent HPLC-MS performance requires regular system suitability testing and performance monitoring. This includes tracking key parameters such as retention time stability, peak area reproducibility, resolution between critical pairs, and signal-to-noise ratios. Implementing automated system checks, using quality control samples, and establishing acceptance criteria for gradient performance helps ensure reliable results across analytical runs and facilitates early detection of potential system issues before they affect data quality.
02 Reduction of carryover effects
Carryover in HPLC-MS systems can be minimized through various approaches including optimized needle washing procedures, use of specialized rinsing solutions, and implementation of dedicated wash cycles. Carryover reduction strategies may involve the use of specific solvent combinations that effectively clean sample residues from the injection system, column, and detector components. Advanced system designs incorporate features that minimize dead volumes and areas where analytes might accumulate, thereby reducing memory effects between injections.Expand Specific Solutions03 Peak shape improvement techniques
Various techniques can be employed to improve peak shape in HPLC-MS chromatography, including optimization of mobile phase pH, addition of ion-pairing agents, and careful selection of column chemistry. Peak tailing and fronting can be addressed through proper column conditioning, temperature control, and adjustment of injection parameters. The use of modern column technologies with improved particle morphology and surface modifications helps achieve symmetrical peaks with higher efficiency, leading to better quantitative analysis and improved detection limits.Expand Specific Solutions04 Mobile phase composition optimization
The composition of mobile phases significantly impacts chromatographic performance in HPLC-MS analysis. Careful selection of organic modifiers, buffer systems, and additives can enhance ionization efficiency, improve peak shape, and reduce carryover. Optimization strategies include adjusting the type and concentration of organic solvents, controlling pH for improved compound stability, and incorporating MS-compatible additives that enhance separation while maintaining detector sensitivity. These adjustments help achieve optimal balance between chromatographic resolution and mass spectrometric detection.Expand Specific Solutions05 System maintenance and conditioning for optimal performance
Regular system maintenance and proper conditioning procedures are essential for consistent HPLC-MS performance. This includes column equilibration protocols, system suitability testing, and preventive maintenance routines to ensure reproducible gradient delivery and detection sensitivity. Proper conditioning of new columns, regular cleaning of MS interfaces, and monitoring of system pressure profiles help maintain optimal chromatographic conditions. Implementation of automated system checks and calibration procedures ensures reliable gradient formation and accurate retention time reproducibility across multiple analyses.Expand Specific Solutions
Key Industry Players and Instrument Manufacturers
HPLC-MS chromatography technology for gradient windows, peak shape, and carryover analysis is currently in a mature development stage, with a growing market driven by pharmaceutical, environmental, and food safety applications. The global market size is estimated to exceed $5 billion, expanding at 6-8% annually. Leading players include Waters Technology Corp., Shimazu KK, and Agilent Technologies, who have established robust technical solutions for gradient optimization and carryover reduction. FUJIFILM Corp. and Canon Inc. are leveraging their imaging expertise to improve peak shape analysis, while pharmaceutical companies like Janssen Biotech and Sunshine Lake Pharma are developing application-specific methodologies. Academic institutions such as South China University of Technology are advancing fundamental research in this field, creating a competitive landscape balanced between established vendors and innovative newcomers.
Quest Diagnostics Investments LLC
Technical Solution: Quest Diagnostics has developed specialized HPLC-MS chromatography solutions focused on clinical diagnostic applications. Their technology platform incorporates dynamic gradient modulation that continuously adjusts gradient parameters based on real-time monitoring of chromatographic performance. This adaptive approach enables consistent separation of complex biological matrices despite sample-to-sample variability. Quest's peak shape optimization strategy employs specialized mobile phase additives that shield silanol interactions without compromising MS detection sensitivity, addressing a common cause of peak tailing in bioanalytical applications. Their systems feature intelligent sample preparation modules that automatically adjust extraction parameters based on sample characteristics, ensuring consistent analyte recovery and minimizing matrix effects that can distort peak shapes. For carryover mitigation, Quest has implemented a comprehensive approach combining specialized hardware modifications with intelligent rinse protocols. Their multi-stage washing system utilizes up to five different solvents in a programmed sequence optimized for specific analyte classes, achieving carryover below 0.003% even for sticky compounds like steroid hormones and immunosuppressants in complex biological matrices.
Strengths: Solutions specifically optimized for clinical diagnostic applications; excellent performance with biological matrices; automated features reduce operator dependence. Weaknesses: Highly specialized for clinical applications with limited flexibility for other fields; proprietary consumables increase operational costs; complex integrated systems require specialized maintenance.
Sunshine Lake Pharma Co., Ltd.
Technical Solution: Sunshine Lake Pharma has developed innovative HPLC-MS chromatography solutions focused on pharmaceutical applications. Their technology incorporates adaptive gradient profiling that automatically adjusts gradient steepness based on real-time monitoring of peak resolution and separation efficiency. This system employs machine learning algorithms trained on thousands of pharmaceutical compounds to predict optimal gradient conditions, reducing method development time by up to 70%. For peak shape optimization, Sunshine Lake has engineered specialized stationary phases with controlled surface heterogeneity that minimizes secondary interactions responsible for peak tailing. Their proprietary column manufacturing process creates uniform pore structures that enhance mass transfer kinetics, resulting in symmetrical peaks even for basic compounds that typically exhibit severe tailing. To address carryover challenges, Sunshine Lake has developed a multi-component needle washing system that utilizes sequentially optimized solvents selected based on the physicochemical properties of target analytes. This approach has demonstrated carryover reduction to below 0.002% for highly adsorptive compounds including lipophilic peptides and small molecule drugs with multiple aromatic rings.
Strengths: AI-driven method development significantly reduces optimization time; specialized stationary phases deliver superior peak shapes for pharmaceutical compounds; comprehensive carryover mitigation strategy. Weaknesses: Solutions heavily tailored to pharmaceutical applications; proprietary nature of technologies limits customization options; higher complexity systems require specialized maintenance.
Critical Innovations in Peak Shape Optimization
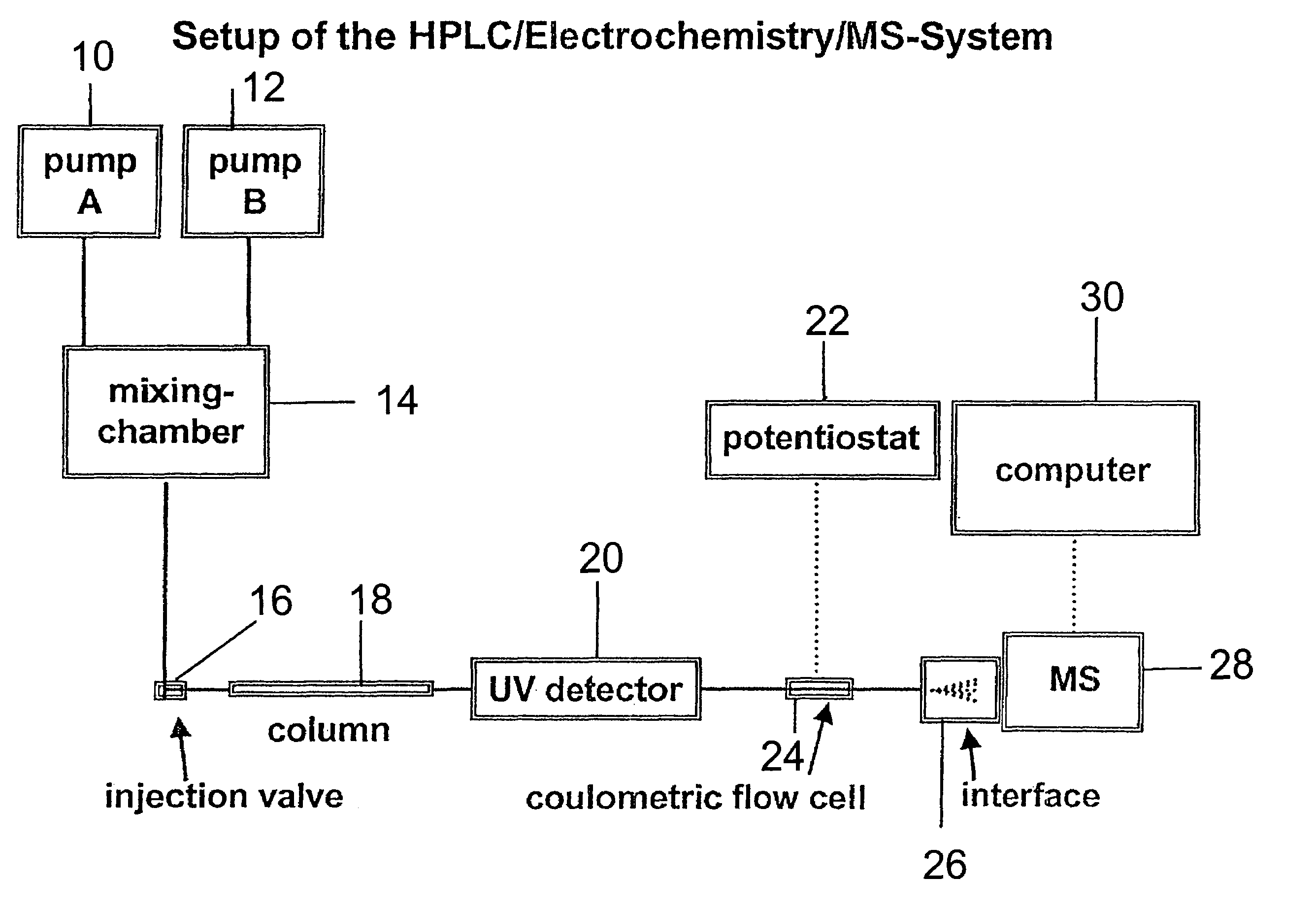
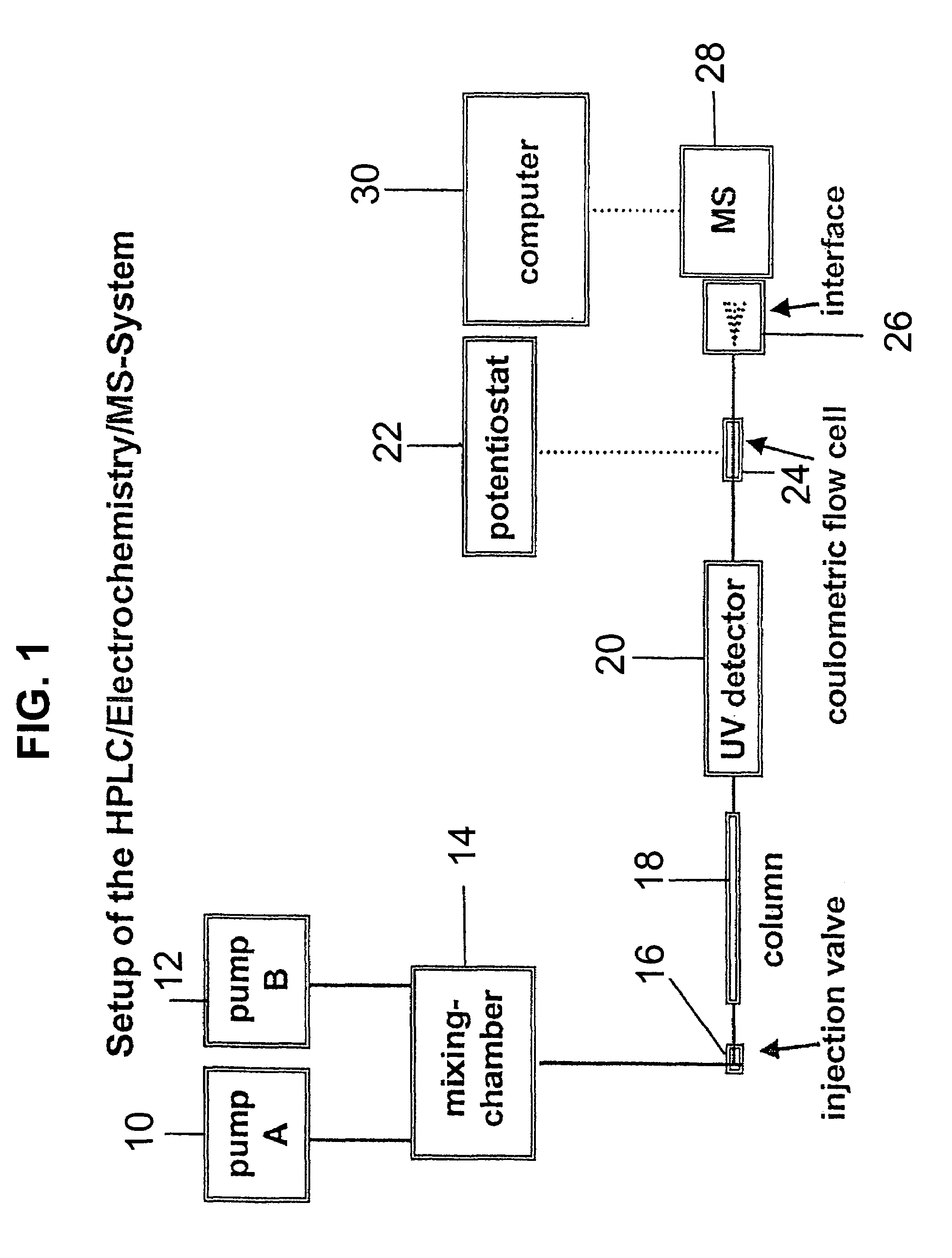
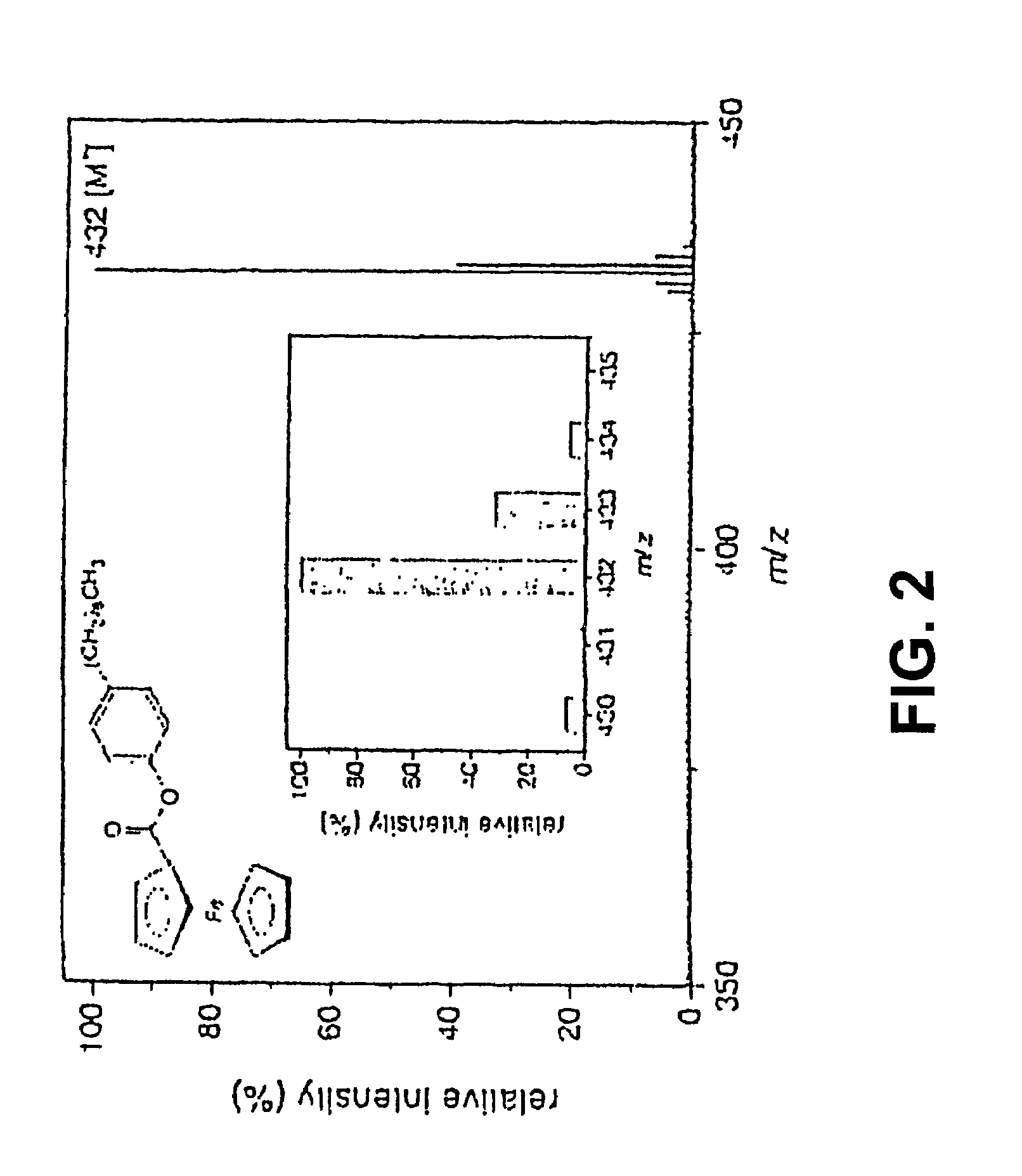
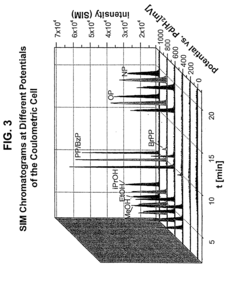
Coupling electrochemistry to mass spectrometry and high performance liquid chromatography
PatentInactiveUS7028537B2
Innovation
- A new HPLC-electrochemistry-MS technique is developed, where a coulometric three-electrode electrochemical cell is inserted between the HPLC column and mass spectrometer, enabling post-column electrochemical oxidation or reduction of analytes, forming charged or strongly polar products compatible with ESI or APCI mass spectrometry, thereby improving ionization efficiency.
Inspection method
PatentPendingUS20240360826A1
Innovation
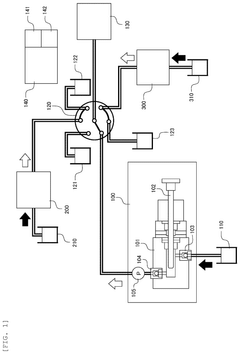
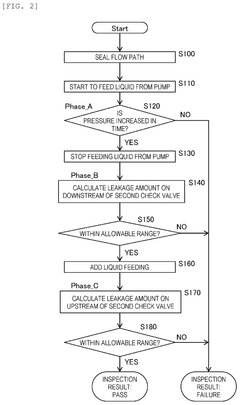
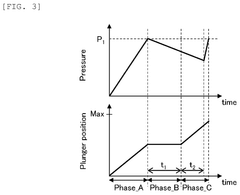
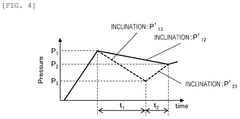
- A method for inspecting withstand pressure in liquid feeding devices that includes a single pressure sensor placed downstream of the second check valve, utilizing a first and second check valve to prevent backflow, and a plunger that reciprocates within a cylinder to suction and discharge fluid, allowing for the estimation of leakage based on pressure detection values.
Method Validation and Reproducibility Standards
Method validation in HPLC-MS chromatography represents a critical process ensuring analytical procedures consistently deliver reliable and accurate results. For gradient windows, peak shape, and carryover analysis, standardized validation protocols must adhere to regulatory frameworks established by organizations such as ICH, FDA, and USP. These frameworks typically require assessment of specificity, linearity, accuracy, precision, detection limit, quantitation limit, and robustness.
Reproducibility standards for gradient elution methods demand particular attention to system suitability parameters. Retention time variability should not exceed ±2% between runs, while peak area reproducibility typically requires relative standard deviation (RSD) values below 2% for major components. Peak shape metrics, including asymmetry factor (As) and tailing factor (Tf), must consistently remain within 0.9-1.5 range to ensure proper integration and quantification.
Carryover validation protocols necessitate blank injections following high-concentration samples, with acceptance criteria typically requiring carryover signals below 0.1% of the preceding sample's response. This becomes especially critical in bioanalytical applications where concentration differences between samples can span several orders of magnitude.
Inter-laboratory reproducibility standards have evolved significantly, with current best practices recommending collaborative trials involving at least six independent laboratories. Statistical evaluation using ANOVA or similar approaches helps establish reproducibility limits and identify sources of variability across different instrument platforms and operator expertise levels.
Method transfer protocols represent another crucial aspect of validation, particularly when methods developed on one HPLC-MS system must be implemented on different instruments. These protocols typically require side-by-side analysis of identical samples on both systems, with acceptance criteria for retention time shifts (≤5%), resolution changes (≤10%), and sensitivity differences (≤15%).
Lifecycle management of validated methods has gained increasing regulatory attention, with current standards emphasizing continuous verification rather than one-time validation. This approach requires periodic system suitability testing, control charting of critical method parameters, and formal revalidation when significant changes occur in instrument components, column lots, or mobile phase preparations.
Quality by Design (QbD) principles have been increasingly incorporated into method validation standards, establishing method operable design regions (MODR) that define the acceptable ranges for critical method parameters. This approach provides greater flexibility while maintaining method performance within predefined acceptance criteria for gradient window parameters, peak shape metrics, and carryover limits.
Reproducibility standards for gradient elution methods demand particular attention to system suitability parameters. Retention time variability should not exceed ±2% between runs, while peak area reproducibility typically requires relative standard deviation (RSD) values below 2% for major components. Peak shape metrics, including asymmetry factor (As) and tailing factor (Tf), must consistently remain within 0.9-1.5 range to ensure proper integration and quantification.
Carryover validation protocols necessitate blank injections following high-concentration samples, with acceptance criteria typically requiring carryover signals below 0.1% of the preceding sample's response. This becomes especially critical in bioanalytical applications where concentration differences between samples can span several orders of magnitude.
Inter-laboratory reproducibility standards have evolved significantly, with current best practices recommending collaborative trials involving at least six independent laboratories. Statistical evaluation using ANOVA or similar approaches helps establish reproducibility limits and identify sources of variability across different instrument platforms and operator expertise levels.
Method transfer protocols represent another crucial aspect of validation, particularly when methods developed on one HPLC-MS system must be implemented on different instruments. These protocols typically require side-by-side analysis of identical samples on both systems, with acceptance criteria for retention time shifts (≤5%), resolution changes (≤10%), and sensitivity differences (≤15%).
Lifecycle management of validated methods has gained increasing regulatory attention, with current standards emphasizing continuous verification rather than one-time validation. This approach requires periodic system suitability testing, control charting of critical method parameters, and formal revalidation when significant changes occur in instrument components, column lots, or mobile phase preparations.
Quality by Design (QbD) principles have been increasingly incorporated into method validation standards, establishing method operable design regions (MODR) that define the acceptable ranges for critical method parameters. This approach provides greater flexibility while maintaining method performance within predefined acceptance criteria for gradient window parameters, peak shape metrics, and carryover limits.
Environmental Impact and Green Chromatography Approaches
The environmental impact of HPLC-MS chromatography has become increasingly important as laboratories worldwide seek to minimize their ecological footprint. Traditional chromatographic methods often rely on large volumes of organic solvents that pose significant environmental hazards through their production, use, and disposal. In gradient HPLC-MS specifically, the continuous changing of mobile phase composition can result in substantial solvent consumption, especially during method development when multiple gradient windows are being optimized.
Recent advances in green chromatography have focused on reducing solvent usage through miniaturization of column dimensions. Narrower columns with smaller particle sizes maintain separation efficiency while dramatically decreasing mobile phase consumption. This approach directly addresses carryover issues by minimizing the volume of potentially contaminating solvents passing through the system.
Peak shape optimization in environmentally friendly HPLC-MS involves the development of stationary phases that perform effectively with greener mobile phases. Water-based mobile phases or those containing bio-derived solvents like ethanol from renewable sources represent significant improvements over traditional acetonitrile or methanol systems. These alternative solvents not only reduce environmental impact but can also enhance MS detection sensitivity in certain applications.
The concept of "solvent footprint" has emerged as a metric for evaluating chromatographic methods. This considers not only the volume of solvents used but also their environmental persistence, toxicity, and energy requirements for production. Gradient window optimization from an environmental perspective seeks to achieve the shortest possible run times with the minimum necessary solvent consumption while maintaining analytical performance.
Recycling technologies for HPLC solvents have advanced considerably, allowing laboratories to recover and purify used mobile phases. This circular approach significantly reduces waste generation and procurement costs. Additionally, modern HPLC-MS systems incorporate solvent-saving features such as automatic standby modes that reduce flow rates during idle periods and smart gradient algorithms that optimize solvent usage based on separation requirements.
Temperature-controlled chromatography offers another avenue for greener separations. Elevated temperature can decrease mobile phase viscosity, allowing for faster flow rates and shorter analysis times without compromising peak shape. This approach reduces overall solvent consumption and energy usage while potentially improving peak shape through enhanced mass transfer kinetics.
The pharmaceutical industry has been particularly proactive in adopting green chromatography approaches, driven by both environmental concerns and regulatory pressures. Quality by Design (QbD) principles now routinely incorporate environmental impact assessments when developing new analytical methods, considering carryover reduction and peak shape optimization within the context of sustainability.
Recent advances in green chromatography have focused on reducing solvent usage through miniaturization of column dimensions. Narrower columns with smaller particle sizes maintain separation efficiency while dramatically decreasing mobile phase consumption. This approach directly addresses carryover issues by minimizing the volume of potentially contaminating solvents passing through the system.
Peak shape optimization in environmentally friendly HPLC-MS involves the development of stationary phases that perform effectively with greener mobile phases. Water-based mobile phases or those containing bio-derived solvents like ethanol from renewable sources represent significant improvements over traditional acetonitrile or methanol systems. These alternative solvents not only reduce environmental impact but can also enhance MS detection sensitivity in certain applications.
The concept of "solvent footprint" has emerged as a metric for evaluating chromatographic methods. This considers not only the volume of solvents used but also their environmental persistence, toxicity, and energy requirements for production. Gradient window optimization from an environmental perspective seeks to achieve the shortest possible run times with the minimum necessary solvent consumption while maintaining analytical performance.
Recycling technologies for HPLC solvents have advanced considerably, allowing laboratories to recover and purify used mobile phases. This circular approach significantly reduces waste generation and procurement costs. Additionally, modern HPLC-MS systems incorporate solvent-saving features such as automatic standby modes that reduce flow rates during idle periods and smart gradient algorithms that optimize solvent usage based on separation requirements.
Temperature-controlled chromatography offers another avenue for greener separations. Elevated temperature can decrease mobile phase viscosity, allowing for faster flow rates and shorter analysis times without compromising peak shape. This approach reduces overall solvent consumption and energy usage while potentially improving peak shape through enhanced mass transfer kinetics.
The pharmaceutical industry has been particularly proactive in adopting green chromatography approaches, driven by both environmental concerns and regulatory pressures. Quality by Design (QbD) principles now routinely incorporate environmental impact assessments when developing new analytical methods, considering carryover reduction and peak shape optimization within the context of sustainability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!