HPLC-MS System Suitability: Calibration, QC And Acceptance
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS System Evolution and Objectives
High-Performance Liquid Chromatography coupled with Mass Spectrometry (HPLC-MS) has undergone significant evolution since its inception in the late 1970s. The integration of these two powerful analytical techniques revolutionized chemical analysis by combining the separation capabilities of HPLC with the identification power of mass spectrometry. Initially, interfaces between these systems posed significant technical challenges due to the incompatibility between liquid chromatography's high-pressure liquid environment and mass spectrometry's high-vacuum requirements.
The 1980s witnessed the development of the first commercially viable HPLC-MS interfaces, including thermospray and particle beam interfaces. However, the true breakthrough came in the 1990s with the introduction of atmospheric pressure ionization techniques, particularly electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), which effectively solved the interface challenges and dramatically improved sensitivity and applicability.
The early 2000s marked the emergence of high-resolution mass spectrometers and ultra-high-performance liquid chromatography (UHPLC), enabling faster analyses with improved resolution and sensitivity. This period also saw the development of more sophisticated system suitability protocols to ensure reliable and reproducible results across different laboratories and instruments.
In recent years, HPLC-MS systems have become increasingly automated and integrated, with advanced software solutions for data acquisition, processing, and reporting. Modern systems incorporate real-time monitoring of system performance parameters and automated calibration procedures, significantly reducing manual intervention and improving operational efficiency.
The primary objective of HPLC-MS system suitability protocols is to ensure that the analytical system consistently performs within predefined specifications, delivering accurate and reliable results. This involves comprehensive calibration procedures to establish the relationship between analyte concentration and detector response, quality control measures to monitor system performance, and acceptance criteria to validate analytical results.
Current technological trends are focused on enhancing system intelligence through machine learning algorithms for predictive maintenance and automated troubleshooting. Additionally, there is growing emphasis on developing standardized protocols for system suitability assessment across different instrument platforms and manufacturers, facilitating method transfer and cross-laboratory comparability.
The evolution trajectory points toward fully integrated analytical ecosystems with seamless connectivity to laboratory information management systems (LIMS) and electronic laboratory notebooks (ELN). Future developments will likely concentrate on miniaturization, reduced environmental impact, and increased accessibility through simplified user interfaces and remote operation capabilities, making advanced analytical capabilities more widely available across various industries and research settings.
The 1980s witnessed the development of the first commercially viable HPLC-MS interfaces, including thermospray and particle beam interfaces. However, the true breakthrough came in the 1990s with the introduction of atmospheric pressure ionization techniques, particularly electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), which effectively solved the interface challenges and dramatically improved sensitivity and applicability.
The early 2000s marked the emergence of high-resolution mass spectrometers and ultra-high-performance liquid chromatography (UHPLC), enabling faster analyses with improved resolution and sensitivity. This period also saw the development of more sophisticated system suitability protocols to ensure reliable and reproducible results across different laboratories and instruments.
In recent years, HPLC-MS systems have become increasingly automated and integrated, with advanced software solutions for data acquisition, processing, and reporting. Modern systems incorporate real-time monitoring of system performance parameters and automated calibration procedures, significantly reducing manual intervention and improving operational efficiency.
The primary objective of HPLC-MS system suitability protocols is to ensure that the analytical system consistently performs within predefined specifications, delivering accurate and reliable results. This involves comprehensive calibration procedures to establish the relationship between analyte concentration and detector response, quality control measures to monitor system performance, and acceptance criteria to validate analytical results.
Current technological trends are focused on enhancing system intelligence through machine learning algorithms for predictive maintenance and automated troubleshooting. Additionally, there is growing emphasis on developing standardized protocols for system suitability assessment across different instrument platforms and manufacturers, facilitating method transfer and cross-laboratory comparability.
The evolution trajectory points toward fully integrated analytical ecosystems with seamless connectivity to laboratory information management systems (LIMS) and electronic laboratory notebooks (ELN). Future developments will likely concentrate on miniaturization, reduced environmental impact, and increased accessibility through simplified user interfaces and remote operation capabilities, making advanced analytical capabilities more widely available across various industries and research settings.
Market Demand Analysis for HPLC-MS Quality Control
The global HPLC-MS quality control market has experienced significant growth in recent years, driven by increasing regulatory requirements and the expanding pharmaceutical and biotechnology sectors. The market size for analytical instrumentation in quality control applications reached approximately $5.7 billion in 2022, with HPLC-MS systems representing a substantial portion of this market.
Pharmaceutical companies constitute the largest end-user segment, accounting for nearly 45% of the total market demand. This dominance stems from stringent regulatory requirements for drug development and manufacturing processes, where system suitability testing is mandatory for ensuring data integrity and product quality. The implementation of quality-by-design approaches in pharmaceutical manufacturing has further accelerated the adoption of advanced HPLC-MS quality control solutions.
Clinical diagnostics represents the fastest-growing segment, with a compound annual growth rate of 8.3%. This growth is primarily attributed to the increasing use of LC-MS in clinical laboratories for therapeutic drug monitoring, toxicology screening, and biomarker analysis. Hospitals and reference laboratories are increasingly investing in HPLC-MS systems with robust quality control protocols to ensure accurate and reliable diagnostic results.
Food and environmental testing sectors collectively contribute about 25% to the overall market demand. These industries face mounting pressure to comply with safety regulations and quality standards, driving the need for sophisticated analytical techniques with proper system suitability protocols.
Regional analysis reveals North America as the dominant market, holding approximately 38% of the global share, followed by Europe (30%) and Asia-Pacific (24%). However, the Asia-Pacific region is projected to witness the highest growth rate due to expanding pharmaceutical manufacturing capabilities, particularly in China and India.
Market trends indicate a growing demand for automated system suitability testing solutions that reduce manual intervention and human error. Software platforms that integrate calibration, quality control, and acceptance criteria management are experiencing increased adoption rates across all end-user segments.
Customer surveys highlight that reliability and reproducibility of results are the primary concerns when selecting HPLC-MS quality control solutions. Approximately 78% of users prioritize robust system suitability features over initial acquisition costs, indicating a value-oriented market approach rather than price sensitivity.
Pharmaceutical companies constitute the largest end-user segment, accounting for nearly 45% of the total market demand. This dominance stems from stringent regulatory requirements for drug development and manufacturing processes, where system suitability testing is mandatory for ensuring data integrity and product quality. The implementation of quality-by-design approaches in pharmaceutical manufacturing has further accelerated the adoption of advanced HPLC-MS quality control solutions.
Clinical diagnostics represents the fastest-growing segment, with a compound annual growth rate of 8.3%. This growth is primarily attributed to the increasing use of LC-MS in clinical laboratories for therapeutic drug monitoring, toxicology screening, and biomarker analysis. Hospitals and reference laboratories are increasingly investing in HPLC-MS systems with robust quality control protocols to ensure accurate and reliable diagnostic results.
Food and environmental testing sectors collectively contribute about 25% to the overall market demand. These industries face mounting pressure to comply with safety regulations and quality standards, driving the need for sophisticated analytical techniques with proper system suitability protocols.
Regional analysis reveals North America as the dominant market, holding approximately 38% of the global share, followed by Europe (30%) and Asia-Pacific (24%). However, the Asia-Pacific region is projected to witness the highest growth rate due to expanding pharmaceutical manufacturing capabilities, particularly in China and India.
Market trends indicate a growing demand for automated system suitability testing solutions that reduce manual intervention and human error. Software platforms that integrate calibration, quality control, and acceptance criteria management are experiencing increased adoption rates across all end-user segments.
Customer surveys highlight that reliability and reproducibility of results are the primary concerns when selecting HPLC-MS quality control solutions. Approximately 78% of users prioritize robust system suitability features over initial acquisition costs, indicating a value-oriented market approach rather than price sensitivity.
Current Challenges in HPLC-MS System Suitability
Despite significant advancements in HPLC-MS technology, several critical challenges persist in system suitability testing that impact the reliability and reproducibility of analytical results. One of the primary challenges is the lack of standardized protocols across different laboratories and industries. While regulatory bodies provide general guidelines, specific implementation varies considerably, leading to inconsistencies in system performance evaluation and acceptance criteria.
Instrument drift represents another significant challenge, particularly in high-throughput environments. Mass spectrometers are susceptible to sensitivity changes over time due to contamination of ion sources, deterioration of detector components, and variations in vacuum conditions. These gradual changes can be difficult to detect through routine system suitability tests but may significantly impact quantitative results.
Matrix effects continue to pose substantial challenges in complex sample analysis. Biological matrices, environmental samples, and food products contain numerous compounds that can suppress or enhance ionization, affecting the accuracy of quantification. Current system suitability tests often fail to adequately account for these matrix effects, particularly when calibration standards are prepared in solvents rather than matched matrices.
Carryover between samples remains problematic, especially for compounds with high retention on column stationary phases or instrument components. Traditional system suitability tests may not detect subtle carryover effects that become significant during analysis of samples with large concentration differences, leading to false positive results or inaccurate quantification.
Data processing algorithms present another layer of complexity. Different vendor software packages employ varied approaches to peak integration, calibration curve fitting, and signal processing. These differences can lead to inconsistent results even when the raw data quality is comparable, making cross-laboratory comparisons challenging.
The increasing complexity of multi-component analyses further complicates system suitability testing. Modern methods often target dozens or hundreds of analytes simultaneously, making it impractical to monitor system performance for each compound. Selecting representative compounds for system suitability testing requires careful consideration but remains somewhat subjective.
Emerging technologies like ultra-high-pressure liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry introduce additional challenges, including faster run times that demand more frequent system suitability checks and more complex data interpretation requirements. The traditional paradigms for system suitability testing may not be optimal for these advanced analytical platforms.
Instrument drift represents another significant challenge, particularly in high-throughput environments. Mass spectrometers are susceptible to sensitivity changes over time due to contamination of ion sources, deterioration of detector components, and variations in vacuum conditions. These gradual changes can be difficult to detect through routine system suitability tests but may significantly impact quantitative results.
Matrix effects continue to pose substantial challenges in complex sample analysis. Biological matrices, environmental samples, and food products contain numerous compounds that can suppress or enhance ionization, affecting the accuracy of quantification. Current system suitability tests often fail to adequately account for these matrix effects, particularly when calibration standards are prepared in solvents rather than matched matrices.
Carryover between samples remains problematic, especially for compounds with high retention on column stationary phases or instrument components. Traditional system suitability tests may not detect subtle carryover effects that become significant during analysis of samples with large concentration differences, leading to false positive results or inaccurate quantification.
Data processing algorithms present another layer of complexity. Different vendor software packages employ varied approaches to peak integration, calibration curve fitting, and signal processing. These differences can lead to inconsistent results even when the raw data quality is comparable, making cross-laboratory comparisons challenging.
The increasing complexity of multi-component analyses further complicates system suitability testing. Modern methods often target dozens or hundreds of analytes simultaneously, making it impractical to monitor system performance for each compound. Selecting representative compounds for system suitability testing requires careful consideration but remains somewhat subjective.
Emerging technologies like ultra-high-pressure liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry introduce additional challenges, including faster run times that demand more frequent system suitability checks and more complex data interpretation requirements. The traditional paradigms for system suitability testing may not be optimal for these advanced analytical platforms.
Established Protocols for System Suitability Testing
01 System suitability parameters for HPLC-MS analysis
System suitability tests are essential for ensuring the reliability and accuracy of HPLC-MS analytical methods. These parameters include retention time, peak resolution, tailing factor, theoretical plate number, and signal-to-noise ratio. Regular assessment of these parameters helps to verify that the chromatographic system is performing adequately before sample analysis, ensuring consistent and reliable results.- System suitability parameters for HPLC-MS analysis: System suitability tests are essential for ensuring the reliability and accuracy of HPLC-MS analytical methods. Key parameters include retention time, peak resolution, tailing factor, theoretical plate count, and signal-to-noise ratio. These parameters help verify that the chromatographic system is performing adequately before sample analysis, ensuring consistent and reproducible results across different analytical runs.
- Calibration and validation procedures for HPLC-MS systems: Proper calibration and validation of HPLC-MS systems are critical for accurate analytical results. This includes establishing calibration curves with reference standards, determining limits of detection and quantification, assessing linearity ranges, and validating method specificity. Regular calibration checks and performance qualification tests ensure that the system maintains its analytical capabilities over time and complies with regulatory requirements.
- Automated system suitability testing solutions: Automated solutions for HPLC-MS system suitability testing streamline the analytical workflow and reduce human error. These systems can automatically inject test samples, monitor critical parameters, evaluate results against predefined acceptance criteria, and generate compliance reports. Software algorithms can detect trends in system performance, enabling predictive maintenance and minimizing unexpected downtime during analytical runs.
- Method development for specific analytes and matrices: Developing optimized HPLC-MS methods for specific analytes and sample matrices requires careful consideration of system suitability parameters. This includes selecting appropriate mobile phases, column chemistry, gradient profiles, and MS detection parameters. Method development focuses on achieving adequate separation of target analytes from matrix interferences while maintaining sensitivity, specificity, and reproducibility across different sample types.
- Troubleshooting and maintenance protocols: Effective troubleshooting and maintenance protocols are essential for maintaining HPLC-MS system suitability. This includes regular preventive maintenance schedules, cleaning procedures for MS ion sources, column conditioning protocols, and systematic approaches for diagnosing common issues like peak tailing, retention time shifts, or sensitivity loss. Proper documentation of maintenance activities and system performance helps ensure data integrity and regulatory compliance.
02 Calibration and standardization procedures for HPLC-MS systems
Proper calibration and standardization are critical for maintaining HPLC-MS system suitability. This involves the use of reference standards, calibration curves, and internal standards to ensure accurate quantification. Regular calibration checks help to detect instrument drift and ensure that the system meets predetermined acceptance criteria, thereby maintaining the validity of analytical results.Expand Specific Solutions03 Automated system suitability testing for HPLC-MS
Automated system suitability testing solutions have been developed to streamline the verification process for HPLC-MS systems. These automated approaches include software algorithms that can evaluate system performance parameters, generate reports, and flag deviations from acceptance criteria. Automation reduces human error, increases efficiency, and ensures consistent application of suitability criteria across multiple analyses.Expand Specific Solutions04 Method development for specific analytes using HPLC-MS
Developing specific HPLC-MS methods for different analytes requires careful optimization of system suitability parameters. This includes selection of appropriate mobile phases, column types, ionization parameters, and detection settings. Method development focuses on achieving optimal separation, sensitivity, and specificity for target compounds while ensuring that system suitability criteria are consistently met.Expand Specific Solutions05 Troubleshooting and maintenance for HPLC-MS system performance
Regular maintenance and troubleshooting procedures are essential for maintaining HPLC-MS system suitability. This includes preventive maintenance schedules, component cleaning protocols, leak detection, and systematic approaches to diagnosing and resolving performance issues. Proper maintenance helps to prevent system failures, extend instrument lifetime, and ensure consistent analytical performance.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The HPLC-MS system suitability market is currently in a mature growth phase, with an estimated global market size exceeding $5 billion and steady annual growth of 5-7%. Leading the competitive landscape is Agilent Technologies, which dominates with comprehensive calibration and QC solutions, followed closely by Waters Technology and Thermo Fisher Scientific. Other significant players include Shimadzu, DH Technologies, and F. Hoffmann-La Roche, each offering specialized system suitability packages. The technology has reached high maturity with standardized protocols, though innovation continues in automation and AI-driven calibration. Pharmaceutical companies like Janssen Pharmaceutica are increasingly partnering with equipment manufacturers to develop industry-specific acceptance criteria, while academic institutions contribute to advancing methodology refinement.
Agilent Technologies, Inc.
Technical Solution: Agilent's HPLC-MS system suitability solution incorporates automated calibration workflows with intelligent diagnostic tools that continuously monitor critical system parameters. Their OpenLab CDS software platform integrates system suitability testing (SST) protocols that automatically verify instrument performance against predefined acceptance criteria before sample analysis begins. The platform features real-time monitoring of retention time stability, peak area precision, and mass accuracy with automated alerts when parameters drift outside acceptable ranges. Agilent's calibration technology employs reference standards with certified purity and concentration to ensure traceability to international standards. Their LC/MSD systems incorporate automated performance verification tests that evaluate sensitivity, linearity, and mass accuracy using proprietary algorithms to detect early signs of performance degradation.
Strengths: Industry-leading integration between hardware and software components provides seamless calibration workflows; extensive validation documentation supports regulatory compliance; predictive maintenance algorithms reduce unplanned downtime. Weaknesses: Higher initial investment compared to some competitors; proprietary calibration standards may increase operational costs; complex systems require specialized training for optimal utilization.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche's HPLC-MS system suitability technology focuses on biopharmaceutical applications with their cobas® analytical platforms. Their approach implements automated calibration verification protocols that assess critical performance parameters including retention time stability, peak area precision, and mass accuracy for complex biological molecules. Roche's system incorporates reference standard mixtures specifically formulated to challenge system performance across multiple protein and peptide analytes. Their calibration methodology includes automated procedures for verifying detector response linearity across the analytical range with statistical evaluation against predefined acceptance criteria based on regulatory guidelines. Roche's quality control software automatically generates comprehensive system suitability reports documenting all performance metrics with pass/fail indicators and trend analysis capabilities. Their technology includes intelligent diagnostic algorithms that track system performance over time, enabling predictive maintenance before critical failures occur.
Strengths: Specialized expertise in biopharmaceutical applications; comprehensive validation documentation supports regulatory compliance; advanced trend analysis capabilities for predictive maintenance. Weaknesses: Solutions primarily optimized for biopharmaceutical applications may have limitations in other fields; proprietary calibration standards increase operational costs; complex systems require specialized training.
Critical Parameters and Acceptance Criteria Analysis
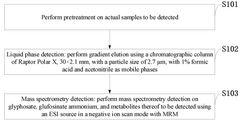
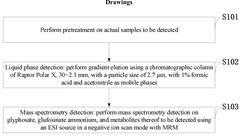
HPLC-ms/ms method for determining glyphosate, glufosinate ammonium, and metabolites thereof
PatentActiveZA202402841B
Innovation
- Simplified sample preparation method combining frozen fruits/vegetables with dry ice grinding, reducing the need for complex extraction procedures while maintaining effective analyte recovery.
- Use of methanol with 1.0% formic acid as extraction solvent, which effectively solubilizes target compounds while providing appropriate pH conditions for subsequent MS/MS analysis.
- Direct filtration approach (0.22 micron) after centrifugation that eliminates multiple clean-up steps typically required for complex matrices like fruits and vegetables.
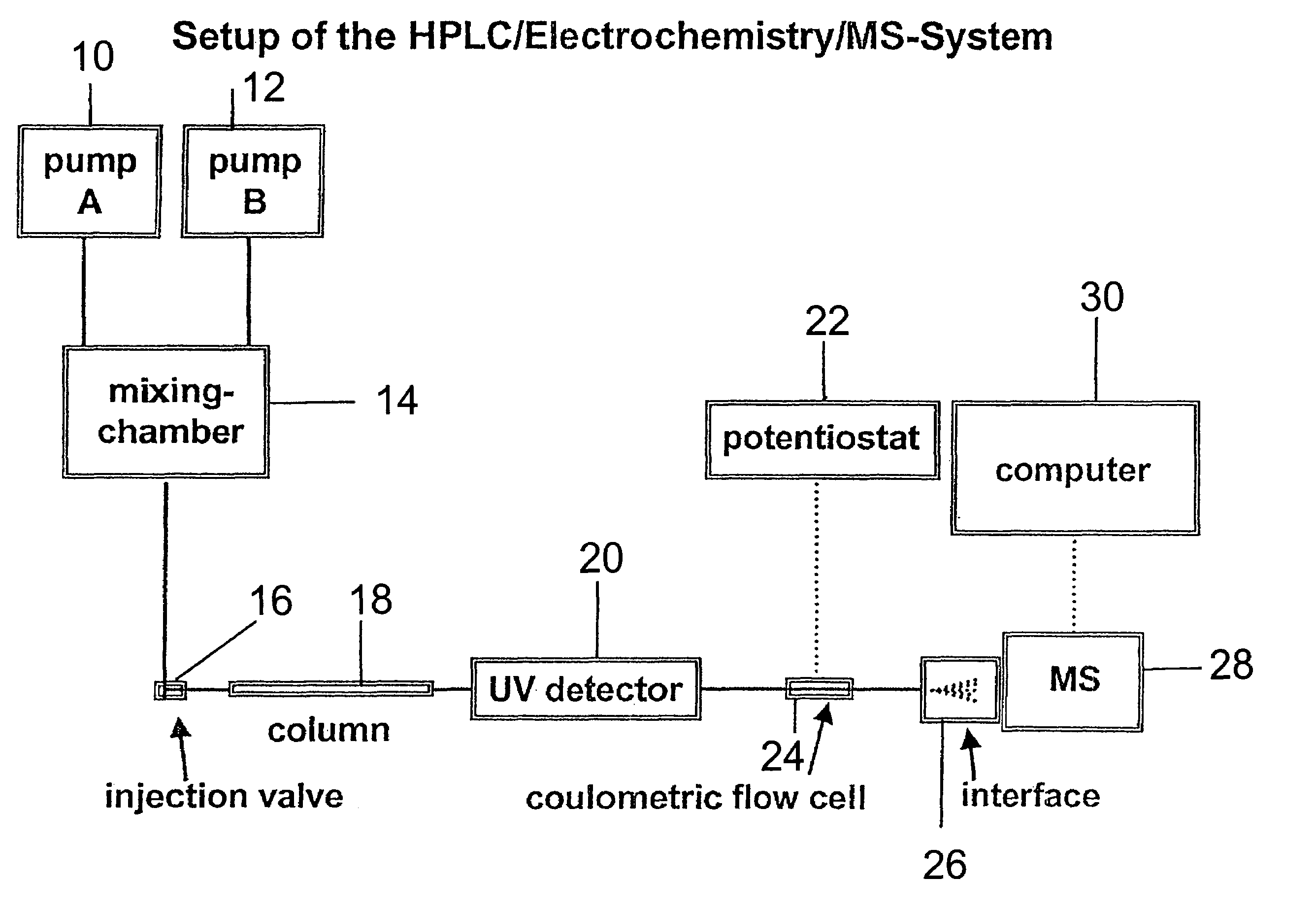
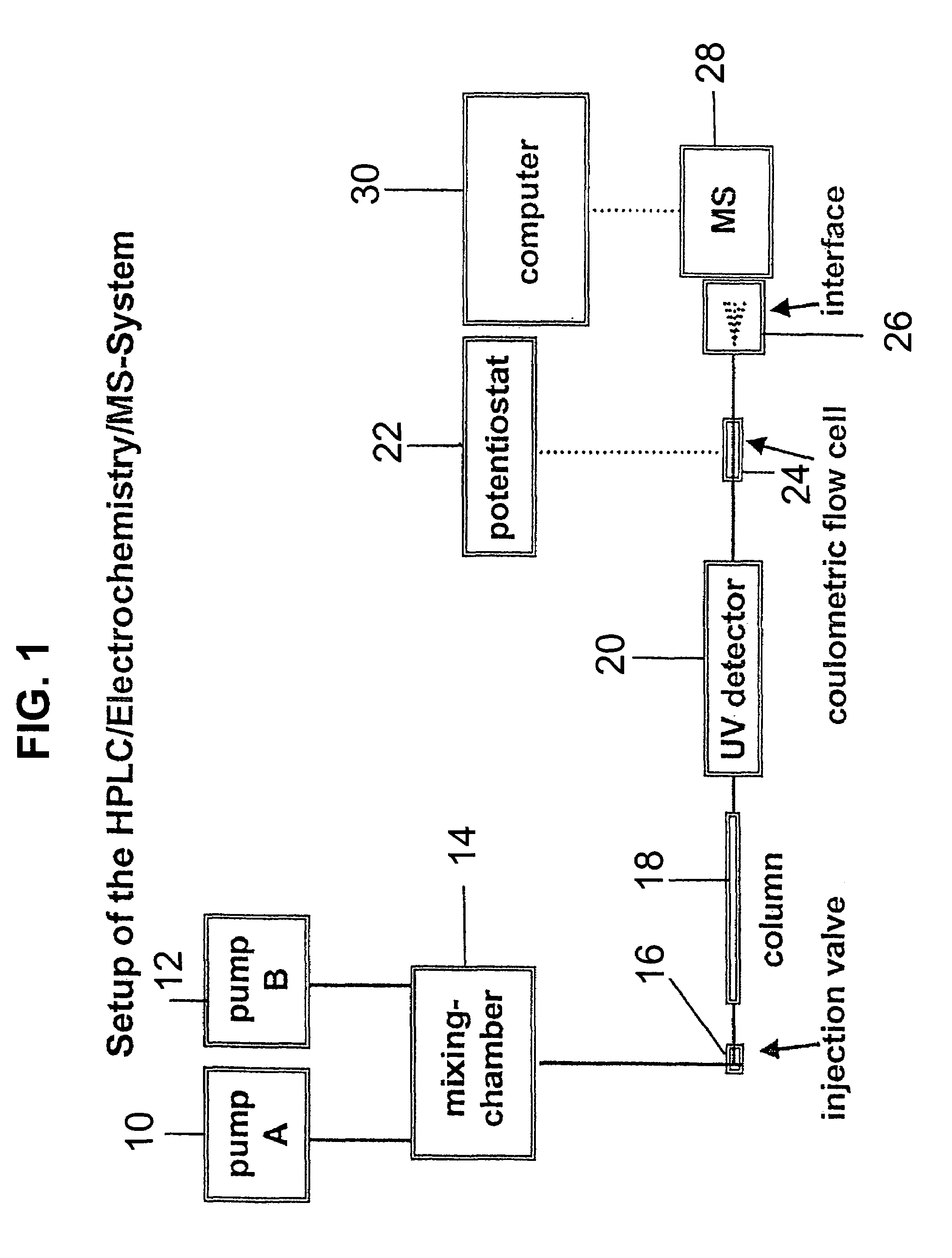
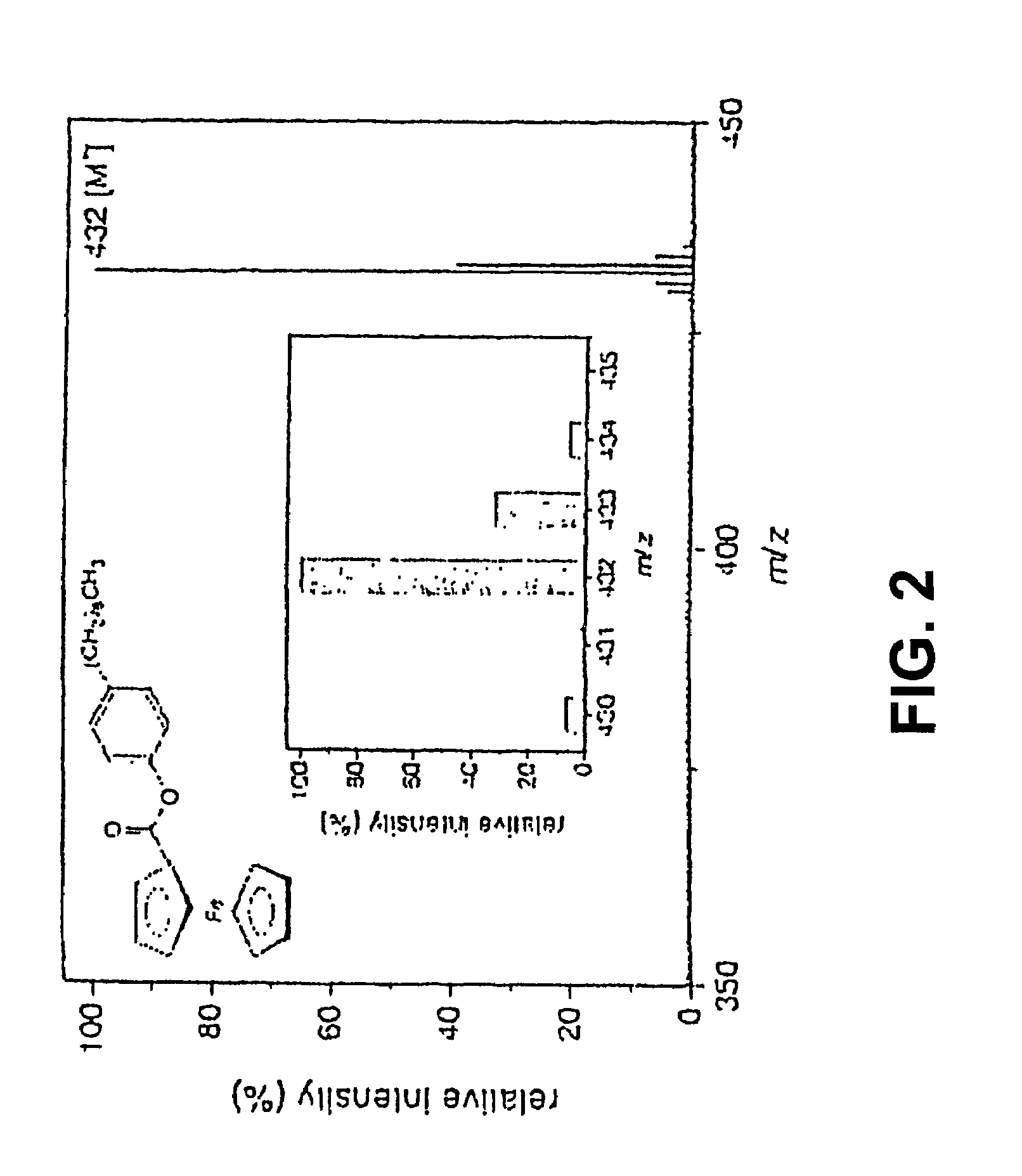
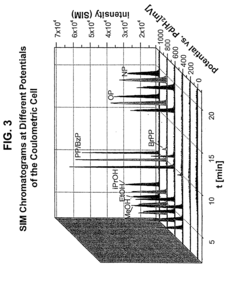
Coupling electrochemistry to mass spectrometry and high performance liquid chromatography
PatentInactiveUS7028537B2
Innovation
- A new HPLC-electrochemistry-MS technique is developed, where a coulometric three-electrode electrochemical cell is inserted between the HPLC column and mass spectrometer, enabling post-column electrochemical oxidation or reduction of analytes, forming charged or strongly polar products compatible with ESI or APCI mass spectrometry, thereby improving ionization efficiency.
Regulatory Compliance Requirements
Regulatory compliance for HPLC-MS system suitability testing is governed by several international standards and guidelines that pharmaceutical and analytical laboratories must adhere to. The primary regulatory frameworks include the FDA's Code of Federal Regulations (21 CFR Part 11 and Part 211), the European Medicines Agency (EMA) guidelines, ICH (International Council for Harmonisation) guidelines particularly Q2(R1) for validation of analytical procedures, and USP (United States Pharmacopeia) chapters <621>, <1058>, and <1065> which specifically address chromatographic methods and mass spectrometry.
These regulations mandate comprehensive documentation of system suitability tests (SST) before sample analysis to ensure the analytical system's performance meets predefined acceptance criteria. For HPLC-MS systems, this includes verification of retention time reproducibility, peak resolution, mass accuracy, ion intensity stability, and signal-to-noise ratios. The FDA requires that these parameters be established during method validation and routinely verified before sample analysis.
Compliance with 21 CFR Part 11 is particularly critical for HPLC-MS systems as it governs electronic records and signatures. This regulation requires implementation of controls including system access restrictions, audit trails, and data integrity measures to prevent unauthorized manipulation of analytical data. Laboratories must maintain complete records of all calibration activities, quality control results, and system suitability tests.
The EMA's guidelines emphasize the importance of establishing appropriate acceptance criteria for system suitability parameters based on method validation data. These criteria must be scientifically justified and documented in standard operating procedures (SOPs). Additionally, the ICH Q2(R1) guideline provides a framework for validating analytical methods, including specificity, accuracy, precision, and linearity, which directly impact system suitability requirements.
ISO/IEC 17025, the international standard for testing and calibration laboratories, requires traceability of measurements to SI units through an unbroken chain of calibrations. For HPLC-MS systems, this means calibration standards must be traceable to certified reference materials, and all measuring equipment must be calibrated using standards with appropriate uncertainty levels.
Regulatory bodies also require laboratories to establish procedures for handling out-of-specification results during system suitability testing. These procedures must include investigation protocols, corrective action plans, and documentation requirements. Failure to meet system suitability criteria necessitates investigation and resolution before proceeding with sample analysis.
These regulations mandate comprehensive documentation of system suitability tests (SST) before sample analysis to ensure the analytical system's performance meets predefined acceptance criteria. For HPLC-MS systems, this includes verification of retention time reproducibility, peak resolution, mass accuracy, ion intensity stability, and signal-to-noise ratios. The FDA requires that these parameters be established during method validation and routinely verified before sample analysis.
Compliance with 21 CFR Part 11 is particularly critical for HPLC-MS systems as it governs electronic records and signatures. This regulation requires implementation of controls including system access restrictions, audit trails, and data integrity measures to prevent unauthorized manipulation of analytical data. Laboratories must maintain complete records of all calibration activities, quality control results, and system suitability tests.
The EMA's guidelines emphasize the importance of establishing appropriate acceptance criteria for system suitability parameters based on method validation data. These criteria must be scientifically justified and documented in standard operating procedures (SOPs). Additionally, the ICH Q2(R1) guideline provides a framework for validating analytical methods, including specificity, accuracy, precision, and linearity, which directly impact system suitability requirements.
ISO/IEC 17025, the international standard for testing and calibration laboratories, requires traceability of measurements to SI units through an unbroken chain of calibrations. For HPLC-MS systems, this means calibration standards must be traceable to certified reference materials, and all measuring equipment must be calibrated using standards with appropriate uncertainty levels.
Regulatory bodies also require laboratories to establish procedures for handling out-of-specification results during system suitability testing. These procedures must include investigation protocols, corrective action plans, and documentation requirements. Failure to meet system suitability criteria necessitates investigation and resolution before proceeding with sample analysis.
Method Validation Strategies
Method validation is a critical component of HPLC-MS system suitability protocols, ensuring analytical methods consistently produce reliable and accurate results. Comprehensive validation strategies must address multiple parameters including specificity, linearity, accuracy, precision, detection limits, quantitation limits, and robustness.
The validation process typically begins with a detailed validation plan that outlines specific acceptance criteria for each parameter. These criteria should be established based on intended use of the method and regulatory requirements applicable to the specific industry, such as ICH guidelines for pharmaceuticals or FDA requirements for clinical diagnostics.
Specificity validation ensures the method can accurately identify and quantify the analyte in the presence of other components. This involves analyzing samples containing known interferences and demonstrating acceptable resolution between the analyte and potential interferents. For HPLC-MS methods, this includes evaluation of matrix effects and potential ion suppression.
Linearity assessment requires analyzing a minimum of five concentration levels spanning 80-120% of the expected working range. The resulting calibration curve should demonstrate a correlation coefficient (r²) exceeding 0.99 for most applications, with residuals randomly distributed around zero.
Accuracy validation involves analyzing samples with known concentrations and calculating the percent recovery. Typically, three concentration levels with multiple replicates are tested, with acceptance criteria generally set at 98-102% recovery for pharmaceutical applications and potentially wider ranges for complex biological matrices.
Precision validation encompasses repeatability (intra-day), intermediate precision (inter-day), and reproducibility (inter-laboratory) assessments. For HPLC-MS methods, relative standard deviation (RSD) values should typically be below 2% for repeatability and below 3% for intermediate precision in pharmaceutical applications.
Robustness testing evaluates method performance under deliberately varied conditions, including changes in mobile phase composition, column temperature, flow rate, and MS parameters. This identifies critical method parameters requiring tight control during routine analysis.
System suitability tests (SSTs) should be incorporated into the validation strategy, establishing criteria for ongoing performance verification. These typically include retention time stability, peak resolution, tailing factor, and signal-to-noise ratio measurements that must be met before sample analysis.
The validation process typically begins with a detailed validation plan that outlines specific acceptance criteria for each parameter. These criteria should be established based on intended use of the method and regulatory requirements applicable to the specific industry, such as ICH guidelines for pharmaceuticals or FDA requirements for clinical diagnostics.
Specificity validation ensures the method can accurately identify and quantify the analyte in the presence of other components. This involves analyzing samples containing known interferences and demonstrating acceptable resolution between the analyte and potential interferents. For HPLC-MS methods, this includes evaluation of matrix effects and potential ion suppression.
Linearity assessment requires analyzing a minimum of five concentration levels spanning 80-120% of the expected working range. The resulting calibration curve should demonstrate a correlation coefficient (r²) exceeding 0.99 for most applications, with residuals randomly distributed around zero.
Accuracy validation involves analyzing samples with known concentrations and calculating the percent recovery. Typically, three concentration levels with multiple replicates are tested, with acceptance criteria generally set at 98-102% recovery for pharmaceutical applications and potentially wider ranges for complex biological matrices.
Precision validation encompasses repeatability (intra-day), intermediate precision (inter-day), and reproducibility (inter-laboratory) assessments. For HPLC-MS methods, relative standard deviation (RSD) values should typically be below 2% for repeatability and below 3% for intermediate precision in pharmaceutical applications.
Robustness testing evaluates method performance under deliberately varied conditions, including changes in mobile phase composition, column temperature, flow rate, and MS parameters. This identifies critical method parameters requiring tight control during routine analysis.
System suitability tests (SSTs) should be incorporated into the validation strategy, establishing criteria for ongoing performance verification. These typically include retention time stability, peak resolution, tailing factor, and signal-to-noise ratio measurements that must be met before sample analysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!