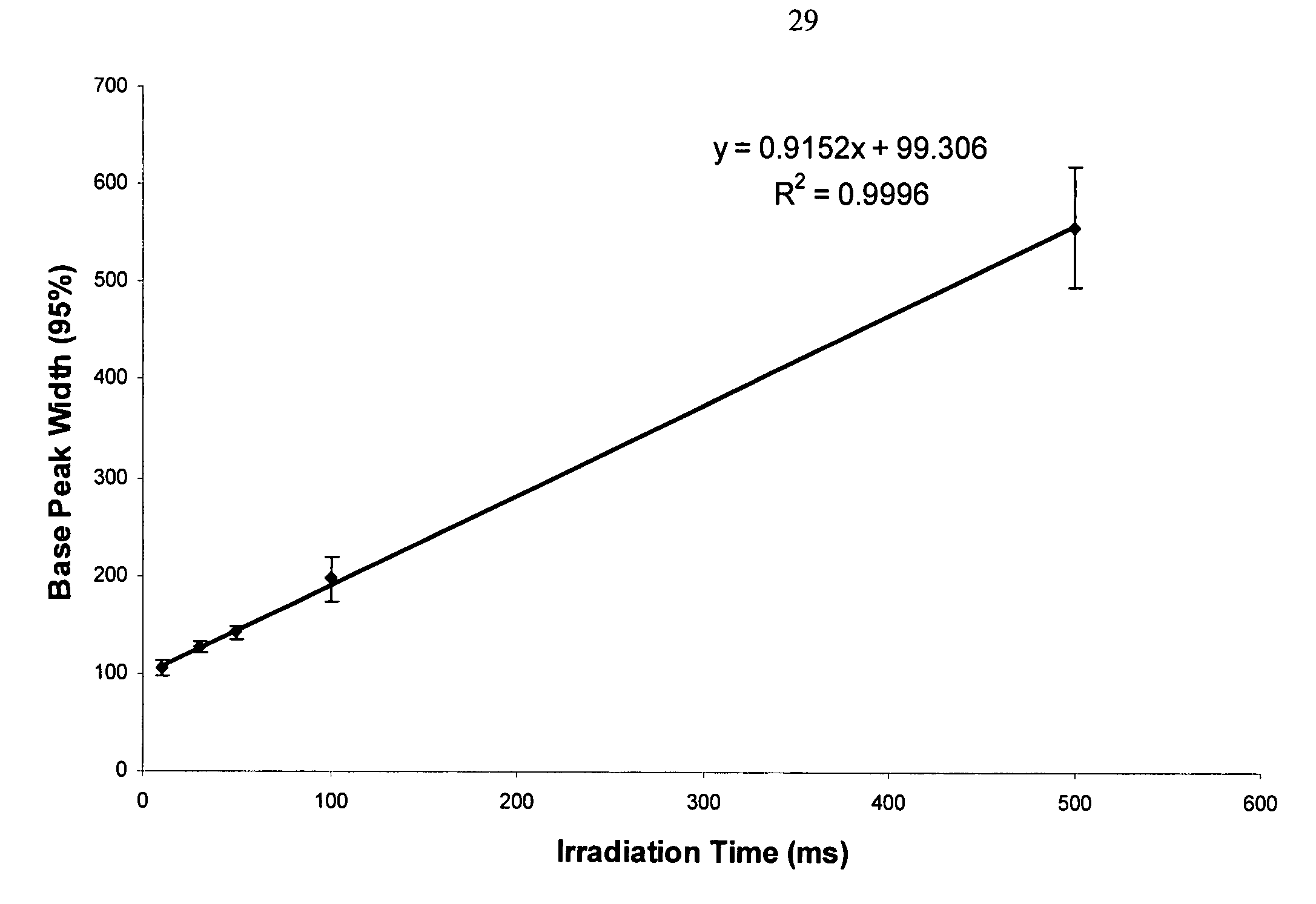
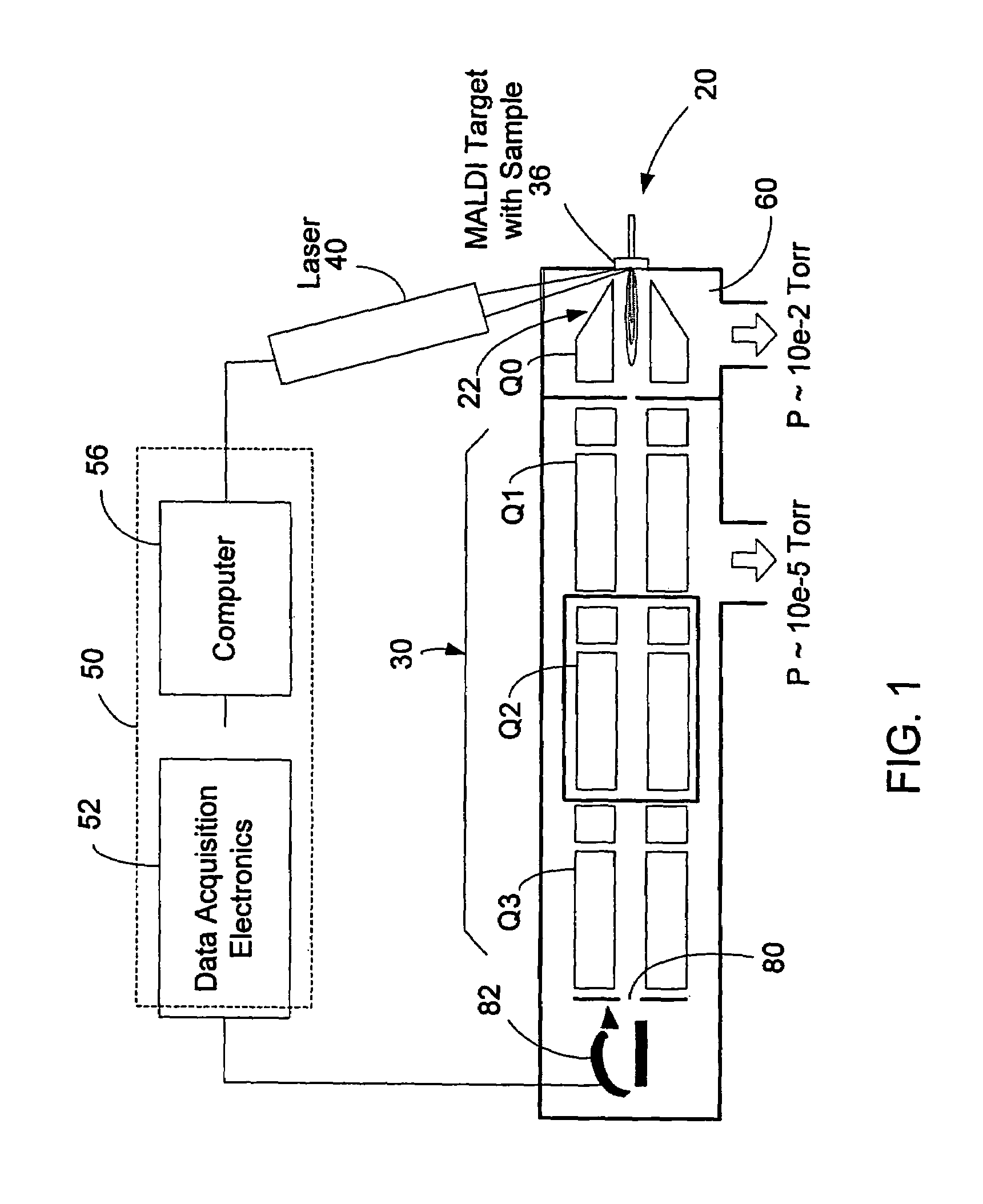
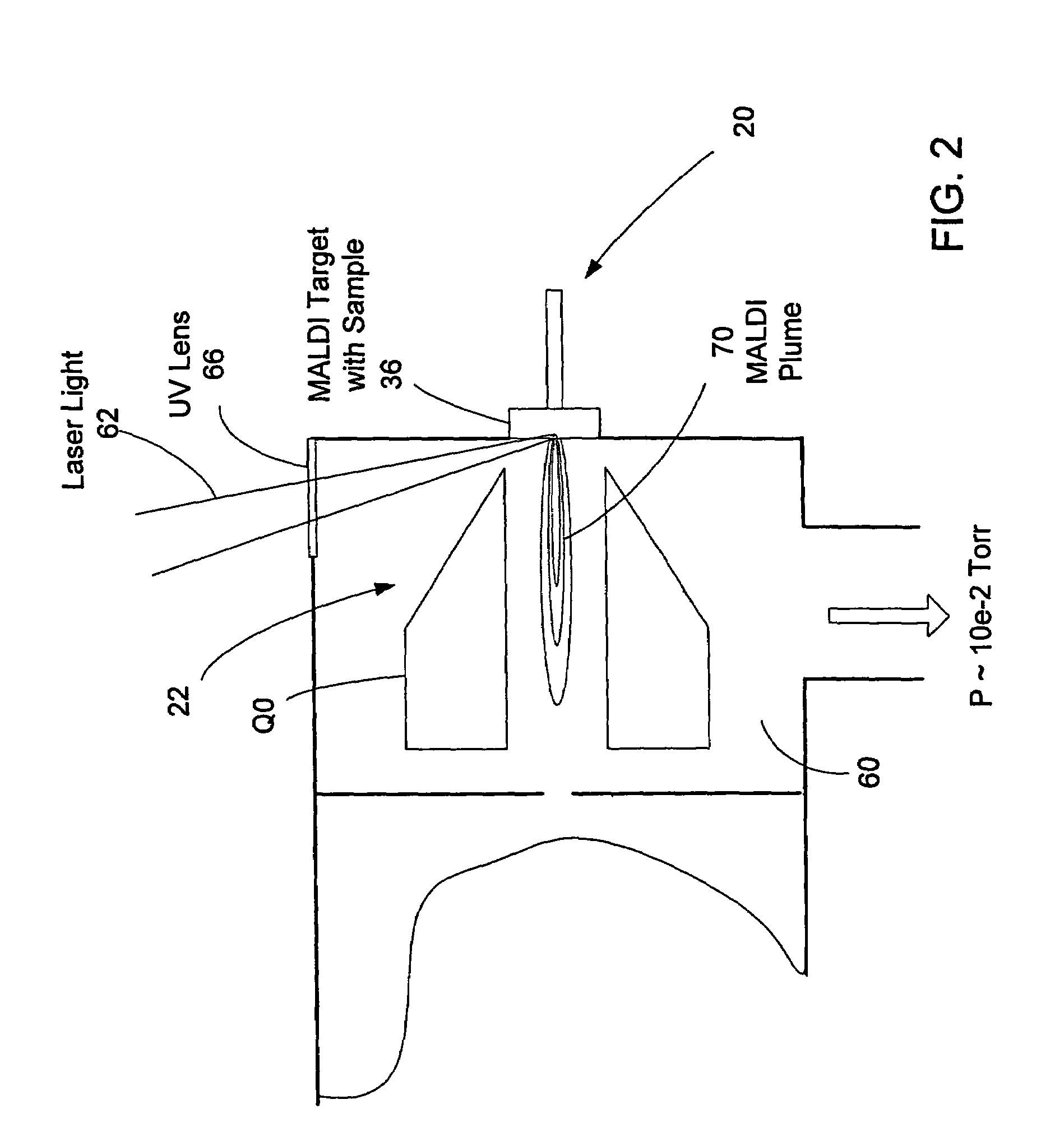
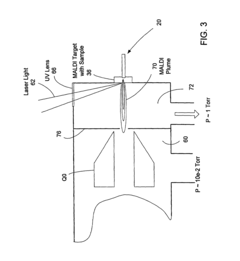
HPLC-MS Quantitation: MRM Transitions, Dwell/Schedule And LOQ
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Quantitation Background and Objectives
High-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) has evolved significantly over the past three decades, transforming from a specialized analytical technique to an essential tool across pharmaceutical, clinical, environmental, and food safety sectors. The integration of these two powerful analytical methods combines the separation capabilities of HPLC with the detection specificity and sensitivity of mass spectrometry, enabling precise quantitation of compounds in complex matrices.
The development trajectory of HPLC-MS quantitation has been marked by continuous technological advancements, particularly in the areas of ionization techniques, mass analyzers, and data processing algorithms. Early systems in the 1990s faced significant limitations in sensitivity, reproducibility, and throughput. However, the introduction of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) techniques revolutionized the field by enabling efficient ionization of a broader range of compounds.
Multiple Reaction Monitoring (MRM) emerged as a breakthrough approach in the early 2000s, allowing for highly selective and sensitive quantitation by monitoring specific precursor-to-product ion transitions. This technique has become the gold standard for targeted quantitative analysis, particularly in regulated environments such as pharmaceutical development and clinical diagnostics.
The optimization of MRM transitions, dwell times, and scheduling parameters represents a critical aspect of method development that directly impacts analytical performance. Properly selected transitions ensure specificity, while optimized dwell times and scheduling maximize sensitivity without compromising chromatographic resolution. These parameters collectively determine the limit of quantitation (LOQ), which is crucial for applications requiring detection of compounds at trace levels.
Current technological trends point toward increased multiplexing capabilities, allowing simultaneous quantitation of hundreds of analytes in a single run. This is facilitated by advances in fast-scanning mass analyzers and sophisticated scheduling algorithms that optimize instrument duty cycles. Additionally, the integration of artificial intelligence and machine learning approaches is beginning to transform method development and data analysis workflows.
The primary objective of this technical research report is to comprehensively evaluate the current state of HPLC-MS quantitation methodologies, with specific focus on optimizing MRM transitions, dwell time parameters, scheduling strategies, and their collective impact on achieving lower limits of quantitation. We aim to identify technological bottlenecks, emerging solutions, and potential innovation pathways that could advance quantitative capabilities beyond current limitations.
Furthermore, this report seeks to establish a framework for systematic method development that balances analytical performance with practical considerations such as throughput, robustness, and transferability across different instrument platforms. This holistic approach will provide valuable insights for both immediate implementation and long-term strategic planning in analytical laboratories across various industries.
The development trajectory of HPLC-MS quantitation has been marked by continuous technological advancements, particularly in the areas of ionization techniques, mass analyzers, and data processing algorithms. Early systems in the 1990s faced significant limitations in sensitivity, reproducibility, and throughput. However, the introduction of electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) techniques revolutionized the field by enabling efficient ionization of a broader range of compounds.
Multiple Reaction Monitoring (MRM) emerged as a breakthrough approach in the early 2000s, allowing for highly selective and sensitive quantitation by monitoring specific precursor-to-product ion transitions. This technique has become the gold standard for targeted quantitative analysis, particularly in regulated environments such as pharmaceutical development and clinical diagnostics.
The optimization of MRM transitions, dwell times, and scheduling parameters represents a critical aspect of method development that directly impacts analytical performance. Properly selected transitions ensure specificity, while optimized dwell times and scheduling maximize sensitivity without compromising chromatographic resolution. These parameters collectively determine the limit of quantitation (LOQ), which is crucial for applications requiring detection of compounds at trace levels.
Current technological trends point toward increased multiplexing capabilities, allowing simultaneous quantitation of hundreds of analytes in a single run. This is facilitated by advances in fast-scanning mass analyzers and sophisticated scheduling algorithms that optimize instrument duty cycles. Additionally, the integration of artificial intelligence and machine learning approaches is beginning to transform method development and data analysis workflows.
The primary objective of this technical research report is to comprehensively evaluate the current state of HPLC-MS quantitation methodologies, with specific focus on optimizing MRM transitions, dwell time parameters, scheduling strategies, and their collective impact on achieving lower limits of quantitation. We aim to identify technological bottlenecks, emerging solutions, and potential innovation pathways that could advance quantitative capabilities beyond current limitations.
Furthermore, this report seeks to establish a framework for systematic method development that balances analytical performance with practical considerations such as throughput, robustness, and transferability across different instrument platforms. This holistic approach will provide valuable insights for both immediate implementation and long-term strategic planning in analytical laboratories across various industries.
Market Demand Analysis for HPLC-MS Quantitation Methods
The global market for HPLC-MS quantitation methods continues to experience robust growth, driven primarily by increasing demand in pharmaceutical research, clinical diagnostics, food safety testing, and environmental monitoring. The market size for liquid chromatography-mass spectrometry equipment reached approximately $4.8 billion in 2022, with projections indicating a compound annual growth rate of 7.2% through 2028.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 45% of the total demand. This is largely attributed to the critical role of HPLC-MS quantitation in drug discovery, development, and quality control processes. The need for precise MRM (Multiple Reaction Monitoring) transitions and lower limits of quantitation (LOQ) has become particularly acute as regulatory requirements for bioanalytical methods continue to tighten globally.
Clinical diagnostics represents the fastest-growing segment, with an estimated growth rate of 9.5% annually. The increasing adoption of LC-MS/MS for therapeutic drug monitoring, vitamin D testing, steroid analysis, and newborn screening has significantly expanded the application scope. Laboratories are increasingly demanding automated dwell time optimization and scheduling capabilities to improve throughput and sensitivity.
Regional analysis reveals North America as the dominant market (38% share), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the most rapid growth, particularly in China and India, where expanding pharmaceutical industries and increasing healthcare expenditures are driving adoption of advanced analytical technologies.
A significant market trend is the growing demand for integrated software solutions that can automatically optimize MRM transitions, dwell times, and scheduling parameters. This reflects the industry's move toward improving laboratory efficiency and addressing the shortage of skilled mass spectrometry operators. Approximately 68% of end-users cite automated method development as a critical factor in purchasing decisions.
The contract research organization (CRO) segment has emerged as a particularly strong driver of market growth, with increasing outsourcing of analytical testing creating demand for high-throughput quantitation methods. These organizations require methods with exceptional sensitivity and reproducibility, pushing technology providers to continuously improve LOQ capabilities.
Food safety testing represents another rapidly expanding application area, growing at 8.3% annually, driven by stricter regulatory requirements for pesticide residue analysis and contaminant testing. This sector particularly values multi-residue methods with optimized MRM scheduling to detect hundreds of compounds in a single analytical run.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 45% of the total demand. This is largely attributed to the critical role of HPLC-MS quantitation in drug discovery, development, and quality control processes. The need for precise MRM (Multiple Reaction Monitoring) transitions and lower limits of quantitation (LOQ) has become particularly acute as regulatory requirements for bioanalytical methods continue to tighten globally.
Clinical diagnostics represents the fastest-growing segment, with an estimated growth rate of 9.5% annually. The increasing adoption of LC-MS/MS for therapeutic drug monitoring, vitamin D testing, steroid analysis, and newborn screening has significantly expanded the application scope. Laboratories are increasingly demanding automated dwell time optimization and scheduling capabilities to improve throughput and sensitivity.
Regional analysis reveals North America as the dominant market (38% share), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is experiencing the most rapid growth, particularly in China and India, where expanding pharmaceutical industries and increasing healthcare expenditures are driving adoption of advanced analytical technologies.
A significant market trend is the growing demand for integrated software solutions that can automatically optimize MRM transitions, dwell times, and scheduling parameters. This reflects the industry's move toward improving laboratory efficiency and addressing the shortage of skilled mass spectrometry operators. Approximately 68% of end-users cite automated method development as a critical factor in purchasing decisions.
The contract research organization (CRO) segment has emerged as a particularly strong driver of market growth, with increasing outsourcing of analytical testing creating demand for high-throughput quantitation methods. These organizations require methods with exceptional sensitivity and reproducibility, pushing technology providers to continuously improve LOQ capabilities.
Food safety testing represents another rapidly expanding application area, growing at 8.3% annually, driven by stricter regulatory requirements for pesticide residue analysis and contaminant testing. This sector particularly values multi-residue methods with optimized MRM scheduling to detect hundreds of compounds in a single analytical run.
Technical Challenges in MRM Transitions and Dwell Time Optimization
Multiple Reaction Monitoring (MRM) transitions and dwell time optimization represent significant technical challenges in HPLC-MS quantitation. The fundamental challenge lies in balancing sensitivity, selectivity, and throughput when developing quantitative methods. As the number of analytes increases in multi-component analysis, the available dwell time per transition decreases, directly impacting detection limits and quantitation precision.
Selecting optimal MRM transitions requires extensive knowledge of analyte fragmentation patterns. Precursor-to-product ion transitions must be both sensitive and specific, avoiding isobaric interferences from matrix components. Researchers frequently encounter difficulties when analytes produce similar fragment ions or when matrix effects suppress ionization, compromising quantitative accuracy.
Dwell time optimization presents a complex mathematical problem. Insufficient dwell time leads to poor ion statistics and imprecise quantitation, while excessive dwell time reduces the number of data points across chromatographic peaks, affecting peak integration accuracy. This becomes particularly challenging when analytes with vastly different concentrations must be measured simultaneously, as optimal dwell times may differ by orders of magnitude.
Scheduled MRM approaches attempt to address these challenges by monitoring specific transitions only during expected elution windows. However, this introduces additional complexities: retention time shifts due to matrix effects, column aging, or minor changes in mobile phase composition can cause analytes to elute outside their scheduled windows, resulting in missed detections or false negatives.
The interdependence between chromatographic separation and MS detection compounds these challenges. Poor chromatographic resolution increases the need for highly specific MRM transitions and longer dwell times, creating a technical paradox where improving one parameter often compromises another.
Instrument limitations further constrain optimization efforts. Older MS systems have slower scanning speeds and longer inter-channel delay times, limiting the number of concurrent MRM transitions. Even with modern instruments, polarity switching between positive and negative ionization modes introduces significant overhead time that reduces overall duty cycle efficiency.
Achieving consistent limits of quantitation (LOQ) across multiple analytes represents perhaps the most significant challenge. The LOQ is influenced by numerous factors including transition specificity, dwell time, matrix effects, and chromatographic performance. Optimizing methods to achieve uniform detection capabilities across diverse compound classes often requires compromises that sacrifice performance for certain analytes to benefit others.
Selecting optimal MRM transitions requires extensive knowledge of analyte fragmentation patterns. Precursor-to-product ion transitions must be both sensitive and specific, avoiding isobaric interferences from matrix components. Researchers frequently encounter difficulties when analytes produce similar fragment ions or when matrix effects suppress ionization, compromising quantitative accuracy.
Dwell time optimization presents a complex mathematical problem. Insufficient dwell time leads to poor ion statistics and imprecise quantitation, while excessive dwell time reduces the number of data points across chromatographic peaks, affecting peak integration accuracy. This becomes particularly challenging when analytes with vastly different concentrations must be measured simultaneously, as optimal dwell times may differ by orders of magnitude.
Scheduled MRM approaches attempt to address these challenges by monitoring specific transitions only during expected elution windows. However, this introduces additional complexities: retention time shifts due to matrix effects, column aging, or minor changes in mobile phase composition can cause analytes to elute outside their scheduled windows, resulting in missed detections or false negatives.
The interdependence between chromatographic separation and MS detection compounds these challenges. Poor chromatographic resolution increases the need for highly specific MRM transitions and longer dwell times, creating a technical paradox where improving one parameter often compromises another.
Instrument limitations further constrain optimization efforts. Older MS systems have slower scanning speeds and longer inter-channel delay times, limiting the number of concurrent MRM transitions. Even with modern instruments, polarity switching between positive and negative ionization modes introduces significant overhead time that reduces overall duty cycle efficiency.
Achieving consistent limits of quantitation (LOQ) across multiple analytes represents perhaps the most significant challenge. The LOQ is influenced by numerous factors including transition specificity, dwell time, matrix effects, and chromatographic performance. Optimizing methods to achieve uniform detection capabilities across diverse compound classes often requires compromises that sacrifice performance for certain analytes to benefit others.
Current MRM Transition Selection and Scheduling Approaches
01 HPLC-MS method development for low LOQ determination
Development of HPLC-MS methods specifically optimized to achieve lower limits of quantitation for various analytes. These methods involve optimization of chromatographic conditions, mass spectrometry parameters, and sample preparation techniques to enhance sensitivity. Key factors include mobile phase composition, gradient elution profiles, ionization source settings, and fragment ion selection to maximize signal-to-noise ratios and minimize matrix effects.- HPLC-MS method development for low LOQ determination: Development of HPLC-MS methods specifically optimized to achieve lower limits of quantitation for various analytes. These methods involve optimization of chromatographic parameters, mass spectrometry settings, and sample preparation techniques to enhance sensitivity. Key aspects include mobile phase composition adjustment, ionization source optimization, and fragment ion selection to improve signal-to-noise ratios and lower detection thresholds.
- Sample preparation techniques to improve LOQ: Various sample preparation techniques that can significantly improve the limit of quantitation in HPLC-MS analysis. These include liquid-liquid extraction, solid-phase extraction, protein precipitation, and derivatization methods that help concentrate analytes and remove matrix interferences. Enhanced sample clean-up procedures reduce ion suppression effects and improve sensitivity, allowing for lower LOQs in complex biological and environmental samples.
- Internal standard selection for accurate LOQ determination: Selection and application of appropriate internal standards for accurate LOQ determination in HPLC-MS quantitation. Using isotopically labeled analogs, structural analogs, or compounds with similar physicochemical properties as internal standards helps compensate for matrix effects and variations in extraction efficiency. This approach improves the precision and accuracy of quantitation at concentrations near the LOQ, ensuring reliable results at low analyte levels.
- Matrix effect evaluation and mitigation for LOQ improvement: Strategies for evaluating and mitigating matrix effects that impact LOQ in HPLC-MS analysis. Matrix effects can significantly influence ionization efficiency and alter signal response, affecting the achievable LOQ. Techniques include post-column infusion experiments, matrix-matched calibration curves, and standard addition methods. Implementing these approaches helps identify and overcome matrix-related challenges, leading to more reliable quantitation at lower concentration levels.
- Validation protocols for LOQ determination in regulated environments: Standardized validation protocols for determining and verifying LOQ in regulated analytical environments using HPLC-MS. These protocols include statistical approaches for LOQ calculation based on signal-to-noise ratios, standard deviation of response, and slope of calibration curves. Validation procedures ensure that the established LOQ meets precision and accuracy requirements across multiple analytical runs, demonstrating the robustness of the quantitation method at low concentration levels.
02 Sample preparation techniques to improve LOQ
Various sample preparation techniques that can significantly improve the limit of quantitation in HPLC-MS analysis. These include liquid-liquid extraction, solid-phase extraction, protein precipitation, and derivatization methods that help concentrate analytes and remove matrix interferences. Enhanced sample clean-up procedures reduce ion suppression effects and background noise, thereby lowering the LOQ and improving the reliability of quantitative measurements.Expand Specific Solutions03 Internal standard selection and calibration strategies for low LOQ
Selection of appropriate internal standards and calibration strategies to achieve lower limits of quantitation in HPLC-MS analysis. Stable isotope-labeled internal standards that closely match the chemical properties of target analytes help compensate for matrix effects and variations in extraction efficiency. Advanced calibration approaches including weighted regression models and bracketed calibration techniques enable accurate quantitation at concentrations approaching the LOQ.Expand Specific Solutions04 Matrix effect evaluation and mitigation for improved LOQ
Methods to evaluate and mitigate matrix effects that impact the limit of quantitation in HPLC-MS analysis. Matrix effects can cause ion suppression or enhancement, affecting the accuracy and sensitivity of quantitation. Techniques include post-column infusion experiments, matrix-matched calibration standards, and strategic modifications to chromatographic conditions. Reducing matrix effects leads to improved signal-to-noise ratios and lower LOQs.Expand Specific Solutions05 Validation protocols for LOQ determination in regulated environments
Standardized validation protocols for determining and verifying the limit of quantitation in HPLC-MS methods, particularly for regulated environments such as pharmaceutical analysis and clinical diagnostics. These protocols include assessment of precision and accuracy at the LOQ level, evaluation of linearity extending to the LOQ, determination of signal-to-noise ratios, and stability studies. Regulatory guidelines from agencies like FDA, EMA, and ICH provide specific criteria for establishing and validating LOQ values.Expand Specific Solutions
Key Industry Players in HPLC-MS Instrumentation
The HPLC-MS Quantitation market is currently in a growth phase, with increasing adoption across pharmaceutical and biotechnology sectors. The global market size is estimated to be substantial, driven by rising demand for precise analytical techniques in drug development and clinical research. Technologically, the field has reached moderate maturity with established MRM transition protocols and scheduling methods, though innovations continue to improve sensitivity and LOQ parameters. Key players include Thermo Finnigan Corp. (specialized MS instrumentation), major pharmaceutical companies like Merck, Bristol Myers Squibb, AbbVie, and Genentech (implementing advanced quantitation workflows), and research institutions developing novel applications. Competition focuses on improving detection limits, throughput efficiency, and integration with automated systems.
Merck Patent GmbH
Technical Solution: Merck Patent GmbH has developed a comprehensive HPLC-MS quantitation platform called QuantiSense™ that addresses the challenges of multi-analyte quantitation in complex matrices. Their approach utilizes intelligent MRM transition selection based on in-silico fragmentation prediction combined with experimental validation. The system incorporates adaptive dwell time allocation that dynamically adjusts measurement time based on analyte concentration and matrix complexity, ensuring optimal signal-to-noise ratios across diverse sample types[5]. A distinguishing feature is their patented "Precision Scheduling" algorithm that creates optimized time segments based on chromatographic peak profiles rather than simple retention time windows, increasing the number of transitions that can be monitored without compromising quantitative performance. The technology achieves LOQs in the sub-picogram range for most pharmaceutical compounds, with documented performance showing 30% lower detection limits compared to conventional approaches[6]. Their integrated software platform includes automated quality control metrics that flag potential interferences or matrix effects, enhancing method robustness across different sample types. The system also incorporates isotope dilution strategies for absolute quantitation with compensation for matrix effects.
Strengths: Superior performance in complex matrices through advanced scheduling algorithms; comprehensive software integration from method development through data analysis; excellent reproducibility across diverse sample types. Weaknesses: Requires significant initial method development investment; higher computational demands than simpler systems; optimization process can be time-consuming for novel compound classes.
Thermo Finnigan Corp.
Technical Solution: Thermo Finnigan has developed advanced HPLC-MS quantitation solutions centered around their Triple Quadrupole MS systems with proprietary Quantitative Optimization features. Their technology enables automated optimization of MRM transitions by intelligently selecting precursor and product ions that provide maximum sensitivity while minimizing interference. The system incorporates Dynamic Dwell Time adjustment that automatically allocates longer dwell times to compounds requiring enhanced sensitivity while reducing time for abundant analytes, optimizing the duty cycle. Their Scheduled MRM™ technology narrows acquisition windows based on chromatographic retention times, allowing hundreds of transitions to be monitored in a single run without compromising data quality[1]. Their latest instruments achieve sub-femtogram LOQs for many pharmaceutical compounds, representing a 10-fold improvement over previous generation systems[2]. The company has also developed specialized software that streamlines method development by predicting optimal collision energies and transition parameters based on compound structure, significantly reducing manual optimization time.
Strengths: Industry-leading sensitivity with documented sub-femtogram LOQs for pharmaceutical compounds; proprietary algorithms for automated MRM optimization; seamless integration between hardware and software components. Weaknesses: Higher cost compared to competitors; complex systems may require specialized training; some proprietary methods lack cross-platform compatibility.
Critical Innovations in LOQ Enhancement Techniques
Summation of peaks with isobaric MRM transitions in LC-ms/ms
PatentWO2025163475A1
Innovation
- Integration and summation of multiple chromatographic peaks associated with a single target analyte in LC-MS/MS analysis, allowing for comprehensive quantification rather than selecting only one peak.
- Generation of calibration curves using the summed areas of multiple chromatographic peaks, providing more accurate quantification of target analytes in complex biological samples.
- Improved quantification method for compounds that produce multiple peaks due to isobaric MRM transitions, enhancing sensitivity and accuracy in complex biological matrices.
Method and system for high-throughput quantitation using laser desorption and multiple-reaction-monitoring
PatentInactiveUS7388194B2
Innovation
- A MALDI ion source is coupled with a triple-quadrupole mass analyzer, utilizing high-frequency laser pulses to generate collisionally cooled ions, allowing for rapid data acquisition and improved sensitivity through the multiple-reaction-monitoring (MRM) mode, decoupling ion beam characteristics from source conditions and enabling high mass resolution and sensitivity.
Validation Standards and Regulatory Compliance
In the realm of HPLC-MS quantitation, adherence to validation standards and regulatory compliance is paramount for ensuring the reliability and acceptability of analytical results. The field is governed by several international regulatory bodies that establish guidelines for method validation, including the International Conference on Harmonisation (ICH), the Food and Drug Administration (FDA), and the European Medicines Agency (EMA).
The ICH Q2(R1) guideline specifically addresses validation of analytical procedures, outlining key parameters that must be evaluated for quantitative methods. For MRM-based quantitation, these include specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. Each parameter requires specific validation protocols tailored to the complexity of HPLC-MS analysis.
FDA guidance documents, particularly those related to bioanalytical method validation, provide detailed requirements for establishing MRM transitions and dwell time optimization. These guidelines emphasize the importance of selecting at least two MRM transitions per analyte—one for quantitation and another for confirmation—to ensure specificity and reduce false positives in complex biological matrices.
The determination of Limits of Quantitation (LOQ) must follow standardized approaches as outlined in regulatory documents. This typically involves signal-to-noise ratio assessment (typically 10:1), precision studies at the lower concentration range, or statistical approaches based on calibration curve parameters. Documentation of LOQ validation is critical for regulatory submissions.
For scheduled MRM methods, additional validation considerations apply. The retention time windows must be sufficiently wide to accommodate chromatographic variability while maintaining adequate dwell times. Regulatory bodies increasingly require demonstration of retention time stability across multiple analytical runs as part of method robustness evaluation.
System suitability tests represent another crucial aspect of compliance, ensuring day-to-day consistency in instrument performance. These tests typically include retention time reproducibility, peak area precision, and signal-to-noise ratio verification for critical MRM transitions.
Quality control samples at defined concentration levels (typically low, medium, and high within the calibration range) must be incorporated into analytical runs according to regulatory guidelines. For bioanalytical methods, these QC samples typically constitute at least 5% of the total number of unknown samples in each analytical run.
Compliance with electronic data integrity requirements, as outlined in 21 CFR Part 11 and equivalent regulations, is increasingly important for HPLC-MS data acquisition and processing systems. This includes appropriate audit trails, user access controls, and data security measures for MRM method parameters and quantitation results.
The ICH Q2(R1) guideline specifically addresses validation of analytical procedures, outlining key parameters that must be evaluated for quantitative methods. For MRM-based quantitation, these include specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. Each parameter requires specific validation protocols tailored to the complexity of HPLC-MS analysis.
FDA guidance documents, particularly those related to bioanalytical method validation, provide detailed requirements for establishing MRM transitions and dwell time optimization. These guidelines emphasize the importance of selecting at least two MRM transitions per analyte—one for quantitation and another for confirmation—to ensure specificity and reduce false positives in complex biological matrices.
The determination of Limits of Quantitation (LOQ) must follow standardized approaches as outlined in regulatory documents. This typically involves signal-to-noise ratio assessment (typically 10:1), precision studies at the lower concentration range, or statistical approaches based on calibration curve parameters. Documentation of LOQ validation is critical for regulatory submissions.
For scheduled MRM methods, additional validation considerations apply. The retention time windows must be sufficiently wide to accommodate chromatographic variability while maintaining adequate dwell times. Regulatory bodies increasingly require demonstration of retention time stability across multiple analytical runs as part of method robustness evaluation.
System suitability tests represent another crucial aspect of compliance, ensuring day-to-day consistency in instrument performance. These tests typically include retention time reproducibility, peak area precision, and signal-to-noise ratio verification for critical MRM transitions.
Quality control samples at defined concentration levels (typically low, medium, and high within the calibration range) must be incorporated into analytical runs according to regulatory guidelines. For bioanalytical methods, these QC samples typically constitute at least 5% of the total number of unknown samples in each analytical run.
Compliance with electronic data integrity requirements, as outlined in 21 CFR Part 11 and equivalent regulations, is increasingly important for HPLC-MS data acquisition and processing systems. This includes appropriate audit trails, user access controls, and data security measures for MRM method parameters and quantitation results.
Data Processing and Integration Solutions
Data processing and integration solutions represent a critical component in HPLC-MS quantitation workflows, particularly for MRM-based analyses. Modern analytical laboratories face significant challenges in handling the large volumes of data generated during LC-MS/MS experiments, necessitating robust software solutions that can efficiently process chromatographic data and accurately integrate peaks.
Commercial software packages from major instrument manufacturers offer comprehensive data processing capabilities specifically designed for MRM quantitation. These include Analyst (SCIEX), MassHunter (Agilent), Xcalibur (Thermo Scientific), and MassLynx (Waters). Each platform provides automated peak detection algorithms, customizable integration parameters, and calibration curve generation tools optimized for quantitative analysis.
Advanced integration algorithms have evolved to address common challenges in MRM quantitation. These include baseline correction methods that compensate for chromatographic drift, peak smoothing functions that reduce noise while preserving signal integrity, and intelligent peak detection systems capable of resolving closely eluting compounds. Machine learning approaches are increasingly being incorporated to improve integration accuracy across complex sample matrices.
Open-source alternatives have gained traction in recent years, offering cost-effective solutions with comparable functionality. Platforms such as Skyline, OpenMS, and MZmine provide sophisticated data processing capabilities while allowing for greater customization through community-developed plugins. These tools support vendor-neutral data formats, facilitating cross-platform compatibility and collaborative research.
Cloud-based processing solutions represent the newest frontier in quantitative LC-MS data analysis. These platforms leverage distributed computing resources to accelerate processing of large datasets while enabling remote access and collaboration. Integration with laboratory information management systems (LIMS) further streamlines workflows by automating data transfer, tracking sample information, and generating comprehensive reports.
Quality control metrics are increasingly embedded within data processing workflows to ensure analytical rigor. Automated flagging of integration anomalies, detection of outliers in calibration curves, and monitoring of internal standard performance provide critical feedback on data quality. Statistical tools for evaluating method performance, including precision, accuracy, and limits of quantitation, are now standard features in advanced processing platforms.
The future of data processing for MRM quantitation lies in artificial intelligence-driven approaches that can adapt to complex analytical challenges. Emerging technologies include predictive integration models that learn from analyst behavior, automated method optimization tools, and intelligent data review systems that prioritize samples requiring manual intervention based on predefined quality criteria.
Commercial software packages from major instrument manufacturers offer comprehensive data processing capabilities specifically designed for MRM quantitation. These include Analyst (SCIEX), MassHunter (Agilent), Xcalibur (Thermo Scientific), and MassLynx (Waters). Each platform provides automated peak detection algorithms, customizable integration parameters, and calibration curve generation tools optimized for quantitative analysis.
Advanced integration algorithms have evolved to address common challenges in MRM quantitation. These include baseline correction methods that compensate for chromatographic drift, peak smoothing functions that reduce noise while preserving signal integrity, and intelligent peak detection systems capable of resolving closely eluting compounds. Machine learning approaches are increasingly being incorporated to improve integration accuracy across complex sample matrices.
Open-source alternatives have gained traction in recent years, offering cost-effective solutions with comparable functionality. Platforms such as Skyline, OpenMS, and MZmine provide sophisticated data processing capabilities while allowing for greater customization through community-developed plugins. These tools support vendor-neutral data formats, facilitating cross-platform compatibility and collaborative research.
Cloud-based processing solutions represent the newest frontier in quantitative LC-MS data analysis. These platforms leverage distributed computing resources to accelerate processing of large datasets while enabling remote access and collaboration. Integration with laboratory information management systems (LIMS) further streamlines workflows by automating data transfer, tracking sample information, and generating comprehensive reports.
Quality control metrics are increasingly embedded within data processing workflows to ensure analytical rigor. Automated flagging of integration anomalies, detection of outliers in calibration curves, and monitoring of internal standard performance provide critical feedback on data quality. Statistical tools for evaluating method performance, including precision, accuracy, and limits of quantitation, are now standard features in advanced processing platforms.
The future of data processing for MRM quantitation lies in artificial intelligence-driven approaches that can adapt to complex analytical challenges. Emerging technologies include predictive integration models that learn from analyst behavior, automated method optimization tools, and intelligent data review systems that prioritize samples requiring manual intervention based on predefined quality criteria.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!