How HPLC-MS Prevents Carryover In Sticky Analyte Methods?
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Carryover Challenges and Objectives
High-performance liquid chromatography-mass spectrometry (HPLC-MS) has evolved significantly over the past three decades, transforming from specialized research equipment to an essential analytical tool across pharmaceutical, environmental, and clinical laboratories. Carryover—the phenomenon where residual analytes from previous injections contaminate subsequent samples—represents one of the most persistent challenges in HPLC-MS methodology, particularly when working with sticky analytes that tend to adhere to system components.
The technical evolution of HPLC-MS carryover prevention has progressed through several distinct phases. Early systems (1990s-2000s) relied primarily on extended washing protocols, which were time-consuming and often ineffective for particularly adhesive compounds. The mid-2000s saw the introduction of specialized hardware modifications, including inert flow paths and specialized injection port designs. Recent advancements (2015-present) have focused on integrated approaches combining optimized hardware, software algorithms for intelligent sample sequencing, and advanced materials science.
Current carryover challenges primarily stem from the physicochemical properties of sticky analytes, including high lipophilicity, strong ionic interactions, and complex matrix effects. These compounds frequently interact with system components through various mechanisms: hydrophobic interactions with stationary phases, ionic binding to metal surfaces, and formation of secondary structures that resist standard cleaning procedures.
The primary objective of modern HPLC-MS carryover prevention research is to develop robust, efficient methodologies that maintain analytical integrity while minimizing system downtime and resource consumption. Specific technical goals include reducing carryover to below 0.05% for even the most challenging compounds, developing universal washing solutions effective across diverse analyte classes, and creating predictive models to anticipate carryover based on molecular properties.
Industry benchmarks indicate that acceptable carryover thresholds vary by application: pharmaceutical quality control typically requires <0.1%, bioanalytical applications <0.5%, and environmental testing <1.0%. However, emerging fields such as ultra-sensitive biomarker detection and single-cell proteomics are pushing these requirements even lower, necessitating innovative approaches to system design and operation.
The technological trajectory suggests convergence toward intelligent, self-optimizing systems that can adapt washing protocols based on analyte properties, implement preventative measures before carryover occurs, and utilize machine learning algorithms to continuously improve performance based on historical data patterns. This evolution represents a shift from reactive to proactive carryover management strategies in HPLC-MS methodology.
The technical evolution of HPLC-MS carryover prevention has progressed through several distinct phases. Early systems (1990s-2000s) relied primarily on extended washing protocols, which were time-consuming and often ineffective for particularly adhesive compounds. The mid-2000s saw the introduction of specialized hardware modifications, including inert flow paths and specialized injection port designs. Recent advancements (2015-present) have focused on integrated approaches combining optimized hardware, software algorithms for intelligent sample sequencing, and advanced materials science.
Current carryover challenges primarily stem from the physicochemical properties of sticky analytes, including high lipophilicity, strong ionic interactions, and complex matrix effects. These compounds frequently interact with system components through various mechanisms: hydrophobic interactions with stationary phases, ionic binding to metal surfaces, and formation of secondary structures that resist standard cleaning procedures.
The primary objective of modern HPLC-MS carryover prevention research is to develop robust, efficient methodologies that maintain analytical integrity while minimizing system downtime and resource consumption. Specific technical goals include reducing carryover to below 0.05% for even the most challenging compounds, developing universal washing solutions effective across diverse analyte classes, and creating predictive models to anticipate carryover based on molecular properties.
Industry benchmarks indicate that acceptable carryover thresholds vary by application: pharmaceutical quality control typically requires <0.1%, bioanalytical applications <0.5%, and environmental testing <1.0%. However, emerging fields such as ultra-sensitive biomarker detection and single-cell proteomics are pushing these requirements even lower, necessitating innovative approaches to system design and operation.
The technological trajectory suggests convergence toward intelligent, self-optimizing systems that can adapt washing protocols based on analyte properties, implement preventative measures before carryover occurs, and utilize machine learning algorithms to continuously improve performance based on historical data patterns. This evolution represents a shift from reactive to proactive carryover management strategies in HPLC-MS methodology.
Market Demand for Carryover-Free Analytical Methods
The analytical chemistry market has witnessed a significant surge in demand for carryover-free methods, particularly in HPLC-MS applications dealing with sticky analytes. This demand is primarily driven by industries requiring high precision and reliability in their analytical processes, including pharmaceuticals, clinical diagnostics, food safety, environmental monitoring, and forensic toxicology.
In the pharmaceutical sector, which represents approximately 40% of the global analytical instrumentation market, there is an increasing need for methods that can accurately quantify drug compounds and metabolites at trace levels without interference from previous samples. The growing focus on biologics and complex molecules has further intensified this demand, as these compounds often exhibit sticky characteristics that make them prone to carryover issues.
Clinical laboratories constitute another major market segment driving demand for carryover-free methods. With the rise in personalized medicine and therapeutic drug monitoring, these facilities require analytical methods capable of processing large sample batches with minimal cross-contamination. The consequences of carryover in clinical settings can be severe, potentially leading to misdiagnosis or improper treatment decisions.
Food safety testing represents a rapidly growing market for advanced analytical methods. Regulatory bodies worldwide have implemented increasingly stringent requirements for detecting contaminants, pesticides, and adulterants in food products. Many of these compounds tend to adsorb to system surfaces, making carryover prevention critical for accurate results and regulatory compliance.
Environmental analysis laboratories face similar challenges when monitoring persistent organic pollutants, which by their nature tend to be sticky and difficult to eliminate from analytical systems. The market demand in this sector is driven by both regulatory requirements and increasing public awareness of environmental contamination issues.
The global market for analytical instruments addressing carryover issues is projected to grow at a compound annual growth rate exceeding the overall analytical instrument market growth. This premium growth rate reflects the critical importance users place on eliminating carryover-related problems.
Vendors who can demonstrate superior carryover prevention in their HPLC-MS systems command premium pricing, with users willing to invest in technologies that reduce method development time and increase sample throughput. The economic benefits of reduced system cleaning time, decreased solvent consumption, and improved data reliability create a compelling value proposition that supports this premium positioning in the market.
In the pharmaceutical sector, which represents approximately 40% of the global analytical instrumentation market, there is an increasing need for methods that can accurately quantify drug compounds and metabolites at trace levels without interference from previous samples. The growing focus on biologics and complex molecules has further intensified this demand, as these compounds often exhibit sticky characteristics that make them prone to carryover issues.
Clinical laboratories constitute another major market segment driving demand for carryover-free methods. With the rise in personalized medicine and therapeutic drug monitoring, these facilities require analytical methods capable of processing large sample batches with minimal cross-contamination. The consequences of carryover in clinical settings can be severe, potentially leading to misdiagnosis or improper treatment decisions.
Food safety testing represents a rapidly growing market for advanced analytical methods. Regulatory bodies worldwide have implemented increasingly stringent requirements for detecting contaminants, pesticides, and adulterants in food products. Many of these compounds tend to adsorb to system surfaces, making carryover prevention critical for accurate results and regulatory compliance.
Environmental analysis laboratories face similar challenges when monitoring persistent organic pollutants, which by their nature tend to be sticky and difficult to eliminate from analytical systems. The market demand in this sector is driven by both regulatory requirements and increasing public awareness of environmental contamination issues.
The global market for analytical instruments addressing carryover issues is projected to grow at a compound annual growth rate exceeding the overall analytical instrument market growth. This premium growth rate reflects the critical importance users place on eliminating carryover-related problems.
Vendors who can demonstrate superior carryover prevention in their HPLC-MS systems command premium pricing, with users willing to invest in technologies that reduce method development time and increase sample throughput. The economic benefits of reduced system cleaning time, decreased solvent consumption, and improved data reliability create a compelling value proposition that supports this premium positioning in the market.
Current Limitations in Sticky Analyte Analysis
Despite significant advancements in HPLC-MS technology, the analysis of sticky analytes continues to present substantial challenges for researchers and laboratory professionals. Sticky analytes, characterized by their tendency to adhere to various surfaces within the analytical system, create persistent carryover issues that compromise data integrity and analytical reliability. Current methodologies struggle with several key limitations that hinder effective analysis.
The most significant limitation is the inherent physicochemical properties of sticky compounds themselves. These analytes, which include certain peptides, proteins, lipids, and highly polar compounds, exhibit strong interactions with stationary phases, tubing, injector components, and even detector surfaces. These interactions occur through various mechanisms including hydrogen bonding, van der Waals forces, and electrostatic attractions, making them particularly difficult to fully elute from the system.
Conventional washing procedures often prove inadequate for completely removing residual sticky analytes. Standard flush solutions typically employ mixtures of organic solvents and water, which may not provide sufficient solubilizing power for compounds with extreme hydrophobicity or hydrophilicity. This results in gradual accumulation of analytes within the system, leading to increasingly problematic carryover effects over time.
Current instrument designs also contribute to carryover challenges. Dead volumes, microchannels, and connection points within HPLC systems create areas where flow dynamics are suboptimal, allowing sticky compounds to accumulate. These design limitations are particularly problematic in high-throughput environments where rapid analysis cycles provide minimal time for thorough system cleaning between injections.
Method development for sticky analytes remains largely empirical rather than systematic. Analysts often resort to trial-and-error approaches to mitigate carryover, lacking comprehensive theoretical frameworks to guide optimization efforts. This results in inconsistent methodologies across laboratories and significant time investment in method development.
Detection sensitivity paradoxically exacerbates carryover challenges. As MS technology advances with increasingly lower detection limits, even minute amounts of carryover become analytically significant. Compounds that previously went undetected now register as interfering peaks, creating false positives or quantification errors in subsequent analyses.
Regulatory and quality control frameworks have not fully adapted to address the specific challenges of sticky analyte analysis. Current guidelines provide limited specific recommendations for carryover mitigation strategies, leaving laboratories to develop their own validation approaches without standardized benchmarks for acceptable performance.
The most significant limitation is the inherent physicochemical properties of sticky compounds themselves. These analytes, which include certain peptides, proteins, lipids, and highly polar compounds, exhibit strong interactions with stationary phases, tubing, injector components, and even detector surfaces. These interactions occur through various mechanisms including hydrogen bonding, van der Waals forces, and electrostatic attractions, making them particularly difficult to fully elute from the system.
Conventional washing procedures often prove inadequate for completely removing residual sticky analytes. Standard flush solutions typically employ mixtures of organic solvents and water, which may not provide sufficient solubilizing power for compounds with extreme hydrophobicity or hydrophilicity. This results in gradual accumulation of analytes within the system, leading to increasingly problematic carryover effects over time.
Current instrument designs also contribute to carryover challenges. Dead volumes, microchannels, and connection points within HPLC systems create areas where flow dynamics are suboptimal, allowing sticky compounds to accumulate. These design limitations are particularly problematic in high-throughput environments where rapid analysis cycles provide minimal time for thorough system cleaning between injections.
Method development for sticky analytes remains largely empirical rather than systematic. Analysts often resort to trial-and-error approaches to mitigate carryover, lacking comprehensive theoretical frameworks to guide optimization efforts. This results in inconsistent methodologies across laboratories and significant time investment in method development.
Detection sensitivity paradoxically exacerbates carryover challenges. As MS technology advances with increasingly lower detection limits, even minute amounts of carryover become analytically significant. Compounds that previously went undetected now register as interfering peaks, creating false positives or quantification errors in subsequent analyses.
Regulatory and quality control frameworks have not fully adapted to address the specific challenges of sticky analyte analysis. Current guidelines provide limited specific recommendations for carryover mitigation strategies, leaving laboratories to develop their own validation approaches without standardized benchmarks for acceptable performance.
Established Strategies for Minimizing Analyte Carryover
01 Mobile phase optimization for carryover reduction
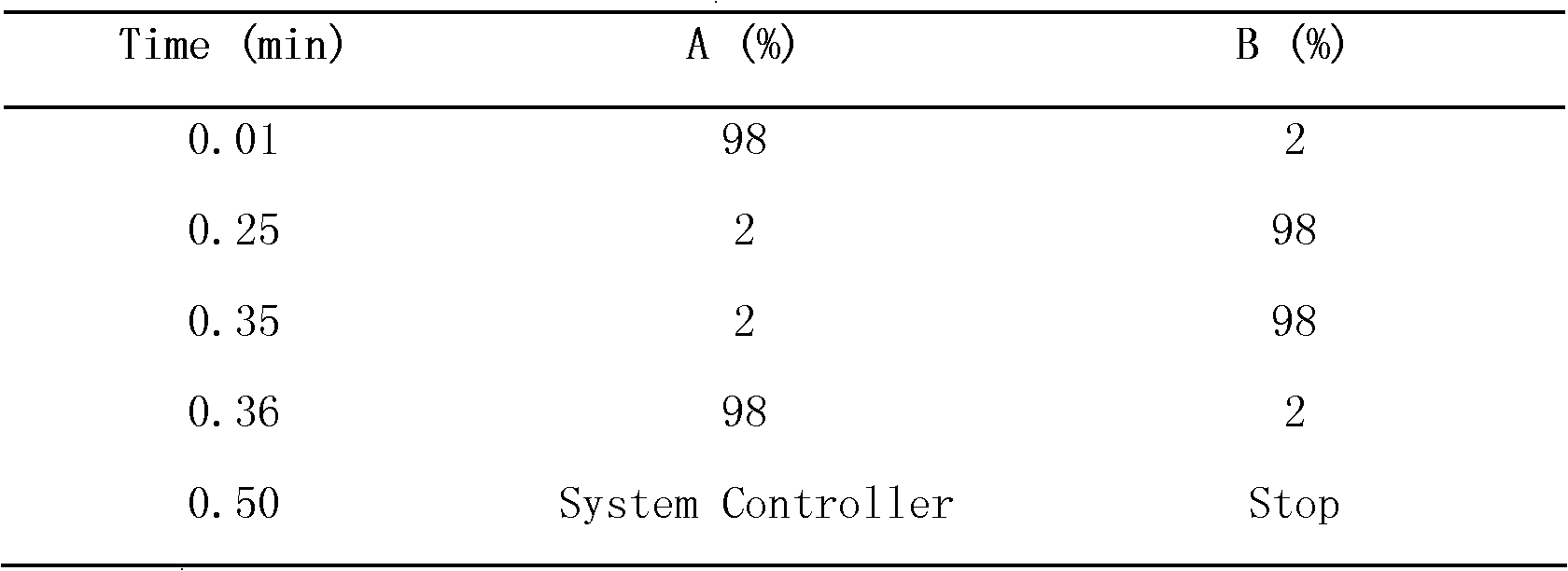
Optimizing the mobile phase composition in HPLC-MS systems can significantly reduce carryover effects. This includes adjusting the pH, organic solvent concentration, and adding specific modifiers that prevent analyte adsorption to system components. Techniques such as gradient elution with high organic content wash steps and the incorporation of additives like formic acid or ammonium acetate can effectively clean the system between injections, minimizing residual analyte retention.- System washing and cleaning procedures: Implementing thorough washing and cleaning procedures between sample analyses can significantly reduce carryover in HPLC-MS systems. This includes using appropriate washing solutions, such as organic solvents or detergents, to flush the system components including injection port, column, and detector. Multiple washing cycles with different solvents of increasing polarity can effectively remove residual analytes from previous injections, preventing their appearance in subsequent analyses.
- Mobile phase optimization: Optimizing the mobile phase composition and gradient profiles can minimize carryover in HPLC-MS systems. This includes adjusting the pH, organic solvent percentage, and buffer concentration to reduce analyte adsorption to system components. Implementing strong wash solvents between injections and using specialized additives that prevent analyte binding to surfaces can significantly reduce carryover effects, especially for sticky or highly hydrophobic compounds.
- Hardware modifications and material selection: Using specialized materials and hardware modifications can reduce carryover in HPLC-MS systems. This includes replacing standard components with inert materials such as PEEK (polyetheretherketone) or specialized metal alloys that minimize analyte adsorption. Modifications to injection valves, sample loops, and connection tubing with low-adsorption materials can significantly reduce carryover, particularly for compounds that tend to bind to metal surfaces or conventional plastics.
- Sample preparation techniques: Implementing appropriate sample preparation techniques can minimize carryover in HPLC-MS analysis. This includes proper dilution of highly concentrated samples, protein precipitation, liquid-liquid extraction, or solid-phase extraction to remove matrix components that might contribute to carryover. Adding competing agents or carrier proteins to the sample can also prevent analytes from adhering to surfaces in the HPLC-MS system, thereby reducing carryover between injections.
- Software and analytical method approaches: Implementing software solutions and analytical method adjustments can help manage carryover in HPLC-MS systems. This includes using blank injections between samples, implementing carryover correction algorithms in data processing, and developing specialized sequence methods that arrange samples from low to high concentration. Statistical approaches can be used to quantify and correct for carryover effects, while machine learning algorithms can predict and compensate for carryover based on sample characteristics and system conditions.
02 System washing and flushing protocols
Implementing comprehensive washing and flushing protocols between sample analyses can effectively prevent carryover in HPLC-MS systems. These protocols typically involve multiple rinse cycles with strong solvents, dedicated needle wash solutions, and extended purging of sample loops and injection ports. Automated washing sequences can be programmed to ensure thorough cleaning of all system components that come into contact with samples.Expand Specific Solutions03 Hardware modifications and specialized components
Specialized hardware components and system modifications can be implemented to minimize carryover in HPLC-MS analysis. These include using inert materials for sample contact surfaces, installing dedicated bypass valves, employing dual needle systems, and utilizing specialized injection port designs. Replacing standard components with low-adsorption alternatives made from materials such as PEEK or titanium can significantly reduce analyte retention on surfaces.Expand Specific Solutions04 Sample preparation techniques
Advanced sample preparation techniques can help prevent carryover issues before samples enter the HPLC-MS system. These include dilution protocols for high-concentration samples, protein precipitation, solid-phase extraction, and the addition of competing agents that prevent analyte adsorption. Proper sample handling and preparation can significantly reduce the risk of contaminating the analytical system with residual analytes.Expand Specific Solutions05 Analytical method development strategies
Comprehensive analytical method development strategies can be employed to minimize carryover effects in HPLC-MS analysis. These include optimizing injection volumes, implementing blank injections between samples, developing specialized detection parameters, and establishing appropriate calibration procedures that account for potential carryover. Method validation protocols specifically designed to assess and mitigate carryover can ensure reliable analytical results even when analyzing complex sample matrices.Expand Specific Solutions
Leading Manufacturers in HPLC-MS Instrumentation
The HPLC-MS carryover prevention market is currently in a growth phase, with increasing demand driven by pharmaceutical, clinical, and food safety applications. The global analytical instrumentation market, including HPLC-MS systems, is estimated to exceed $5 billion, with carryover prevention solutions representing a significant segment. Leading players like Agilent Technologies, Roche, and Thermo Fisher (via acquired Dionex) have developed mature technologies including advanced wash protocols, specialized column chemistries, and innovative sample preparation techniques. Emerging competitors such as Optimize Technologies are focusing on specialized hardware solutions, while pharmaceutical companies like LG Chem and WuXi AppTec are developing application-specific methodologies. The technology has reached commercial maturity but continues to evolve with innovations in surface treatments, automated cleaning systems, and machine learning-based method optimization.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed a comprehensive approach to prevent carryover in HPLC-MS analysis of sticky analytes through their InfinityLab LC/MSD systems. Their technology incorporates multi-stage washing protocols with specialized solvents that target both hydrophilic and hydrophobic residues. The Agilent Jet Wash self-cleaning ion source automatically performs inter-sample cleaning to minimize contamination. Their systems feature specialized flow path components with minimal dead volume and inert surface treatments that reduce analyte adsorption. Agilent's needle wash stations utilize programmable wash cycles with multiple solvents and extended dwell times specifically optimized for sticky compounds. Additionally, their InfinityLab Flex Bench LC-MS incorporates intelligent system design with heated columns and connection capillaries to prevent precipitation of analytes during analysis.
Strengths: Comprehensive integrated solution with automated cleaning protocols; specialized surface treatments for reduced adsorption; flexible multi-solvent washing capabilities. Weaknesses: Higher initial investment cost; may require specialized consumables; some solutions may increase analysis time due to extended washing cycles.
Hitachi High-Tech America, Inc.
Technical Solution: Hitachi High-Tech has developed specialized HPLC-MS systems with advanced carryover prevention technologies for sticky analytes. Their approach centers on their LaChrom and Chromaster series that feature specialized flow path components with minimal dead volume and proprietary surface treatments to reduce analyte adsorption. Hitachi's autosampler technology incorporates programmable multi-solvent washing protocols with adjustable parameters for wash volume, cycles, and dwell time. Their systems feature specialized needle designs with optimized geometry and surface treatments to minimize sample retention. Hitachi has also developed specialized mobile phase additives and column regeneration protocols specifically for challenging compounds. Their intelligent control software includes features for monitoring system performance and automatically implementing enhanced washing protocols when carryover is detected. Additionally, Hitachi offers specialized consumables with inert surfaces specifically designed to minimize interaction with sticky analytes.
Strengths: Robust autosampler design with comprehensive washing capabilities; specialized surface treatments throughout the flow path; intelligent software for carryover detection. Weaknesses: May require method-specific optimization; some solutions increase analysis time; less widespread adoption compared to other major vendors.
Key Innovations in Sample Path Materials and Design
High performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) system and method
PatentActiveCN102253141A
Innovation
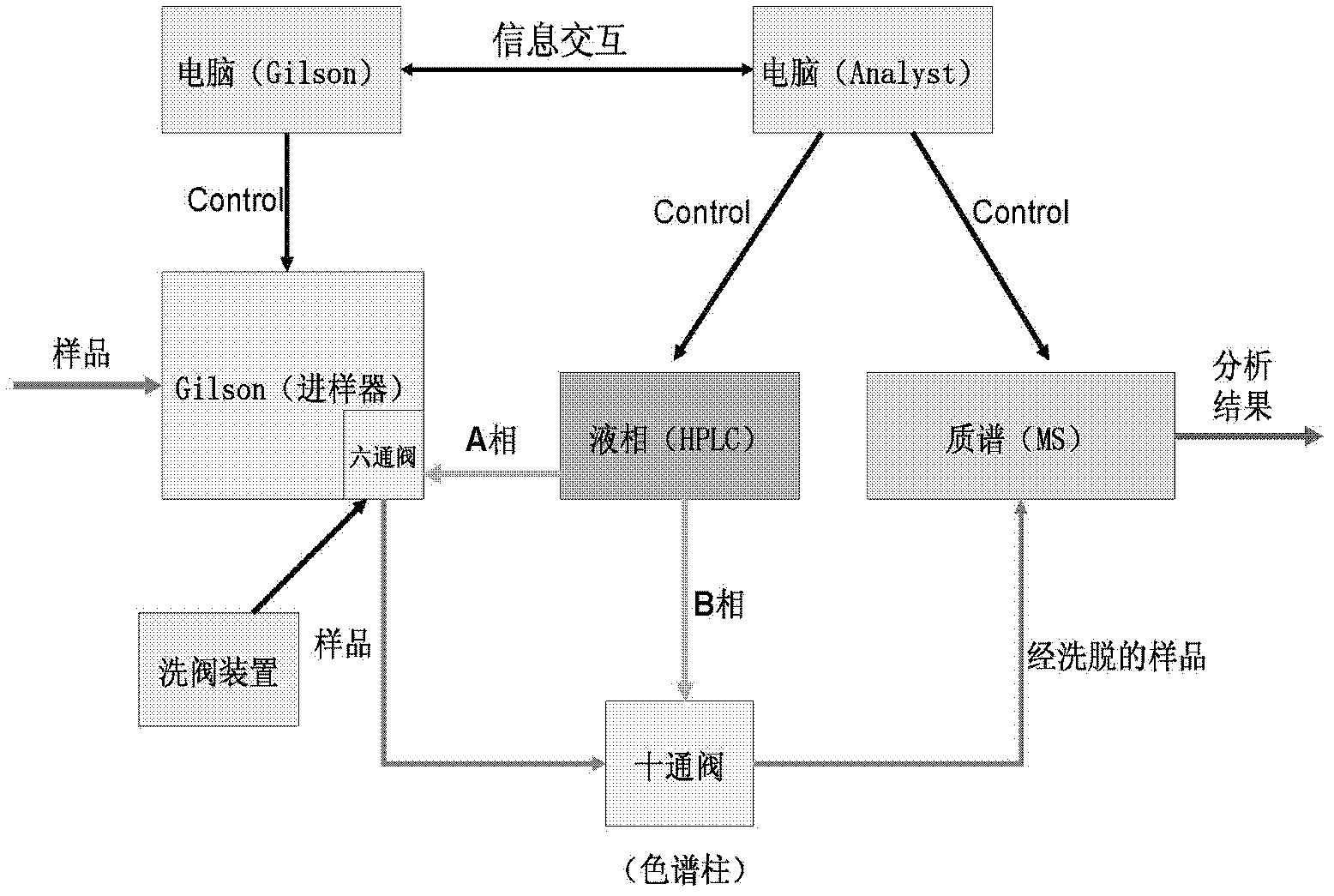
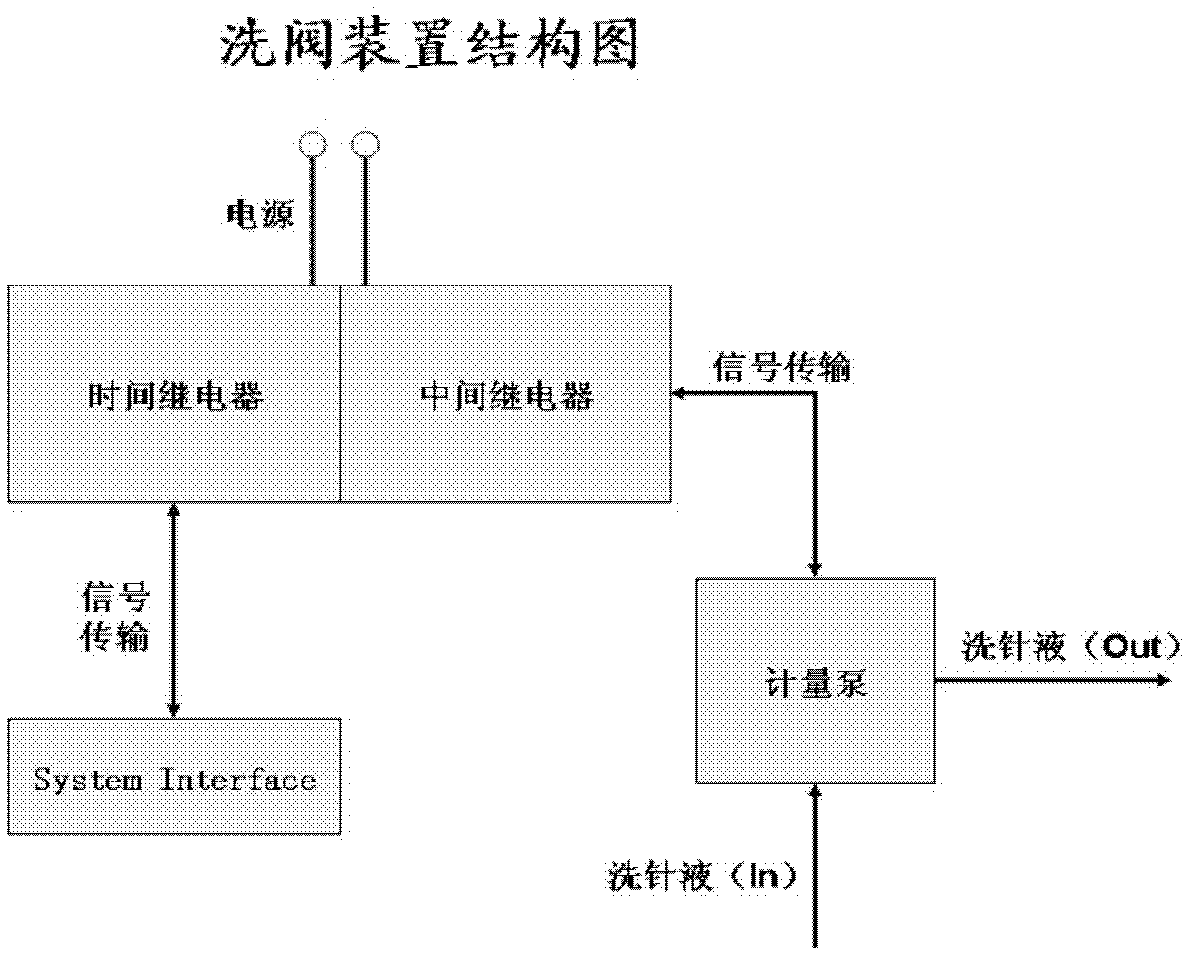
- Using a system including a high performance liquid chromatograph, mass spectrometer, pulse injector, electronic pulse device and automatic data processing module, the Jerson autosampler and electronic pulse backflush valve are used to reduce the injection volume and time and improve analysis The detection sensitivity of the substance was measured, and data were automatically processed through DiscoveryQuant 2.0 and Microsoft Excel VBA.
Method for detecting beauvericin and ennifusin in edible fungi
PatentPendingCN116165302A
Innovation
- Using high-performance liquid chromatography tandem mass spectrometry, fresh edible fungus samples were dried and ground into powder, and ultrasonic extraction was performed using a methanol aqueous solution containing formic acid, combined with a reversed-phase solid-phase extraction column and HPLC-MS/MS detection to detect Beauveria bassiana. Quantitative analysis of fusarium and fusariin.
Method Validation and Quality Control Procedures
Method validation and quality control procedures are essential components in developing robust HPLC-MS methods that effectively prevent carryover of sticky analytes. These procedures ensure that analytical methods consistently deliver reliable results by systematically identifying and mitigating carryover risks.
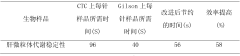
Comprehensive validation protocols must include specific carryover assessment tests. These typically involve analyzing blank samples immediately after high-concentration standards or samples to quantify any residual analyte signal. Acceptance criteria should be established based on the lowest level of quantification (LLOQ), with carryover generally required to be less than 20% of the LLOQ signal to ensure minimal impact on subsequent analyses.
Quality control samples at varying concentrations (low, medium, and high) should be strategically positioned throughout analytical batches to monitor system performance and detect any developing carryover issues. This approach allows analysts to identify when carryover begins to affect results before it compromises entire sample sets, enabling timely intervention.
System suitability tests represent another critical component of quality control procedures. These tests should include specific parameters designed to detect carryover, such as monitoring peak asymmetry, which can indicate adsorption issues, and evaluating baseline stability between injections. Establishing clear acceptance criteria for these parameters ensures consistent system performance.
Regular preventive maintenance schedules must be documented and followed as part of quality control procedures. These should include specific cleaning protocols for components prone to accumulating sticky analytes, such as injector needles, sample loops, and column frits. Documentation of maintenance activities provides traceability and helps identify correlations between maintenance events and carryover reduction.
Automated quality control checks can be implemented within data processing software to flag potential carryover issues. These algorithms can compare blank sample signals to predetermined thresholds and alert analysts when carryover exceeds acceptable limits, allowing for immediate corrective action.
Method transfer protocols should explicitly address carryover prevention strategies when implementing methods across different laboratories or instruments. This ensures consistency in carryover control regardless of the specific hardware configuration or analyst experience level, maintaining data integrity across multiple testing environments.
Comprehensive validation protocols must include specific carryover assessment tests. These typically involve analyzing blank samples immediately after high-concentration standards or samples to quantify any residual analyte signal. Acceptance criteria should be established based on the lowest level of quantification (LLOQ), with carryover generally required to be less than 20% of the LLOQ signal to ensure minimal impact on subsequent analyses.
Quality control samples at varying concentrations (low, medium, and high) should be strategically positioned throughout analytical batches to monitor system performance and detect any developing carryover issues. This approach allows analysts to identify when carryover begins to affect results before it compromises entire sample sets, enabling timely intervention.
System suitability tests represent another critical component of quality control procedures. These tests should include specific parameters designed to detect carryover, such as monitoring peak asymmetry, which can indicate adsorption issues, and evaluating baseline stability between injections. Establishing clear acceptance criteria for these parameters ensures consistent system performance.
Regular preventive maintenance schedules must be documented and followed as part of quality control procedures. These should include specific cleaning protocols for components prone to accumulating sticky analytes, such as injector needles, sample loops, and column frits. Documentation of maintenance activities provides traceability and helps identify correlations between maintenance events and carryover reduction.
Automated quality control checks can be implemented within data processing software to flag potential carryover issues. These algorithms can compare blank sample signals to predetermined thresholds and alert analysts when carryover exceeds acceptable limits, allowing for immediate corrective action.
Method transfer protocols should explicitly address carryover prevention strategies when implementing methods across different laboratories or instruments. This ensures consistency in carryover control regardless of the specific hardware configuration or analyst experience level, maintaining data integrity across multiple testing environments.
Cost-Benefit Analysis of Carryover Prevention Solutions
When evaluating carryover prevention solutions for HPLC-MS analysis of sticky analytes, a comprehensive cost-benefit analysis reveals significant financial implications across multiple dimensions. Initial investment costs vary considerably among different approaches, with system modifications like specialized column switching valves requiring substantial capital expenditure ($15,000-30,000), while procedural changes such as optimized wash protocols demand minimal direct investment but increased consumable usage.
Operational expenses represent a critical consideration in the long-term economics of carryover prevention. Advanced autosampler technologies with dedicated wash stations increase power consumption by approximately 15-20% compared to standard systems, while specialized cleaning solvents may cost 2-3 times more than conventional mobile phases. These recurring costs accumulate significantly over the instrument's lifecycle, potentially exceeding the initial investment within 2-3 years of regular operation.
Laboratory productivity factors substantially impact the overall economic equation. Automated carryover prevention solutions typically reduce manual intervention by 60-75%, freeing valuable analyst time for other tasks. However, more complex prevention protocols may extend run times by 10-30%, reducing daily sample throughput. This throughput reduction must be carefully balanced against the improved data quality and reduced need for sample reanalysis.
The financial benefits of effective carryover prevention extend beyond direct laboratory costs. Reliable analytical results minimize costly reanalysis cycles, which typically account for 5-15% of laboratory expenses in methods plagued by carryover issues. Furthermore, enhanced data integrity reduces regulatory compliance risks, potentially avoiding significant remediation costs that can reach hundreds of thousands of dollars in regulated environments.
Return on investment calculations indicate that comprehensive carryover prevention strategies typically achieve breakeven within 12-18 months in high-throughput environments. The most economically efficient approaches combine targeted hardware modifications with optimized procedural controls, achieving approximately 85-95% carryover reduction while minimizing both capital expenditure and operational costs.
Risk mitigation value must also be quantified when evaluating prevention strategies. Failed analyses due to carryover can delay critical decision-making in pharmaceutical development, potentially costing $10,000-50,000 per day in delayed product development. In clinical settings, carryover-induced errors may necessitate patient recall and retesting, incurring both direct costs and reputational damage that far exceeds the investment in prevention technologies.
Operational expenses represent a critical consideration in the long-term economics of carryover prevention. Advanced autosampler technologies with dedicated wash stations increase power consumption by approximately 15-20% compared to standard systems, while specialized cleaning solvents may cost 2-3 times more than conventional mobile phases. These recurring costs accumulate significantly over the instrument's lifecycle, potentially exceeding the initial investment within 2-3 years of regular operation.
Laboratory productivity factors substantially impact the overall economic equation. Automated carryover prevention solutions typically reduce manual intervention by 60-75%, freeing valuable analyst time for other tasks. However, more complex prevention protocols may extend run times by 10-30%, reducing daily sample throughput. This throughput reduction must be carefully balanced against the improved data quality and reduced need for sample reanalysis.
The financial benefits of effective carryover prevention extend beyond direct laboratory costs. Reliable analytical results minimize costly reanalysis cycles, which typically account for 5-15% of laboratory expenses in methods plagued by carryover issues. Furthermore, enhanced data integrity reduces regulatory compliance risks, potentially avoiding significant remediation costs that can reach hundreds of thousands of dollars in regulated environments.
Return on investment calculations indicate that comprehensive carryover prevention strategies typically achieve breakeven within 12-18 months in high-throughput environments. The most economically efficient approaches combine targeted hardware modifications with optimized procedural controls, achieving approximately 85-95% carryover reduction while minimizing both capital expenditure and operational costs.
Risk mitigation value must also be quantified when evaluating prevention strategies. Failed analyses due to carryover can delay critical decision-making in pharmaceutical development, potentially costing $10,000-50,000 per day in delayed product development. In clinical settings, carryover-induced errors may necessitate patient recall and retesting, incurring both direct costs and reputational damage that far exceeds the investment in prevention technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!