HPLC-MS Sample Prep: Protein Precipitation, SPE And Recovery
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Sample Prep Evolution and Objectives
High-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) has evolved significantly over the past decades, transforming from a specialized analytical technique to a cornerstone methodology in pharmaceutical research, clinical diagnostics, and various scientific disciplines. The journey began in the 1970s with rudimentary HPLC systems that were later integrated with mass spectrometers, creating powerful analytical platforms capable of identifying and quantifying complex biological compounds with unprecedented precision.
Sample preparation, a critical pre-analytical step in HPLC-MS workflows, has undergone parallel evolution to address the increasing demands for sensitivity, specificity, and throughput. Early methods relied heavily on manual processes with limited standardization, resulting in variable recovery rates and analytical inconsistencies. The introduction of protein precipitation techniques in the 1980s marked a significant advancement, allowing for the removal of interfering proteins from biological matrices such as plasma and serum.
Solid-phase extraction (SPE) emerged in the 1990s as a more sophisticated approach, offering improved selectivity and recovery compared to traditional liquid-liquid extraction methods. The development of various sorbent materials, including reversed-phase, ion-exchange, and mixed-mode phases, expanded the application scope of SPE across diverse analyte classes and sample matrices.
The early 2000s witnessed the automation of sample preparation workflows, reducing manual intervention and enhancing reproducibility. Parallel processing platforms and robotic systems enabled high-throughput analysis, addressing the growing demands of pharmaceutical development and clinical laboratories. Concurrently, miniaturization trends led to micro-extraction techniques that required smaller sample volumes and reduced solvent consumption, aligning with green chemistry principles.
Recent technological advancements have focused on integrating multiple sample preparation steps into unified workflows, minimizing sample loss and contamination risks. Novel materials such as molecularly imprinted polymers (MIPs) and nanostructured sorbents have pushed the boundaries of extraction efficiency and selectivity. Additionally, the development of online SPE systems directly coupled to HPLC-MS has streamlined analytical processes, reducing analysis time and enhancing sensitivity.
The primary objectives of modern HPLC-MS sample preparation include maximizing analyte recovery while effectively removing matrix interferences, ensuring method robustness across diverse sample types, minimizing manual handling to reduce variability, and developing environmentally sustainable protocols with reduced solvent consumption. These goals drive ongoing innovation in protein precipitation methodologies, SPE technologies, and recovery enhancement strategies, ultimately aiming to improve the accuracy, precision, and applicability of HPLC-MS analyses across scientific and clinical domains.
Sample preparation, a critical pre-analytical step in HPLC-MS workflows, has undergone parallel evolution to address the increasing demands for sensitivity, specificity, and throughput. Early methods relied heavily on manual processes with limited standardization, resulting in variable recovery rates and analytical inconsistencies. The introduction of protein precipitation techniques in the 1980s marked a significant advancement, allowing for the removal of interfering proteins from biological matrices such as plasma and serum.
Solid-phase extraction (SPE) emerged in the 1990s as a more sophisticated approach, offering improved selectivity and recovery compared to traditional liquid-liquid extraction methods. The development of various sorbent materials, including reversed-phase, ion-exchange, and mixed-mode phases, expanded the application scope of SPE across diverse analyte classes and sample matrices.
The early 2000s witnessed the automation of sample preparation workflows, reducing manual intervention and enhancing reproducibility. Parallel processing platforms and robotic systems enabled high-throughput analysis, addressing the growing demands of pharmaceutical development and clinical laboratories. Concurrently, miniaturization trends led to micro-extraction techniques that required smaller sample volumes and reduced solvent consumption, aligning with green chemistry principles.
Recent technological advancements have focused on integrating multiple sample preparation steps into unified workflows, minimizing sample loss and contamination risks. Novel materials such as molecularly imprinted polymers (MIPs) and nanostructured sorbents have pushed the boundaries of extraction efficiency and selectivity. Additionally, the development of online SPE systems directly coupled to HPLC-MS has streamlined analytical processes, reducing analysis time and enhancing sensitivity.
The primary objectives of modern HPLC-MS sample preparation include maximizing analyte recovery while effectively removing matrix interferences, ensuring method robustness across diverse sample types, minimizing manual handling to reduce variability, and developing environmentally sustainable protocols with reduced solvent consumption. These goals drive ongoing innovation in protein precipitation methodologies, SPE technologies, and recovery enhancement strategies, ultimately aiming to improve the accuracy, precision, and applicability of HPLC-MS analyses across scientific and clinical domains.
Market Demand Analysis for Advanced Sample Preparation
The global market for advanced sample preparation technologies in HPLC-MS analysis has been experiencing robust growth, driven primarily by increasing demand for high-precision analytical methods in pharmaceutical research, clinical diagnostics, and food safety testing. Current market valuations indicate that the sample preparation segment for chromatography represents approximately 20% of the overall analytical instrumentation market, with consistent annual growth rates between 5-7% over the past five years.
Protein precipitation, solid-phase extraction (SPE), and recovery optimization techniques have become critical components in the analytical workflow, particularly as sensitivity requirements continue to escalate across industries. Pharmaceutical companies are increasingly investing in advanced sample preparation technologies to enhance drug development processes, reduce time-to-market, and comply with stringent regulatory requirements for bioanalytical method validation.
The clinical diagnostics sector demonstrates particularly strong demand growth, with hospitals and reference laboratories seeking more efficient sample preparation methods to handle increasing test volumes while maintaining accuracy. This sector's demand is projected to grow at nearly twice the rate of the overall market due to the expanding role of LC-MS in clinical applications such as therapeutic drug monitoring, vitamin D testing, and steroid analysis.
Contract research organizations (CROs) represent another significant market segment, as they handle large volumes of samples requiring consistent preparation methodologies. These organizations prioritize technologies that offer automation capabilities, reproducibility, and high-throughput processing to maintain competitive advantages in the outsourced research landscape.
Regional analysis reveals that North America currently dominates the market share for advanced sample preparation technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly in China and India, where expanding pharmaceutical industries and increasing adoption of LC-MS technologies in research institutions are creating substantial market opportunities.
End-user feedback indicates growing demand for integrated sample preparation solutions that reduce manual handling steps, minimize sample loss, and increase reproducibility. Specifically, there is heightened interest in automated systems that can perform multiple preparation techniques within a single platform, addressing the workflow bottlenecks commonly associated with manual sample preparation processes.
The market is also witnessing increased demand for consumables and reagents optimized for specific analytical challenges, such as phospholipid removal columns for plasma analysis and specialized protein precipitation reagents that maximize analyte recovery while effectively removing matrix interferences.
Protein precipitation, solid-phase extraction (SPE), and recovery optimization techniques have become critical components in the analytical workflow, particularly as sensitivity requirements continue to escalate across industries. Pharmaceutical companies are increasingly investing in advanced sample preparation technologies to enhance drug development processes, reduce time-to-market, and comply with stringent regulatory requirements for bioanalytical method validation.
The clinical diagnostics sector demonstrates particularly strong demand growth, with hospitals and reference laboratories seeking more efficient sample preparation methods to handle increasing test volumes while maintaining accuracy. This sector's demand is projected to grow at nearly twice the rate of the overall market due to the expanding role of LC-MS in clinical applications such as therapeutic drug monitoring, vitamin D testing, and steroid analysis.
Contract research organizations (CROs) represent another significant market segment, as they handle large volumes of samples requiring consistent preparation methodologies. These organizations prioritize technologies that offer automation capabilities, reproducibility, and high-throughput processing to maintain competitive advantages in the outsourced research landscape.
Regional analysis reveals that North America currently dominates the market share for advanced sample preparation technologies, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly in China and India, where expanding pharmaceutical industries and increasing adoption of LC-MS technologies in research institutions are creating substantial market opportunities.
End-user feedback indicates growing demand for integrated sample preparation solutions that reduce manual handling steps, minimize sample loss, and increase reproducibility. Specifically, there is heightened interest in automated systems that can perform multiple preparation techniques within a single platform, addressing the workflow bottlenecks commonly associated with manual sample preparation processes.
The market is also witnessing increased demand for consumables and reagents optimized for specific analytical challenges, such as phospholipid removal columns for plasma analysis and specialized protein precipitation reagents that maximize analyte recovery while effectively removing matrix interferences.
Current Challenges in Protein Precipitation and SPE
Despite significant advancements in HPLC-MS sample preparation techniques, protein precipitation and solid-phase extraction (SPE) continue to face several critical challenges that impact analytical performance and reliability. The complexity of biological matrices presents a fundamental obstacle, as diverse proteins, lipids, salts, and metabolites can interfere with target analyte isolation. This matrix complexity often leads to inconsistent precipitation efficiency across different sample types, making standardization difficult.
Recovery rates remain problematic in both protein precipitation and SPE workflows. Analyte loss during precipitation can be substantial, particularly for hydrophilic compounds that may co-precipitate with proteins or remain bound to protein structures. Similarly, SPE methods struggle with recovery optimization across compounds with varying physicochemical properties, creating a challenging balance between selective retention and complete elution.
Method reproducibility presents another significant hurdle. Minor variations in precipitation conditions—including solvent composition, temperature, pH, and incubation time—can dramatically affect precipitation efficiency and selectivity. For SPE, batch-to-batch variability in sorbent materials and manufacturing inconsistencies contribute to method reliability issues, particularly in multi-laboratory settings or long-term studies.
Automation compatibility remains limited for protein precipitation techniques, which often involve manual steps like vortexing, centrifugation, and supernatant transfer that are difficult to standardize across operators. While SPE has seen greater automation progress, challenges persist in miniaturization and integration with high-throughput workflows without compromising extraction performance.
Environmental and economic considerations are increasingly important. Traditional protein precipitation methods rely heavily on organic solvents that pose disposal challenges and environmental concerns. SPE cartridges generate significant plastic waste and often require substantial solvent volumes, contradicting sustainability initiatives in analytical laboratories.
Emerging complex analytes, including large biomolecules, peptides, and protein biomarkers, present unique challenges for conventional precipitation and SPE approaches. These analytes often exhibit unpredictable behavior during sample preparation, with issues including non-specific binding, conformational changes, and degradation that compromise analytical integrity.
The integration of these sample preparation techniques with increasingly sensitive MS detection systems reveals additional challenges. As detection limits continue to decrease, even minor contaminants or matrix effects become significant, requiring more selective and efficient sample clean-up strategies that maintain high recovery while eliminating interfering compounds.
Recovery rates remain problematic in both protein precipitation and SPE workflows. Analyte loss during precipitation can be substantial, particularly for hydrophilic compounds that may co-precipitate with proteins or remain bound to protein structures. Similarly, SPE methods struggle with recovery optimization across compounds with varying physicochemical properties, creating a challenging balance between selective retention and complete elution.
Method reproducibility presents another significant hurdle. Minor variations in precipitation conditions—including solvent composition, temperature, pH, and incubation time—can dramatically affect precipitation efficiency and selectivity. For SPE, batch-to-batch variability in sorbent materials and manufacturing inconsistencies contribute to method reliability issues, particularly in multi-laboratory settings or long-term studies.
Automation compatibility remains limited for protein precipitation techniques, which often involve manual steps like vortexing, centrifugation, and supernatant transfer that are difficult to standardize across operators. While SPE has seen greater automation progress, challenges persist in miniaturization and integration with high-throughput workflows without compromising extraction performance.
Environmental and economic considerations are increasingly important. Traditional protein precipitation methods rely heavily on organic solvents that pose disposal challenges and environmental concerns. SPE cartridges generate significant plastic waste and often require substantial solvent volumes, contradicting sustainability initiatives in analytical laboratories.
Emerging complex analytes, including large biomolecules, peptides, and protein biomarkers, present unique challenges for conventional precipitation and SPE approaches. These analytes often exhibit unpredictable behavior during sample preparation, with issues including non-specific binding, conformational changes, and degradation that compromise analytical integrity.
The integration of these sample preparation techniques with increasingly sensitive MS detection systems reveals additional challenges. As detection limits continue to decrease, even minor contaminants or matrix effects become significant, requiring more selective and efficient sample clean-up strategies that maintain high recovery while eliminating interfering compounds.
Current Protein Precipitation and SPE Methodologies
01 Solid phase extraction techniques for sample preparation
Solid phase extraction (SPE) is a widely used technique for sample preparation in HPLC-MS analysis. It involves the separation of analytes from a liquid sample by sorption onto a solid phase, followed by elution with a suitable solvent. This technique can significantly improve sample recovery by removing interfering compounds and concentrating the analytes of interest. Various sorbent materials can be used depending on the nature of the analytes, including C18, ion exchange resins, and molecularly imprinted polymers.- Solid phase extraction techniques for sample preparation: Solid phase extraction (SPE) is a widely used technique for sample preparation in HPLC-MS analysis to improve recovery rates. This method involves the use of specialized sorbents to selectively retain analytes while removing interfering matrix components. The technique can be optimized by selecting appropriate sorbent materials, conditioning protocols, and elution solvents to maximize recovery of target compounds. Various modifications of SPE, including online SPE and micro-SPE, have been developed to enhance efficiency and recovery rates for different types of analytes.
- Protein precipitation methods for biological samples: Protein precipitation is a critical step in preparing biological samples for HPLC-MS analysis, particularly for plasma, serum, or tissue samples. This technique involves the use of organic solvents (such as acetonitrile, methanol), acids, or salts to precipitate proteins while keeping analytes in solution. The efficiency of protein removal directly impacts analyte recovery and instrument performance. Optimized protocols include specific solvent-to-sample ratios, temperature conditions, and centrifugation parameters to maximize analyte recovery while ensuring complete protein removal.
- Liquid-liquid extraction optimization for recovery enhancement: Liquid-liquid extraction (LLE) techniques are fundamental in HPLC-MS sample preparation, particularly for complex matrices. The method involves partitioning analytes between two immiscible liquid phases based on their solubility differences. Recovery rates can be significantly improved by optimizing parameters such as solvent selection, pH adjustment, salt addition, and extraction time. Modified approaches like salting-out assisted liquid-liquid extraction (SALLE) and dispersive liquid-liquid microextraction (DLLME) have been developed to enhance recovery efficiency while reducing solvent consumption.
- Internal standard calibration for accurate recovery assessment: The use of internal standards is essential for accurate quantification and recovery assessment in HPLC-MS analysis. Isotopically labeled analogs of target compounds are particularly effective as they compensate for variations in extraction efficiency, matrix effects, and instrument response. The internal standard should be added at the earliest possible stage of sample preparation to account for all potential losses during the process. This approach enables precise calculation of absolute recovery rates and improves the reliability of quantitative results across different sample batches.
- Matrix-matched calibration and standard addition methods: Matrix-matched calibration and standard addition methods are advanced approaches to compensate for matrix effects and improve recovery in complex samples. These techniques involve preparing calibration standards in blank matrix identical or similar to the sample matrix, or adding known amounts of analytes to the sample itself. This compensates for matrix-induced signal suppression or enhancement that can affect recovery calculations. The methods are particularly valuable for environmental, food, and biological samples where matrix complexity significantly impacts analyte recovery and detection.
02 Protein precipitation methods for biological samples
Protein precipitation is a crucial step in preparing biological samples for HPLC-MS analysis. This technique involves the addition of organic solvents, acids, or salts to precipitate proteins, which can interfere with chromatographic separation and mass spectrometric detection. Commonly used precipitants include acetonitrile, methanol, and trichloroacetic acid. The efficiency of protein removal directly impacts sample recovery and analytical sensitivity. Optimized precipitation protocols can significantly enhance the recovery of target analytes from complex biological matrices.Expand Specific Solutions03 Liquid-liquid extraction optimization for improved recovery
Liquid-liquid extraction (LLE) is a fundamental sample preparation technique that separates compounds based on their relative solubilities in two immiscible liquids. For HPLC-MS applications, optimizing parameters such as solvent selection, pH adjustment, salt addition, and extraction time can significantly improve analyte recovery. Modified LLE techniques, including salting-out assisted liquid-liquid extraction (SALLE) and dispersive liquid-liquid microextraction (DLLME), have been developed to enhance extraction efficiency while reducing solvent consumption and processing time.Expand Specific Solutions04 Internal standard selection and matrix effect compensation
The selection of appropriate internal standards is critical for accurate quantification and improved recovery in HPLC-MS analysis. Stable isotope-labeled internal standards that closely match the physicochemical properties of target analytes can effectively compensate for matrix effects and variations in extraction efficiency. Matrix effects, which can suppress or enhance ionization in the mass spectrometer, significantly impact analyte recovery. Various approaches to assess and mitigate matrix effects include post-extraction addition, standard addition, and matrix-matched calibration.Expand Specific Solutions05 Automated sample preparation systems for reproducible recovery
Automated sample preparation systems offer improved reproducibility and recovery for HPLC-MS analysis compared to manual methods. These systems can perform multiple sample preparation steps including extraction, filtration, derivatization, and dilution with minimal human intervention. Automation reduces operator-dependent variability, minimizes sample loss during transfer steps, and ensures consistent extraction conditions across batches. Various platforms ranging from simple liquid handling workstations to fully integrated robotic systems have been developed to enhance sample throughput while maintaining high recovery rates.Expand Specific Solutions
Key Industry Players in HPLC-MS Sample Prep
HPLC-MS sample preparation for protein analysis is currently in a growth phase, with the market expanding due to increasing applications in pharmaceutical, biotechnology, and clinical research. The global market size for protein sample preparation is estimated to reach $1.5 billion by 2025, driven by rising demand for proteomics research. Technologically, the field is maturing with established methods like protein precipitation and solid-phase extraction (SPE), but innovations continue to emerge. Leading companies like Agilent Technologies, Biotage AB, and EMD Millipore have developed advanced solutions offering improved recovery rates and workflow efficiency. Newer entrants such as DPX Technologies and Proteoform Scientific are introducing innovative approaches to enhance protein recovery and purity, while established pharmaceutical companies like Amgen and Novo Nordisk are investing in optimizing these techniques for their drug development pipelines.
Dpx Technologies LLC
Technical Solution: DPX Technologies has revolutionized HPLC-MS sample preparation with their patented Dispersive Pipette XTRaction (DPX) technology. This innovative approach uses loosely packed sorbent beds within pipette tips to enable rapid solid-phase extraction with significantly reduced processing times. For protein precipitation applications, DPX has developed specialized MEP (Molecularly Engineered Polymers) sorbents that selectively bind proteins while allowing small molecules to pass through, achieving efficient cleanup without traditional precipitation and centrifugation steps. Their technology enables complete sample preparation in as little as 2-3 minutes per sample, compared to 30+ minutes with conventional methods. DPX's approach also dramatically reduces solvent consumption (typically 200-500 μL versus several mL with traditional SPE), making it more environmentally friendly and cost-effective. The company has demonstrated recovery rates exceeding 90% for many analyte classes, with exceptional reproducibility (RSD values typically <5%). Their solutions are compatible with both manual processing and automated liquid handling platforms, offering flexibility across different laboratory settings.
Strengths: Extremely rapid processing times increase laboratory throughput. Minimal solvent usage reduces operational costs and environmental impact. Weaknesses: The dispersive extraction approach may provide less thorough cleanup than traditional SPE for certain highly complex matrices.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced HPLC-MS sample preparation solutions focusing on automated protein precipitation and solid-phase extraction (SPE) workflows. Their Bravo Automated Liquid Handling Platform integrates with AssayMAP microchromatography technology to provide high-throughput protein sample preparation with minimal manual intervention. The system employs specialized cartridges packed with proprietary sorbents that enable efficient protein cleanup while maintaining high recovery rates (typically >85% for most proteins). Agilent's approach combines traditional protein precipitation methods with innovative SPE technologies that reduce matrix effects and improve analytical sensitivity. Their RapidFire system further enhances throughput by enabling direct coupling of SPE to MS analysis without chromatographic separation, reducing sample preparation time from hours to minutes while maintaining data quality comparable to traditional HPLC-MS methods.
Strengths: High throughput automation reduces manual handling errors and increases reproducibility. Integration of multiple sample prep techniques in unified workflows improves efficiency. Weaknesses: Proprietary consumables increase operational costs, and the sophisticated instrumentation requires specialized training and maintenance.
Critical Technologies for Recovery Enhancement
Compositions and methods for combining protein precipitation and solid phase extraction
PatentActiveUS10928366B2
Innovation
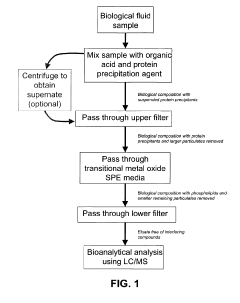
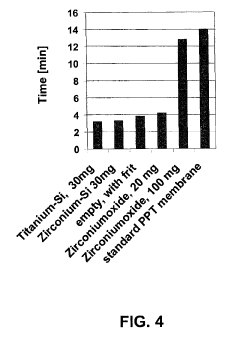
- A novel SPE media is developed by bonding transition metal oxides like zirconia, titania, or ceria onto porous silica substrates, which selectively bind phosphate-containing compounds, including phospholipids, thereby reducing ion-suppression and improving the selectivity of sample preparation.
Device for solid phase extraction and method for purifying samples prior to analysis
PatentInactiveEP1485179A1
Innovation
- The SPE device features a tapered well geometry with a large bed height to top diameter ratio and spherical filters, allowing for efficient retention and minimal elution volumes, eliminating the need for evaporation and reconstitution steps by eluting target compounds in small volumes of organic solvent, which can be directly diluted with aqueous solutions for analysis.
Method Validation Standards and Protocols
Method validation is a critical component in the development and implementation of HPLC-MS sample preparation techniques involving protein precipitation, solid-phase extraction (SPE), and recovery assessment. The validation process must adhere to established regulatory guidelines such as those provided by the FDA, EMA, and ICH, which outline specific criteria for analytical method validation in bioanalytical applications.
The validation of protein precipitation methods requires assessment of several key parameters. Recovery efficiency must be evaluated across multiple concentration levels, typically at the lower limit of quantification (LLOQ), middle, and upper concentration range. Consistency in recovery rates is often more critical than achieving 100% recovery, as consistent extraction allows for reliable quantification through calibration.
Matrix effects represent another crucial validation parameter, particularly for complex biological samples. Ion suppression or enhancement can significantly impact the accuracy of HPLC-MS analysis. These effects should be quantified using post-extraction spike methods compared against neat solution standards, with acceptance criteria typically requiring matrix factor variations below 15%.
For SPE methods, additional validation parameters include breakthrough volume determination and wash solvent optimization. Breakthrough volume validation ensures that analytes are effectively retained during the loading step, while wash solvent validation confirms that interferences are removed without premature elution of target analytes.
Selectivity and specificity validation must demonstrate that the sample preparation method can effectively isolate the analytes of interest from potential interferences. This typically involves analyzing blank matrix samples from multiple sources to ensure no significant interfering peaks co-elute with the analytes of interest.
Stability testing represents another critical aspect of method validation, encompassing bench-top stability, freeze-thaw stability, and long-term storage stability. These tests ensure that analytes remain stable throughout the sample preparation process and subsequent analysis.
Robustness testing evaluates the method's reliability when faced with small but deliberate variations in procedural parameters. For protein precipitation, this might include variations in solvent composition or precipitation time, while for SPE, variations in conditioning steps or elution solvent composition may be evaluated.
The validation protocol should also include system suitability tests to ensure consistent chromatographic performance, including parameters such as retention time reproducibility, peak shape, and resolution between closely eluting compounds.
The validation of protein precipitation methods requires assessment of several key parameters. Recovery efficiency must be evaluated across multiple concentration levels, typically at the lower limit of quantification (LLOQ), middle, and upper concentration range. Consistency in recovery rates is often more critical than achieving 100% recovery, as consistent extraction allows for reliable quantification through calibration.
Matrix effects represent another crucial validation parameter, particularly for complex biological samples. Ion suppression or enhancement can significantly impact the accuracy of HPLC-MS analysis. These effects should be quantified using post-extraction spike methods compared against neat solution standards, with acceptance criteria typically requiring matrix factor variations below 15%.
For SPE methods, additional validation parameters include breakthrough volume determination and wash solvent optimization. Breakthrough volume validation ensures that analytes are effectively retained during the loading step, while wash solvent validation confirms that interferences are removed without premature elution of target analytes.
Selectivity and specificity validation must demonstrate that the sample preparation method can effectively isolate the analytes of interest from potential interferences. This typically involves analyzing blank matrix samples from multiple sources to ensure no significant interfering peaks co-elute with the analytes of interest.
Stability testing represents another critical aspect of method validation, encompassing bench-top stability, freeze-thaw stability, and long-term storage stability. These tests ensure that analytes remain stable throughout the sample preparation process and subsequent analysis.
Robustness testing evaluates the method's reliability when faced with small but deliberate variations in procedural parameters. For protein precipitation, this might include variations in solvent composition or precipitation time, while for SPE, variations in conditioning steps or elution solvent composition may be evaluated.
The validation protocol should also include system suitability tests to ensure consistent chromatographic performance, including parameters such as retention time reproducibility, peak shape, and resolution between closely eluting compounds.
Automation Trends in Sample Preparation
Automation in sample preparation for HPLC-MS analysis has evolved significantly in recent years, particularly for protein precipitation, solid-phase extraction (SPE), and recovery processes. The integration of robotics and automated liquid handling systems has revolutionized laboratory workflows, reducing manual intervention while enhancing reproducibility and throughput. Modern automated systems can now perform multiple sample preparation steps in sequence, from protein precipitation to SPE and final sample reconstitution, with minimal human oversight.
Microfluidic technologies represent a major advancement in this field, enabling miniaturization of sample preparation processes. These systems utilize small channels and chambers to perform precipitation and extraction on microscale volumes, significantly reducing sample and solvent requirements while maintaining analytical performance. The integration of microfluidic chips with detection systems creates powerful lab-on-a-chip solutions that streamline complex bioanalytical workflows.
Machine learning algorithms are increasingly being incorporated into automated sample preparation systems, optimizing parameters for protein precipitation and SPE based on sample characteristics. These intelligent systems can adapt protocols in real-time, adjusting variables such as solvent ratios, mixing times, and extraction conditions to maximize protein recovery and minimize matrix effects. This adaptive approach represents a paradigm shift from traditional fixed protocols to dynamic, sample-specific methodologies.
High-throughput screening facilities have adopted parallel processing architectures, where multiple samples undergo preparation simultaneously. These systems typically feature 96-well or 384-well plate formats with coordinated liquid handling robots that can process hundreds of samples per day. The integration of barcode tracking and laboratory information management systems (LIMS) ensures sample traceability throughout the preparation workflow, reducing errors and enhancing data integrity.
Online sample preparation represents another significant trend, where sample preparation is directly coupled to analytical instrumentation. These hyphenated systems perform protein precipitation and SPE automatically before injection into the HPLC-MS system, eliminating manual transfer steps and reducing sample exposure to environmental contaminants. The development of column-switching technologies and trap-and-elute configurations has further enhanced this approach, allowing for automated clean-up and pre-concentration of samples.
Miniaturized SPE formats, including pipette tips with embedded sorbent materials and micro-SPE cartridges, are gaining popularity for their compatibility with automated liquid handlers. These formats enable selective extraction and concentration of target analytes from complex biological matrices with minimal sample volume, addressing the challenges of limited sample availability in clinical and biomarker research applications.
Microfluidic technologies represent a major advancement in this field, enabling miniaturization of sample preparation processes. These systems utilize small channels and chambers to perform precipitation and extraction on microscale volumes, significantly reducing sample and solvent requirements while maintaining analytical performance. The integration of microfluidic chips with detection systems creates powerful lab-on-a-chip solutions that streamline complex bioanalytical workflows.
Machine learning algorithms are increasingly being incorporated into automated sample preparation systems, optimizing parameters for protein precipitation and SPE based on sample characteristics. These intelligent systems can adapt protocols in real-time, adjusting variables such as solvent ratios, mixing times, and extraction conditions to maximize protein recovery and minimize matrix effects. This adaptive approach represents a paradigm shift from traditional fixed protocols to dynamic, sample-specific methodologies.
High-throughput screening facilities have adopted parallel processing architectures, where multiple samples undergo preparation simultaneously. These systems typically feature 96-well or 384-well plate formats with coordinated liquid handling robots that can process hundreds of samples per day. The integration of barcode tracking and laboratory information management systems (LIMS) ensures sample traceability throughout the preparation workflow, reducing errors and enhancing data integrity.
Online sample preparation represents another significant trend, where sample preparation is directly coupled to analytical instrumentation. These hyphenated systems perform protein precipitation and SPE automatically before injection into the HPLC-MS system, eliminating manual transfer steps and reducing sample exposure to environmental contaminants. The development of column-switching technologies and trap-and-elute configurations has further enhanced this approach, allowing for automated clean-up and pre-concentration of samples.
Miniaturized SPE formats, including pipette tips with embedded sorbent materials and micro-SPE cartridges, are gaining popularity for their compatibility with automated liquid handlers. These formats enable selective extraction and concentration of target analytes from complex biological matrices with minimal sample volume, addressing the challenges of limited sample availability in clinical and biomarker research applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!