HPLC-MS Ionization: ESI/APCI/APPI Selection, Source Conditions And Robustness
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC-MS Ionization Technology Background and Objectives
High-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) has evolved significantly since its inception in the 1970s, revolutionizing analytical chemistry across pharmaceutical, environmental, food safety, and clinical diagnostics sectors. The ionization interface between HPLC and MS represents a critical technological component that determines analytical sensitivity, selectivity, and applicability to diverse compound classes.
The evolution of ionization techniques has progressed from early thermospray and particle beam interfaces to the current dominance of atmospheric pressure ionization (API) methods. Electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photoionization (APPI) have emerged as the three principal API techniques, each with distinct mechanisms and optimal application domains.
ESI, developed by John Fenn in the 1980s, has become the most widely utilized ionization technique due to its exceptional capability to analyze polar, thermally labile, and high molecular weight compounds. APCI, introduced commercially in the early 1990s, excels with moderately polar to non-polar compounds and offers complementary coverage to ESI. APPI, the newest addition introduced in the early 2000s, extends the analytical range to highly non-polar compounds previously challenging to ionize.
The technological trajectory has been driven by demands for increased sensitivity, broader compound coverage, enhanced robustness, and improved quantitative performance. Recent innovations focus on dual and multimode ionization sources that combine multiple ionization mechanisms in a single platform, allowing simultaneous analysis of compounds with diverse physicochemical properties.
Current technical challenges include optimizing source conditions for maximum sensitivity while maintaining robustness, reducing matrix effects in complex samples, improving ionization efficiency for challenging compounds, and developing intelligent software algorithms for automated source parameter optimization.
The primary objective of this technical research is to establish systematic guidelines for selecting the optimal ionization technique (ESI, APCI, or APPI) based on analyte properties and analytical requirements. Additionally, we aim to identify critical source parameters that influence ionization efficiency and robustness across diverse sample matrices and develop strategies to enhance method transferability between instruments and laboratories.
Future technological developments are expected to focus on miniaturization of ionization sources, ambient ionization techniques requiring minimal sample preparation, integration with novel separation technologies, and machine learning approaches for predictive ionization behavior modeling. These advancements will further expand the application scope of HPLC-MS in emerging fields such as single-cell analysis, high-throughput screening, and point-of-care diagnostics.
The evolution of ionization techniques has progressed from early thermospray and particle beam interfaces to the current dominance of atmospheric pressure ionization (API) methods. Electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photoionization (APPI) have emerged as the three principal API techniques, each with distinct mechanisms and optimal application domains.
ESI, developed by John Fenn in the 1980s, has become the most widely utilized ionization technique due to its exceptional capability to analyze polar, thermally labile, and high molecular weight compounds. APCI, introduced commercially in the early 1990s, excels with moderately polar to non-polar compounds and offers complementary coverage to ESI. APPI, the newest addition introduced in the early 2000s, extends the analytical range to highly non-polar compounds previously challenging to ionize.
The technological trajectory has been driven by demands for increased sensitivity, broader compound coverage, enhanced robustness, and improved quantitative performance. Recent innovations focus on dual and multimode ionization sources that combine multiple ionization mechanisms in a single platform, allowing simultaneous analysis of compounds with diverse physicochemical properties.
Current technical challenges include optimizing source conditions for maximum sensitivity while maintaining robustness, reducing matrix effects in complex samples, improving ionization efficiency for challenging compounds, and developing intelligent software algorithms for automated source parameter optimization.
The primary objective of this technical research is to establish systematic guidelines for selecting the optimal ionization technique (ESI, APCI, or APPI) based on analyte properties and analytical requirements. Additionally, we aim to identify critical source parameters that influence ionization efficiency and robustness across diverse sample matrices and develop strategies to enhance method transferability between instruments and laboratories.
Future technological developments are expected to focus on miniaturization of ionization sources, ambient ionization techniques requiring minimal sample preparation, integration with novel separation technologies, and machine learning approaches for predictive ionization behavior modeling. These advancements will further expand the application scope of HPLC-MS in emerging fields such as single-cell analysis, high-throughput screening, and point-of-care diagnostics.
Market Applications and Analytical Demands
The HPLC-MS ionization market is experiencing robust growth driven by increasing demand across pharmaceutical, biotechnology, environmental monitoring, food safety, and clinical diagnostics sectors. The global HPLC-MS market was valued at approximately $4.2 billion in 2022, with a projected compound annual growth rate of 7.8% through 2028, significantly influenced by advancements in ionization technologies.
Pharmaceutical and biotechnology industries represent the largest application segments, collectively accounting for over 60% of the market share. These sectors require highly sensitive and selective analytical methods for drug discovery, development, and quality control processes. The demand for robust ionization techniques capable of analyzing complex biological matrices, including proteins, peptides, and small molecules, continues to drive innovation in ESI, APCI, and APPI technologies.
Environmental monitoring applications have seen substantial growth, particularly in the analysis of persistent organic pollutants, pesticides, and emerging contaminants. Regulatory agencies worldwide have implemented increasingly stringent requirements for detection limits and analytical precision, necessitating more robust and sensitive ionization methods. This sector demands ionization techniques capable of handling diverse sample matrices with minimal matrix effects.
Food safety testing represents another rapidly expanding application area, with particular emphasis on detecting pesticide residues, mycotoxins, and adulterants. The complexity of food matrices presents unique challenges for ionization source stability and robustness, driving demand for advanced source designs and optimization strategies.
Clinical diagnostics and forensic toxicology applications require high-throughput capabilities combined with exceptional sensitivity and specificity. The analysis of biological fluids for therapeutic drug monitoring, doping control, and toxicology screening necessitates ionization techniques that can handle high salt concentrations and biological interferences while maintaining consistent performance.
Across all application areas, there is a growing demand for multi-mode ionization sources that can rapidly switch between ESI, APCI, and APPI without requiring hardware changes or significant downtime. This flexibility allows laboratories to analyze diverse compound classes within a single analytical run, improving efficiency and reducing operational costs.
The analytical demands driving ionization technology development include improved sensitivity for trace analysis, enhanced robustness for complex matrices, reduced matrix effects, broader compound coverage, and simplified method development. Additionally, there is increasing pressure for "green" analytical chemistry approaches that minimize solvent consumption and waste generation, influencing ionization source design and operating parameters.
Pharmaceutical and biotechnology industries represent the largest application segments, collectively accounting for over 60% of the market share. These sectors require highly sensitive and selective analytical methods for drug discovery, development, and quality control processes. The demand for robust ionization techniques capable of analyzing complex biological matrices, including proteins, peptides, and small molecules, continues to drive innovation in ESI, APCI, and APPI technologies.
Environmental monitoring applications have seen substantial growth, particularly in the analysis of persistent organic pollutants, pesticides, and emerging contaminants. Regulatory agencies worldwide have implemented increasingly stringent requirements for detection limits and analytical precision, necessitating more robust and sensitive ionization methods. This sector demands ionization techniques capable of handling diverse sample matrices with minimal matrix effects.
Food safety testing represents another rapidly expanding application area, with particular emphasis on detecting pesticide residues, mycotoxins, and adulterants. The complexity of food matrices presents unique challenges for ionization source stability and robustness, driving demand for advanced source designs and optimization strategies.
Clinical diagnostics and forensic toxicology applications require high-throughput capabilities combined with exceptional sensitivity and specificity. The analysis of biological fluids for therapeutic drug monitoring, doping control, and toxicology screening necessitates ionization techniques that can handle high salt concentrations and biological interferences while maintaining consistent performance.
Across all application areas, there is a growing demand for multi-mode ionization sources that can rapidly switch between ESI, APCI, and APPI without requiring hardware changes or significant downtime. This flexibility allows laboratories to analyze diverse compound classes within a single analytical run, improving efficiency and reducing operational costs.
The analytical demands driving ionization technology development include improved sensitivity for trace analysis, enhanced robustness for complex matrices, reduced matrix effects, broader compound coverage, and simplified method development. Additionally, there is increasing pressure for "green" analytical chemistry approaches that minimize solvent consumption and waste generation, influencing ionization source design and operating parameters.
Current Ionization Techniques and Technical Barriers
The current landscape of HPLC-MS ionization techniques is dominated by three major approaches: Electrospray Ionization (ESI), Atmospheric Pressure Chemical Ionization (APCI), and Atmospheric Pressure Photoionization (APPI). Each technique has established its niche in analytical applications based on compound polarity and molecular characteristics.
ESI remains the most widely adopted technique, particularly effective for polar and ionic compounds. It operates by applying high voltage to a liquid sample in a capillary, creating charged droplets that eventually yield gas-phase ions. While ESI excels with proteins, peptides, and polar metabolites, it struggles with non-polar compounds and exhibits significant matrix effects in complex samples.
APCI addresses some ESI limitations by utilizing a corona discharge needle to ionize vaporized solvent molecules, which subsequently transfer charge to analyte molecules. This technique performs admirably with moderately polar to non-polar compounds but faces challenges with thermally labile molecules and high molecular weight compounds exceeding 1,500 Da.
APPI, the newest among these techniques, employs UV photons to ionize compounds directly or through dopant-mediated processes. It demonstrates superior performance with highly non-polar compounds but requires careful optimization of dopant selection and concentration.
Despite technological advancements, several technical barriers persist across these ionization methods. Ion suppression remains a significant challenge, particularly in ESI, where co-eluting matrix components can dramatically reduce analyte signal intensity. This phenomenon introduces quantification errors and compromises detection limits in complex biological and environmental samples.
Source contamination and carryover present ongoing operational challenges, requiring frequent maintenance and cleaning protocols that increase instrument downtime and operational costs. The accumulation of non-volatile components at ion source interfaces progressively degrades performance and reproducibility.
Robustness and reproducibility issues continue to plague routine applications. Environmental factors such as temperature fluctuations and humidity variations significantly impact day-to-day performance, particularly for ESI. These variations necessitate frequent recalibration and complicate inter-laboratory method transfer.
The optimization paradox represents another significant barrier - parameters optimized for one analyte often compromise the detection of others in multi-analyte methods. This creates substantial challenges for comprehensive metabolomics and exposomics applications where diverse chemical classes must be analyzed simultaneously.
Emerging technical challenges include the need for improved ionization efficiency for extremely low abundance analytes and the development of more universal ionization techniques capable of detecting a broader range of chemical compounds in a single analytical run.
ESI remains the most widely adopted technique, particularly effective for polar and ionic compounds. It operates by applying high voltage to a liquid sample in a capillary, creating charged droplets that eventually yield gas-phase ions. While ESI excels with proteins, peptides, and polar metabolites, it struggles with non-polar compounds and exhibits significant matrix effects in complex samples.
APCI addresses some ESI limitations by utilizing a corona discharge needle to ionize vaporized solvent molecules, which subsequently transfer charge to analyte molecules. This technique performs admirably with moderately polar to non-polar compounds but faces challenges with thermally labile molecules and high molecular weight compounds exceeding 1,500 Da.
APPI, the newest among these techniques, employs UV photons to ionize compounds directly or through dopant-mediated processes. It demonstrates superior performance with highly non-polar compounds but requires careful optimization of dopant selection and concentration.
Despite technological advancements, several technical barriers persist across these ionization methods. Ion suppression remains a significant challenge, particularly in ESI, where co-eluting matrix components can dramatically reduce analyte signal intensity. This phenomenon introduces quantification errors and compromises detection limits in complex biological and environmental samples.
Source contamination and carryover present ongoing operational challenges, requiring frequent maintenance and cleaning protocols that increase instrument downtime and operational costs. The accumulation of non-volatile components at ion source interfaces progressively degrades performance and reproducibility.
Robustness and reproducibility issues continue to plague routine applications. Environmental factors such as temperature fluctuations and humidity variations significantly impact day-to-day performance, particularly for ESI. These variations necessitate frequent recalibration and complicate inter-laboratory method transfer.
The optimization paradox represents another significant barrier - parameters optimized for one analyte often compromise the detection of others in multi-analyte methods. This creates substantial challenges for comprehensive metabolomics and exposomics applications where diverse chemical classes must be analyzed simultaneously.
Emerging technical challenges include the need for improved ionization efficiency for extremely low abundance analytes and the development of more universal ionization techniques capable of detecting a broader range of chemical compounds in a single analytical run.
Comparative Analysis of ESI/APCI/APPI Technologies
01 Ionization technique selection criteria for HPLC-MS
Selection of appropriate ionization techniques (ESI, APCI, APPI) for HPLC-MS depends on the chemical properties of analytes. ESI is suitable for polar and ionic compounds, APCI works well for moderately polar to non-polar compounds, while APPI is effective for highly non-polar compounds. The selection criteria include molecular weight, polarity, thermal stability, and volatility of the target analytes. Proper technique selection enhances sensitivity, selectivity, and reproducibility of analytical methods.- Ionization technique selection criteria for HPLC-MS: Selection of appropriate ionization techniques (ESI, APCI, APPI) for HPLC-MS depends on the analyte properties. ESI is suitable for polar and ionic compounds, APCI works well for moderately polar to non-polar compounds, while APPI is effective for non-polar compounds. The selection criteria include molecular weight, polarity, thermal stability, and volatility of the analytes. Proper technique selection enhances sensitivity, selectivity, and reproducibility of analytical methods.
- Source condition optimization for robust HPLC-MS analysis: Optimizing source conditions is critical for robust HPLC-MS analysis. Key parameters include gas flow rates, temperatures, voltages, and pressures. For ESI, spray voltage, capillary temperature, and sheath gas flow rate are crucial. For APCI, vaporizer temperature and corona discharge current require careful adjustment. For APPI, lamp energy and dopant selection significantly impact ionization efficiency. Systematic optimization of these parameters ensures consistent analyte response and method robustness across different sample matrices.
- Dual and multimode ionization techniques for enhanced coverage: Dual and multimode ionization techniques combine the advantages of different ionization methods to enhance analytical coverage. Systems that can switch between or simultaneously use ESI, APCI, and APPI provide comprehensive detection of compounds with varying physicochemical properties. These approaches are particularly valuable for complex samples containing analytes with diverse polarities and structures. Multimode ionization reduces the need for multiple analyses and improves overall method efficiency.
- Method development strategies for ionization robustness: Developing robust HPLC-MS methods requires systematic approaches to ensure consistent ionization performance. This includes design of experiments (DoE) for parameter optimization, use of internal standards to compensate for matrix effects, and implementation of quality control samples. Strategies such as mobile phase modification, pH adjustment, and addition of ionization modifiers can enhance ionization efficiency and stability. Regular system suitability testing and performance monitoring are essential for maintaining method robustness over time.
- Advanced ionization source designs and modifications: Innovations in ionization source design improve HPLC-MS performance and robustness. These include heated electrospray interfaces, orthogonal spray configurations, and ion funnels that enhance ion transmission. Modified source geometries reduce contamination and carryover while improving desolvation efficiency. Specialized interfaces for nano-flow applications and high-throughput screening have been developed. These advancements address common challenges such as matrix effects, signal suppression, and instrument contamination, leading to more reliable analytical results.
02 Source condition optimization for robust HPLC-MS analysis
Optimizing source conditions is critical for robust HPLC-MS analysis. Key parameters include gas flow rates, temperatures, voltages, and pressures. For ESI, spray voltage, capillary temperature, and sheath gas flow rate significantly impact ionization efficiency. For APCI, vaporizer temperature and corona discharge current are crucial. For APPI, lamp energy and dopant selection affect performance. Systematic optimization of these parameters ensures consistent ionization, reduces matrix effects, and improves method robustness across different sample types.Expand Specific Solutions03 Dual and multimode ionization techniques for comprehensive analysis
Dual and multimode ionization techniques allow simultaneous or rapid switching between different ionization modes (ESI/APCI/APPI) during a single analysis. These approaches provide comprehensive coverage of compounds with varying physicochemical properties without requiring separate analyses. Advanced source designs enable seamless transitions between ionization modes, improving analytical throughput and expanding the range of detectable compounds. This is particularly valuable for complex samples containing analytes with diverse chemical characteristics.Expand Specific Solutions04 Robustness enhancement strategies for HPLC-MS methods
Strategies to enhance robustness in HPLC-MS methods include hardware modifications, software solutions, and methodological approaches. Implementation of reference standards, internal calibration, automated system suitability tests, and regular preventive maintenance improve long-term stability. Advanced algorithms for signal processing and noise reduction help maintain consistent performance. Design of experiments (DoE) approaches identify critical parameters affecting method robustness. These strategies ensure reliable results across different instruments, operators, and environmental conditions.Expand Specific Solutions05 Novel ionization source designs for improved sensitivity and stability
Innovative ionization source designs address limitations of conventional ESI, APCI, and APPI sources. These include modified spray geometries, heated ion transfer tubes, improved desolvation systems, and novel electrode configurations. Advanced designs minimize contamination, reduce ion suppression, and enhance ion transmission efficiency. Some innovations incorporate multiple ionization mechanisms in a single source or utilize auxiliary technologies like ultrasonic nebulization. These developments result in improved sensitivity, wider dynamic range, and greater operational stability for challenging applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The HPLC-MS ionization technology market is currently in a mature growth phase, characterized by established techniques (ESI, APCI, APPI) with ongoing refinements focused on robustness and application-specific optimization. The global market size for mass spectrometry exceeds $4.5 billion, with HPLC-MS systems representing a significant segment experiencing 5-7% annual growth. Leading players include established analytical instrument manufacturers like Agilent Technologies and Thermo Fisher Scientific (via Thermo Finnigan), who offer comprehensive ionization solutions with high technical maturity. Academic institutions (Tsinghua University, University of North Carolina) collaborate with industry to advance fundamental research, while specialized companies like Micromass (Waters subsidiary) focus on innovative ionization technologies. The competitive landscape shows a balance between large integrated providers and niche specialists developing application-specific ionization solutions.
Micromass UK Ltd.
Technical Solution: Micromass (now part of Waters Corporation) has developed the ZSpray™ dual-orthogonal sampling technology for HPLC-MS ionization that significantly reduces contamination and maintenance requirements. Their ionization source design features a unique Z-shaped ion path where the initial spray is perpendicular to the sampling cone, allowing only charged particles to make the turn toward the mass analyzer while neutrals continue straight and are pumped away[1]. This architecture dramatically improves robustness when analyzing complex biological and environmental samples. Micromass's StepWave™ ion-transfer technology further enhances sensitivity by efficiently capturing ions while rejecting neutral contaminants. Their UniSpray™ ionization source utilizes a high-velocity charged solvent stream impacting a target to generate smaller, more highly charged droplets than conventional ESI, extending the range of compounds that can be effectively ionized[2]. For challenging non-polar compounds, Micromass offers atmospheric pressure photoionization (APPI) with optimized lamp geometry and gas flows that achieve ionization efficiencies up to 10 times higher than APCI for certain analyte classes[3].
Strengths: Exceptional source robustness in complex matrices; reduced maintenance requirements; excellent sensitivity for a wide compound range; efficient switching between ionization modes. Weaknesses: More complex initial setup and optimization; higher gas consumption than some competing designs; specialized training required for maximum performance.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed a comprehensive suite of ionization technologies for HPLC-MS applications, including their Jet Stream ESI technology that utilizes heated sheath gas to improve desolvation efficiency and ion focusing. Their dual-mode ESI/APCI source allows seamless switching between ionization modes without hardware changes, maximizing analytical flexibility[1]. Agilent's multimode source (MMI) integrates ESI, APCI, and APPI capabilities in a single unit, enabling simultaneous ionization through different mechanisms for improved compound coverage[2]. Their Agilent 6400 Series Triple Quadrupole LC/MS systems feature optimized ion optics and collision cell technology that enhance sensitivity while maintaining robustness across diverse sample matrices. Agilent has also pioneered source condition optimization software that automatically tunes parameters like nebulizer pressure, drying gas temperature, and capillary voltage based on compound characteristics[3].
Strengths: Superior robustness in high-salt matrices; excellent source-to-source reproducibility; comprehensive software for method development and optimization. Weaknesses: Higher initial investment compared to some competitors; some specialized ionization techniques require additional hardware modules; optimization complexity for multi-compound analyses.
Critical Patents and Innovations in Ionization Source Design
Ion source for a mass spectrometer
PatentInactiveEP1819423A2
Innovation
- An atmospheric pressure ionization source that can ionize both liquid and gaseous effluents, using a combination of electric discharge, photoionization, and electrospray methods, with the introduction of a dry purge gas to minimize water and organic contaminants, and reactive gases to selectively ionize compounds, allowing rapid switching between LC/MS and GC/MS operations on a single instrument.
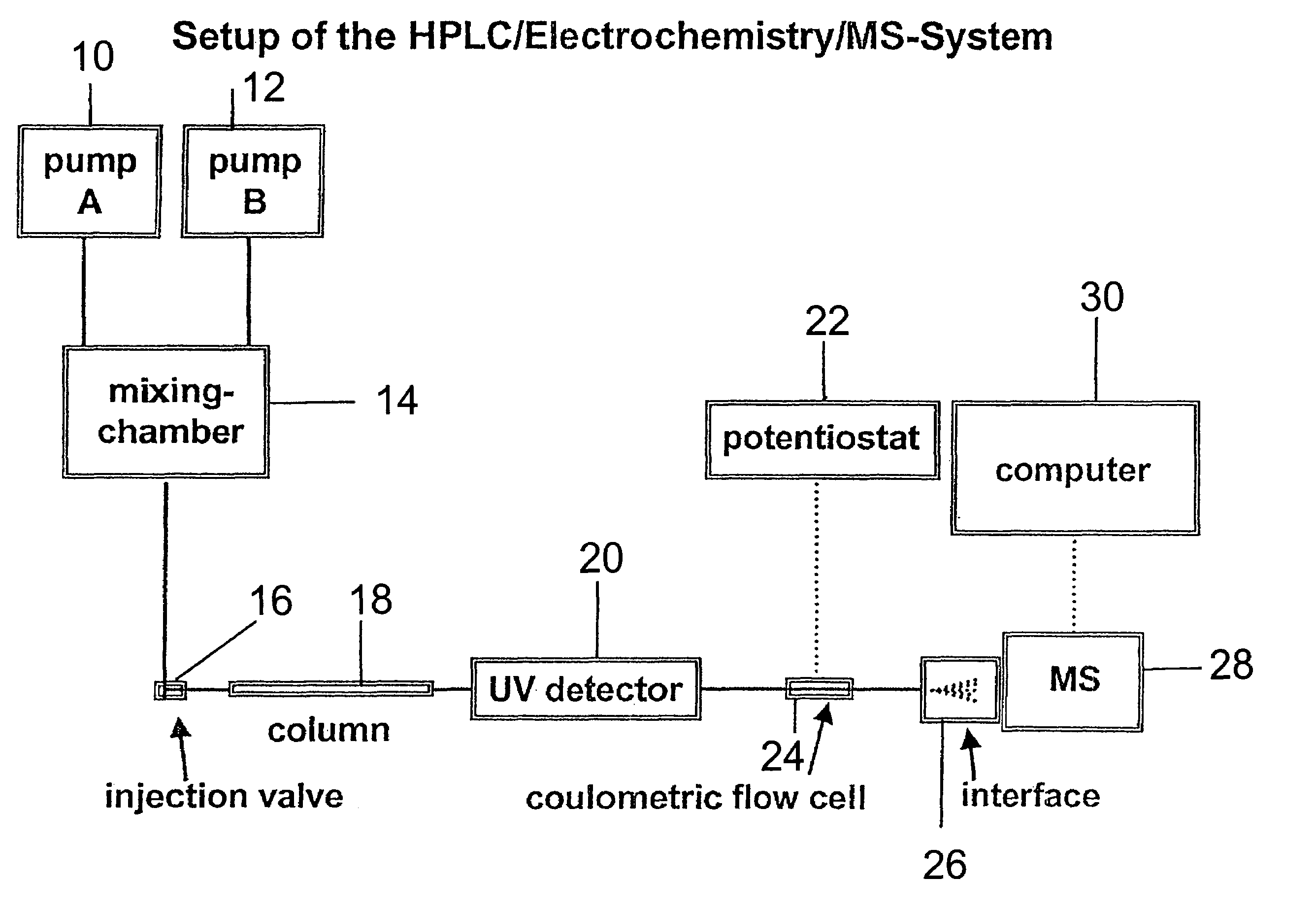
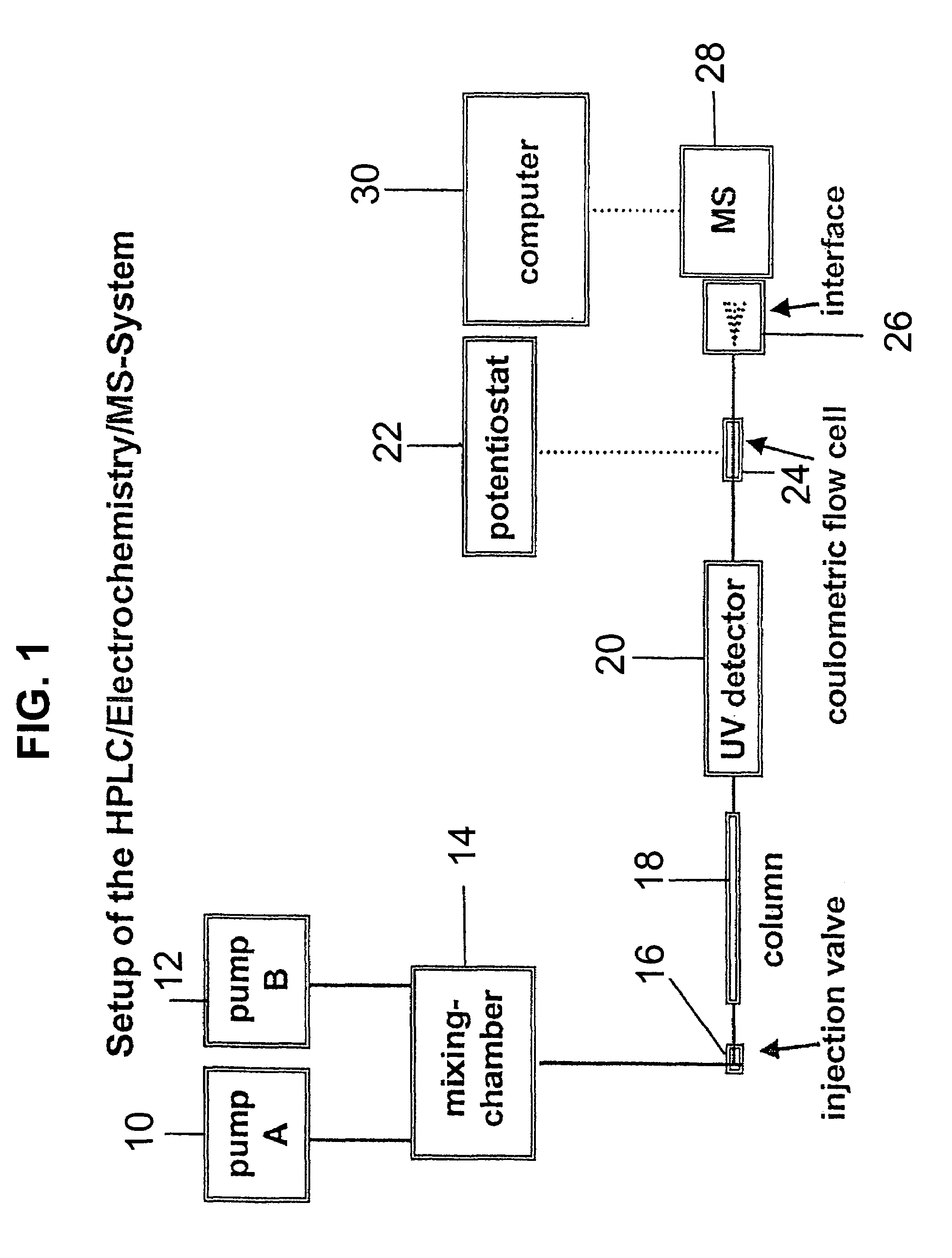
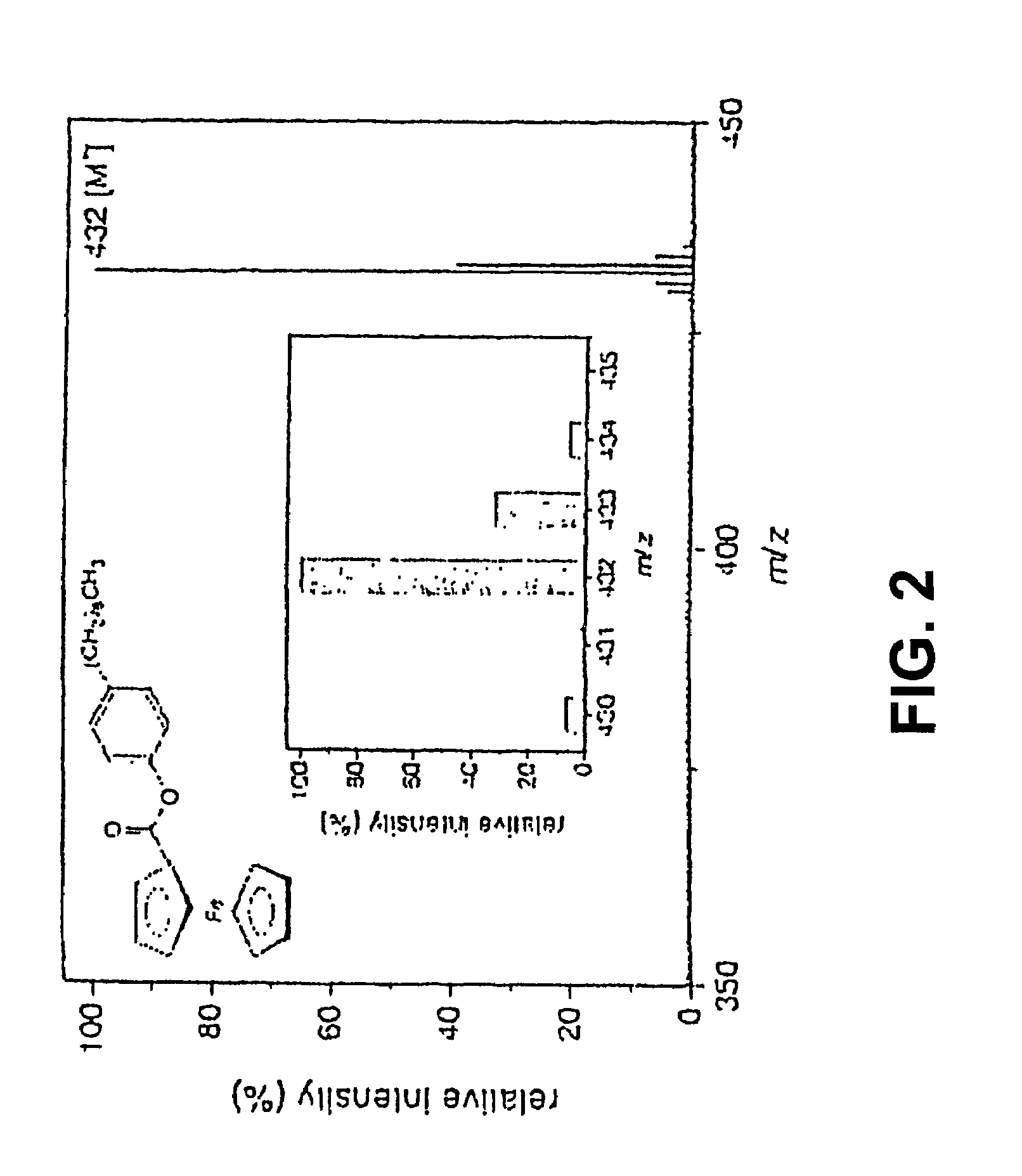
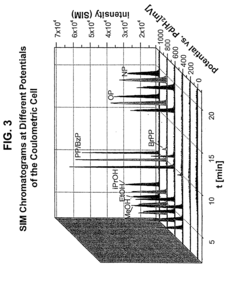
Coupling electrochemistry to mass spectrometry and high performance liquid chromatography
PatentInactiveUS7028537B2
Innovation
- A new HPLC-electrochemistry-MS technique is developed, where a coulometric three-electrode electrochemical cell is inserted between the HPLC column and mass spectrometer, enabling post-column electrochemical oxidation or reduction of analytes, forming charged or strongly polar products compatible with ESI or APCI mass spectrometry, thereby improving ionization efficiency.
Method Validation and Quality Control Strategies
Method validation and quality control are critical components in ensuring the reliability and reproducibility of HPLC-MS ionization techniques. A comprehensive validation strategy for ESI, APCI, and APPI methods must address several key parameters to establish analytical robustness.
Validation protocols should begin with specificity testing to confirm that the selected ionization technique can accurately identify and quantify target analytes without interference from matrix components. This is particularly important when analyzing complex biological samples where ion suppression or enhancement can significantly impact results.
Linearity assessment across a wide concentration range is essential for each ionization technique, as ESI, APCI, and APPI may exhibit different linear dynamic ranges depending on the analyte properties. Establishing calibration curves with appropriate internal standards helps compensate for ionization variability between sample batches.
Precision evaluation at multiple levels (intra-day and inter-day) provides critical information about method reproducibility. For ionization techniques, this should include assessment of retention time stability, peak area reproducibility, and mass accuracy consistency. APCI and APPI typically demonstrate better robustness than ESI when analyzing samples with high salt content.
Accuracy determination through recovery studies using spiked samples at different concentration levels helps identify potential matrix effects specific to each ionization technique. This is particularly important when transitioning between different sample types or matrices.
Limits of detection (LOD) and quantification (LOQ) should be established for each ionization mode, as sensitivity can vary significantly between ESI, APCI, and APPI depending on analyte polarity and volatility. Regular system suitability tests using standard mixtures help monitor instrument performance and detect potential issues before they affect analytical results.
Quality control strategies should incorporate the use of quality control samples at low, medium, and high concentration levels within each analytical batch. Monitoring these QC samples using statistical process control charts enables early detection of systematic errors or drift in ionization efficiency.
Robustness testing should specifically address source condition parameters critical to each ionization technique, including temperature stability, gas flow rates, voltage settings, and source geometry. This helps establish acceptable operating ranges and identifies critical parameters requiring strict control during routine analysis.
Validation protocols should begin with specificity testing to confirm that the selected ionization technique can accurately identify and quantify target analytes without interference from matrix components. This is particularly important when analyzing complex biological samples where ion suppression or enhancement can significantly impact results.
Linearity assessment across a wide concentration range is essential for each ionization technique, as ESI, APCI, and APPI may exhibit different linear dynamic ranges depending on the analyte properties. Establishing calibration curves with appropriate internal standards helps compensate for ionization variability between sample batches.
Precision evaluation at multiple levels (intra-day and inter-day) provides critical information about method reproducibility. For ionization techniques, this should include assessment of retention time stability, peak area reproducibility, and mass accuracy consistency. APCI and APPI typically demonstrate better robustness than ESI when analyzing samples with high salt content.
Accuracy determination through recovery studies using spiked samples at different concentration levels helps identify potential matrix effects specific to each ionization technique. This is particularly important when transitioning between different sample types or matrices.
Limits of detection (LOD) and quantification (LOQ) should be established for each ionization mode, as sensitivity can vary significantly between ESI, APCI, and APPI depending on analyte polarity and volatility. Regular system suitability tests using standard mixtures help monitor instrument performance and detect potential issues before they affect analytical results.
Quality control strategies should incorporate the use of quality control samples at low, medium, and high concentration levels within each analytical batch. Monitoring these QC samples using statistical process control charts enables early detection of systematic errors or drift in ionization efficiency.
Robustness testing should specifically address source condition parameters critical to each ionization technique, including temperature stability, gas flow rates, voltage settings, and source geometry. This helps establish acceptable operating ranges and identifies critical parameters requiring strict control during routine analysis.
Environmental and Safety Considerations for MS Laboratories
Mass spectrometry laboratories present unique environmental and safety challenges due to the combination of chemical, electrical, and mechanical hazards. When operating HPLC-MS systems with various ionization sources (ESI, APCI, APPI), specific safety protocols must be implemented to protect personnel and minimize environmental impact.
The primary safety concern with ESI sources is the high voltage (typically 2-5 kV) required for operation. Laboratories must install proper electrical insulation and grounding systems, with clear warning signs indicating high voltage areas. Regular inspection of electrical connections is essential to prevent electrical hazards, particularly in environments with potential solvent vapors.
APCI and APPI sources present additional risks due to their operation at elevated temperatures (400-500°C) and the use of corona discharge needles or UV lamps. Thermal insulation around these components is critical to prevent accidental burns. For APPI specifically, UV radiation shielding must be verified to protect operators from harmful exposure.
Solvent waste management represents a significant environmental consideration across all ionization techniques. HPLC-MS systems generate substantial volumes of organic solvent waste, often containing acetonitrile, methanol, and other potentially harmful chemicals. Laboratories must implement proper waste segregation systems and contract with certified disposal services to ensure environmental compliance.
Ventilation requirements differ based on the ionization source. APCI and APPI generate more volatile organic compounds (VOCs) than ESI, necessitating enhanced laboratory ventilation systems. Fume hoods should be positioned strategically near MS instruments, with airflow rates meeting or exceeding 100 feet per minute at the hood face to effectively remove potentially harmful vapors.
Gas management presents another critical safety aspect. Nitrogen generators or compressed gas cylinders used for nebulization and desolvation must be secured properly with appropriate regulators and safety systems. Laboratories using hydrogen as a carrier gas require specialized detectors and emergency shutdown protocols due to explosion risks.
Personal protective equipment (PPE) policies should be tailored to the specific ionization techniques in use. Standard laboratory PPE includes safety glasses, lab coats, and appropriate gloves resistant to the solvents being used. When maintaining APCI or APPI sources, heat-resistant gloves should be available.
Emergency response planning must account for the specific hazards of MS laboratories. This includes procedures for electrical emergencies, chemical spills, and fire response. Specialized fire extinguishers suitable for electrical and chemical fires should be readily accessible, and staff should receive regular training on their use.
The primary safety concern with ESI sources is the high voltage (typically 2-5 kV) required for operation. Laboratories must install proper electrical insulation and grounding systems, with clear warning signs indicating high voltage areas. Regular inspection of electrical connections is essential to prevent electrical hazards, particularly in environments with potential solvent vapors.
APCI and APPI sources present additional risks due to their operation at elevated temperatures (400-500°C) and the use of corona discharge needles or UV lamps. Thermal insulation around these components is critical to prevent accidental burns. For APPI specifically, UV radiation shielding must be verified to protect operators from harmful exposure.
Solvent waste management represents a significant environmental consideration across all ionization techniques. HPLC-MS systems generate substantial volumes of organic solvent waste, often containing acetonitrile, methanol, and other potentially harmful chemicals. Laboratories must implement proper waste segregation systems and contract with certified disposal services to ensure environmental compliance.
Ventilation requirements differ based on the ionization source. APCI and APPI generate more volatile organic compounds (VOCs) than ESI, necessitating enhanced laboratory ventilation systems. Fume hoods should be positioned strategically near MS instruments, with airflow rates meeting or exceeding 100 feet per minute at the hood face to effectively remove potentially harmful vapors.
Gas management presents another critical safety aspect. Nitrogen generators or compressed gas cylinders used for nebulization and desolvation must be secured properly with appropriate regulators and safety systems. Laboratories using hydrogen as a carrier gas require specialized detectors and emergency shutdown protocols due to explosion risks.
Personal protective equipment (PPE) policies should be tailored to the specific ionization techniques in use. Standard laboratory PPE includes safety glasses, lab coats, and appropriate gloves resistant to the solvents being used. When maintaining APCI or APPI sources, heat-resistant gloves should be available.
Emergency response planning must account for the specific hazards of MS laboratories. This includes procedures for electrical emergencies, chemical spills, and fire response. Specialized fire extinguishers suitable for electrical and chemical fires should be readily accessible, and staff should receive regular training on their use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!