How Solenoid Valve Materials Impact Biocompatibility in Medical Applications
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solenoid Valve Biocompatibility Background
Solenoid valves have become integral components in various medical applications, playing crucial roles in fluid control systems within medical devices and equipment. The biocompatibility of these valves is of paramount importance, as they often come into direct or indirect contact with biological tissues, fluids, and substances in medical settings.
The concept of biocompatibility in relation to solenoid valves emerged as medical technology advanced and the use of these components in healthcare applications expanded. Historically, solenoid valves were primarily used in industrial settings, where biocompatibility was not a significant concern. However, as their potential for medical applications became apparent, researchers and manufacturers began to focus on developing materials and designs that could meet the stringent requirements of the medical field.
Biocompatibility, in the context of solenoid valves, refers to the ability of the valve materials to interact with biological systems without causing adverse effects. This includes ensuring that the materials do not leach harmful substances, trigger immune responses, or promote bacterial growth. The importance of biocompatibility has grown in parallel with the increasing complexity and invasiveness of medical procedures and devices.
The materials used in solenoid valves for medical applications have evolved significantly over time. Early valves often utilized materials that were not specifically designed for medical use, leading to potential complications and limitations in their application. As the field progressed, specialized materials were developed and adopted, including medical-grade stainless steels, certain polymers, and advanced coatings.
The impact of solenoid valve materials on biocompatibility extends beyond just the valve body. It encompasses all components that may come into contact with biological substances, including seals, gaskets, and internal mechanisms. Each of these elements must be carefully considered and selected to ensure overall biocompatibility of the valve system.
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established guidelines and standards for biocompatibility in medical devices, including components like solenoid valves. These regulations have significantly influenced the development and selection of materials used in medical-grade solenoid valves, driving innovation in material science and manufacturing processes.
The ongoing research in this field continues to push the boundaries of biocompatible materials for solenoid valves. Scientists and engineers are exploring novel materials and surface treatments that not only meet current biocompatibility standards but also offer improved performance, durability, and compatibility with a wider range of medical applications.
The concept of biocompatibility in relation to solenoid valves emerged as medical technology advanced and the use of these components in healthcare applications expanded. Historically, solenoid valves were primarily used in industrial settings, where biocompatibility was not a significant concern. However, as their potential for medical applications became apparent, researchers and manufacturers began to focus on developing materials and designs that could meet the stringent requirements of the medical field.
Biocompatibility, in the context of solenoid valves, refers to the ability of the valve materials to interact with biological systems without causing adverse effects. This includes ensuring that the materials do not leach harmful substances, trigger immune responses, or promote bacterial growth. The importance of biocompatibility has grown in parallel with the increasing complexity and invasiveness of medical procedures and devices.
The materials used in solenoid valves for medical applications have evolved significantly over time. Early valves often utilized materials that were not specifically designed for medical use, leading to potential complications and limitations in their application. As the field progressed, specialized materials were developed and adopted, including medical-grade stainless steels, certain polymers, and advanced coatings.
The impact of solenoid valve materials on biocompatibility extends beyond just the valve body. It encompasses all components that may come into contact with biological substances, including seals, gaskets, and internal mechanisms. Each of these elements must be carefully considered and selected to ensure overall biocompatibility of the valve system.
Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established guidelines and standards for biocompatibility in medical devices, including components like solenoid valves. These regulations have significantly influenced the development and selection of materials used in medical-grade solenoid valves, driving innovation in material science and manufacturing processes.
The ongoing research in this field continues to push the boundaries of biocompatible materials for solenoid valves. Scientists and engineers are exploring novel materials and surface treatments that not only meet current biocompatibility standards but also offer improved performance, durability, and compatibility with a wider range of medical applications.
Medical Device Market Analysis
The global medical device market has been experiencing significant growth, driven by technological advancements, an aging population, and increasing healthcare expenditure. In 2021, the market was valued at approximately $495 billion and is projected to reach $719 billion by 2029, growing at a CAGR of 5.5% during the forecast period. This growth is particularly evident in the segment of solenoid valves used in medical applications, which play a crucial role in various medical devices and equipment.
The demand for biocompatible solenoid valves in medical applications has been steadily increasing due to the rising prevalence of chronic diseases, the growing need for minimally invasive surgeries, and the expansion of point-of-care diagnostics. These valves are essential components in medical devices such as ventilators, anesthesia machines, infusion pumps, and diagnostic equipment. The COVID-19 pandemic has further accelerated the demand for medical devices incorporating solenoid valves, particularly in respiratory care equipment.
North America currently dominates the medical device market, accounting for approximately 40% of the global market share. This is attributed to the presence of major medical device manufacturers, advanced healthcare infrastructure, and high healthcare spending in the region. Europe follows closely, with a market share of around 25%, driven by stringent regulatory standards and a focus on innovative medical technologies. The Asia-Pacific region is expected to witness the fastest growth in the coming years, with China and India emerging as key markets due to improving healthcare access and increasing investments in medical infrastructure.
The solenoid valve segment within the medical device market is characterized by intense competition among key players such as Emerson Electric Co., Parker Hannifin Corporation, and SMC Corporation. These companies are investing heavily in research and development to improve the biocompatibility of solenoid valve materials, addressing the growing demand for safer and more efficient medical devices. The focus on biocompatibility has led to the development of advanced materials such as PEEK (polyetheretherketone), PTFE (polytetrafluoroethylene), and medical-grade stainless steel, which offer improved chemical resistance and reduced risk of contamination.
Market trends indicate a shift towards miniaturization and integration of solenoid valves in medical devices, enabling the development of more compact and portable equipment. Additionally, there is a growing emphasis on smart valves with enhanced control capabilities and IoT integration, allowing for remote monitoring and predictive maintenance. These advancements are expected to drive the adoption of solenoid valves in emerging medical applications, such as wearable drug delivery systems and robotic-assisted surgeries.
The demand for biocompatible solenoid valves in medical applications has been steadily increasing due to the rising prevalence of chronic diseases, the growing need for minimally invasive surgeries, and the expansion of point-of-care diagnostics. These valves are essential components in medical devices such as ventilators, anesthesia machines, infusion pumps, and diagnostic equipment. The COVID-19 pandemic has further accelerated the demand for medical devices incorporating solenoid valves, particularly in respiratory care equipment.
North America currently dominates the medical device market, accounting for approximately 40% of the global market share. This is attributed to the presence of major medical device manufacturers, advanced healthcare infrastructure, and high healthcare spending in the region. Europe follows closely, with a market share of around 25%, driven by stringent regulatory standards and a focus on innovative medical technologies. The Asia-Pacific region is expected to witness the fastest growth in the coming years, with China and India emerging as key markets due to improving healthcare access and increasing investments in medical infrastructure.
The solenoid valve segment within the medical device market is characterized by intense competition among key players such as Emerson Electric Co., Parker Hannifin Corporation, and SMC Corporation. These companies are investing heavily in research and development to improve the biocompatibility of solenoid valve materials, addressing the growing demand for safer and more efficient medical devices. The focus on biocompatibility has led to the development of advanced materials such as PEEK (polyetheretherketone), PTFE (polytetrafluoroethylene), and medical-grade stainless steel, which offer improved chemical resistance and reduced risk of contamination.
Market trends indicate a shift towards miniaturization and integration of solenoid valves in medical devices, enabling the development of more compact and portable equipment. Additionally, there is a growing emphasis on smart valves with enhanced control capabilities and IoT integration, allowing for remote monitoring and predictive maintenance. These advancements are expected to drive the adoption of solenoid valves in emerging medical applications, such as wearable drug delivery systems and robotic-assisted surgeries.
Biocompatible Material Challenges
The development of biocompatible materials for solenoid valves in medical applications faces several significant challenges. One of the primary concerns is the potential for material degradation and leaching when in contact with biological fluids or tissues. This can lead to the release of potentially harmful substances into the patient's body, compromising both the safety and efficacy of the medical device.
Another major challenge lies in achieving the right balance between mechanical properties and biocompatibility. Materials that exhibit excellent biocompatibility may not always possess the necessary strength, durability, or flexibility required for optimal valve performance. This trade-off often necessitates extensive research and development to identify or engineer materials that can meet both criteria simultaneously.
Sterilization compatibility presents another hurdle in the selection of biocompatible materials for solenoid valves. Medical devices must withstand rigorous sterilization processes, such as autoclaving, gamma irradiation, or ethylene oxide treatment, without compromising their structural integrity or biocompatibility. Some materials may degrade or alter their properties under these harsh conditions, limiting their applicability in medical settings.
The long-term stability of biocompatible materials is also a critical concern. Materials must maintain their properties and performance over extended periods, often for the entire lifetime of the implanted device. This requirement poses challenges in predicting and preventing material fatigue, wear, or chemical changes that may occur over time in the biological environment.
Furthermore, the regulatory landscape surrounding biocompatible materials adds complexity to their development and implementation. Stringent approval processes and evolving standards require extensive testing and documentation to demonstrate both short-term and long-term safety. This can significantly increase development time and costs for new materials or material combinations.
Lastly, the challenge of scalability and cost-effectiveness cannot be overlooked. While certain exotic materials may exhibit excellent biocompatibility and performance characteristics, their high cost or limited availability may render them impractical for widespread use in medical devices. Striking a balance between material performance, biocompatibility, and economic viability remains an ongoing challenge in the field.
Addressing these challenges requires a multidisciplinary approach, combining expertise in materials science, bioengineering, and medical device design. Continued research into novel materials, surface modification techniques, and advanced manufacturing processes will be crucial in overcoming these hurdles and advancing the development of biocompatible solenoid valves for medical applications.
Another major challenge lies in achieving the right balance between mechanical properties and biocompatibility. Materials that exhibit excellent biocompatibility may not always possess the necessary strength, durability, or flexibility required for optimal valve performance. This trade-off often necessitates extensive research and development to identify or engineer materials that can meet both criteria simultaneously.
Sterilization compatibility presents another hurdle in the selection of biocompatible materials for solenoid valves. Medical devices must withstand rigorous sterilization processes, such as autoclaving, gamma irradiation, or ethylene oxide treatment, without compromising their structural integrity or biocompatibility. Some materials may degrade or alter their properties under these harsh conditions, limiting their applicability in medical settings.
The long-term stability of biocompatible materials is also a critical concern. Materials must maintain their properties and performance over extended periods, often for the entire lifetime of the implanted device. This requirement poses challenges in predicting and preventing material fatigue, wear, or chemical changes that may occur over time in the biological environment.
Furthermore, the regulatory landscape surrounding biocompatible materials adds complexity to their development and implementation. Stringent approval processes and evolving standards require extensive testing and documentation to demonstrate both short-term and long-term safety. This can significantly increase development time and costs for new materials or material combinations.
Lastly, the challenge of scalability and cost-effectiveness cannot be overlooked. While certain exotic materials may exhibit excellent biocompatibility and performance characteristics, their high cost or limited availability may render them impractical for widespread use in medical devices. Striking a balance between material performance, biocompatibility, and economic viability remains an ongoing challenge in the field.
Addressing these challenges requires a multidisciplinary approach, combining expertise in materials science, bioengineering, and medical device design. Continued research into novel materials, surface modification techniques, and advanced manufacturing processes will be crucial in overcoming these hurdles and advancing the development of biocompatible solenoid valves for medical applications.
Current Biocompatible Solutions
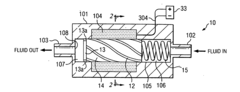
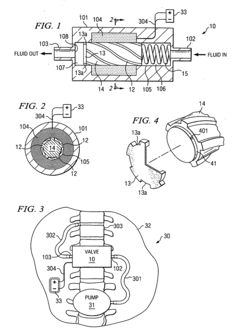
01 Biocompatible materials for solenoid valve components
Solenoid valves used in medical or biological applications require biocompatible materials for their components. These materials are selected to minimize adverse reactions when in contact with living tissues or fluids. Common biocompatible materials include certain grades of stainless steel, titanium alloys, and specialized polymers that meet regulatory standards for medical devices.- Biocompatible materials for solenoid valve components: Solenoid valves used in medical or biological applications require biocompatible materials for their components. These materials are selected to minimize adverse reactions when in contact with living tissues or fluids. Common biocompatible materials include certain grades of stainless steel, titanium alloys, and specialized polymers that resist corrosion and do not leach harmful substances.
- Coatings to enhance biocompatibility: To improve the biocompatibility of solenoid valve materials, various coating techniques can be employed. These coatings act as a barrier between the base material and the biological environment, reducing the risk of adverse reactions. Examples include diamond-like carbon coatings, ceramic coatings, and specialized polymer coatings that can be applied to metal components to enhance their biocompatibility and durability in biological settings.
- Material selection for specific biological applications: The choice of materials for solenoid valves in biological applications depends on the specific requirements of the system. For instance, valves used in drug delivery systems may require different materials compared to those used in diagnostic equipment. Factors such as chemical resistance, sterilization compatibility, and long-term stability in biological environments are considered when selecting materials for these specialized applications.
- Testing and validation of biocompatibility: Rigorous testing and validation procedures are essential to ensure the biocompatibility of solenoid valve materials. These tests may include in vitro cytotoxicity assays, sensitization tests, and long-term implantation studies. Compliance with regulatory standards such as ISO 10993 for biological evaluation of medical devices is often required to demonstrate the safety and biocompatibility of the materials used in solenoid valves for biological applications.
- Design considerations for biocompatible solenoid valves: The design of solenoid valves for biological applications must take into account the biocompatibility of materials as well as other factors such as ease of sterilization, minimization of dead spaces where biological materials can accumulate, and resistance to fouling. Special attention is given to sealing materials and lubricants to ensure they do not compromise the overall biocompatibility of the valve system.
02 Coatings to enhance biocompatibility
To improve the biocompatibility of solenoid valve materials, specialized coatings can be applied to the valve components. These coatings may include inert materials or surface treatments that reduce the risk of corrosion, prevent leaching of potentially harmful substances, and improve overall compatibility with biological systems.Expand Specific Solutions03 Design considerations for biocompatible solenoid valves
The design of solenoid valves for biocompatible applications involves careful consideration of material selection, surface finish, and overall construction. This includes minimizing crevices or areas where biological materials could accumulate, ensuring smooth fluid pathways, and incorporating features that facilitate easy cleaning and sterilization.Expand Specific Solutions04 Testing and certification of biocompatible solenoid valves
Solenoid valves intended for use in biocompatible applications undergo rigorous testing and certification processes. These may include in vitro and in vivo biocompatibility tests, chemical analysis of materials, and long-term stability studies. Compliance with relevant standards and regulations, such as ISO 10993 for biocompatibility evaluation, is essential for these valves.Expand Specific Solutions05 Maintenance and cleaning of biocompatible solenoid valves
Proper maintenance and cleaning procedures are crucial for maintaining the biocompatibility of solenoid valves in medical or biological applications. This includes using compatible cleaning agents, following specific sterilization protocols, and regular inspection for wear or degradation that could compromise biocompatibility. The valve design should facilitate easy disassembly and reassembly for thorough cleaning.Expand Specific Solutions
Key Medical Valve Manufacturers
The biocompatibility of solenoid valve materials in medical applications is a critical area of research, currently in a growth phase. The market for biocompatible solenoid valves is expanding, driven by increasing demand in healthcare and life sciences sectors. Companies like Boston Scientific Scimed and SWS Hemodialysis Care are at the forefront, developing advanced materials and technologies. The technical maturity varies, with established players like Robert Bosch GmbH and Toyobo Co., Ltd. offering proven solutions, while newer entrants like Mirus LLC are innovating with novel materials inspired by aerospace technology. Academic institutions such as Zhejiang University and the University of Tokyo are contributing to fundamental research, pushing the boundaries of material science in this field.
Robert Bosch GmbH
Technical Solution: Bosch has leveraged its automotive and industrial expertise to develop biocompatible solenoid valve solutions for medical applications, particularly in diagnostics and laboratory automation. Their approach focuses on using high-purity stainless steel alloys and engineered plastics that meet USP Class VI and ISO 10993 standards for biocompatibility[13]. The company has implemented advanced surface treatment processes, such as electropolishing and passivation, to enhance the corrosion resistance and reduce protein binding on valve surfaces[15]. Bosch has also developed miniaturized valve designs that incorporate biocompatible materials while maintaining high precision and reliability, crucial for point-of-care diagnostic devices and automated liquid handling systems[17].
Strengths: Strong background in precision engineering, expertise in miniaturization, and robust quality control processes. Weaknesses: Less experience in long-term implantable medical devices compared to specialized medical technology companies.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific has pioneered the use of advanced polymer blends in their solenoid valve designs for medical devices, particularly in urology and gastroenterology applications. Their approach focuses on developing materials with optimized surface properties to reduce bacterial adhesion and biofilm formation[2]. The company has implemented a multi-layer coating technology that combines hydrophilic and hydrophobic elements to create a biocompatible interface between the valve and biological fluids[4]. Boston Scientific has also invested in the development of self-cleaning valve mechanisms that utilize specially engineered materials to prevent accumulation of biological debris, thereby maintaining long-term biocompatibility in implantable devices[6].
Strengths: Diverse application range across multiple medical specialties, innovative surface modification techniques, and focus on long-term biocompatibility. Weaknesses: Complexity in manufacturing multi-layer coatings and potential regulatory challenges with novel materials.
Innovative Material Technologies
System and method for implantation of devices having unknown biocompatible materials
PatentInactiveUS20100004637A1
Innovation
- A magnetically controlled solenoid/valve system where the portions of the plunger contacting the deliverable composition are coated with a known biocompatible material, such as titanium nitride, to ensure biocompatibility and wear resistance, allowing for the safe use of off-the-shelf devices by isolating potential biocompatibility concerns.
Material for producing products having fibrinolytic and/or antibacterial properties
PatentInactiveEP1328306A2
Innovation
- A material comprising a body tissue-compatible matrix with a molecular sieve that contains water of crystallization, which partially dehydrates and is loaded with fibrinolytic and/or antibacterial active substances, allowing controlled adsorption and desorption upon contact with body tissue, ensuring a continuous supply and optimal release mechanism.
Regulatory Compliance Framework
The regulatory compliance framework for solenoid valve materials in medical applications is a critical aspect that manufacturers and healthcare providers must navigate to ensure patient safety and product efficacy. The primary regulatory bodies overseeing this domain include the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the International Organization for Standardization (ISO).
In the United States, the FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating medical devices, including solenoid valves used in medical applications. The FDA classifies medical devices into three categories based on their risk level, with Class III being the highest risk. Solenoid valves may fall into different classes depending on their specific use and the level of patient contact.
The FDA's 510(k) premarket notification process is often applicable for solenoid valves, requiring manufacturers to demonstrate that their device is substantially equivalent to a legally marketed predicate device. This process includes providing detailed information about the materials used in the valve, their biocompatibility, and any potential risks associated with their use in medical applications.
In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide the regulatory framework for medical devices. These regulations emphasize the importance of biocompatibility and require manufacturers to conduct thorough risk assessments and provide clinical evidence of safety and performance.
The ISO 10993 series of standards, particularly ISO 10993-1, provides guidelines for evaluating the biocompatibility of medical devices. This standard outlines a systematic approach to biological evaluation and risk management, including the selection of appropriate tests based on the nature and duration of body contact.
Manufacturers must also comply with Good Manufacturing Practices (GMP) and Quality Management System (QMS) requirements, such as those outlined in ISO 13485. These standards ensure that the production processes for solenoid valves and their components meet stringent quality and safety criteria.
Material selection for solenoid valves must adhere to specific regulatory guidelines. For instance, materials that come into direct or indirect contact with patients must undergo biocompatibility testing as per ISO 10993 or equivalent standards. This includes cytotoxicity, sensitization, and irritation tests, among others, depending on the nature and duration of contact with the human body.
Regulatory bodies also require manufacturers to implement post-market surveillance systems to monitor the performance and safety of their devices once they are in use. This ongoing process helps identify any long-term biocompatibility issues that may not have been apparent during initial testing and approval phases.
In the United States, the FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating medical devices, including solenoid valves used in medical applications. The FDA classifies medical devices into three categories based on their risk level, with Class III being the highest risk. Solenoid valves may fall into different classes depending on their specific use and the level of patient contact.
The FDA's 510(k) premarket notification process is often applicable for solenoid valves, requiring manufacturers to demonstrate that their device is substantially equivalent to a legally marketed predicate device. This process includes providing detailed information about the materials used in the valve, their biocompatibility, and any potential risks associated with their use in medical applications.
In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) provide the regulatory framework for medical devices. These regulations emphasize the importance of biocompatibility and require manufacturers to conduct thorough risk assessments and provide clinical evidence of safety and performance.
The ISO 10993 series of standards, particularly ISO 10993-1, provides guidelines for evaluating the biocompatibility of medical devices. This standard outlines a systematic approach to biological evaluation and risk management, including the selection of appropriate tests based on the nature and duration of body contact.
Manufacturers must also comply with Good Manufacturing Practices (GMP) and Quality Management System (QMS) requirements, such as those outlined in ISO 13485. These standards ensure that the production processes for solenoid valves and their components meet stringent quality and safety criteria.
Material selection for solenoid valves must adhere to specific regulatory guidelines. For instance, materials that come into direct or indirect contact with patients must undergo biocompatibility testing as per ISO 10993 or equivalent standards. This includes cytotoxicity, sensitization, and irritation tests, among others, depending on the nature and duration of contact with the human body.
Regulatory bodies also require manufacturers to implement post-market surveillance systems to monitor the performance and safety of their devices once they are in use. This ongoing process helps identify any long-term biocompatibility issues that may not have been apparent during initial testing and approval phases.
Long-term Implant Considerations
When considering solenoid valve materials for long-term implantable medical devices, biocompatibility becomes a critical factor that extends beyond initial implantation. The prolonged exposure of these materials to the human body necessitates a comprehensive evaluation of their long-term performance and potential effects on surrounding tissues.
One of the primary considerations is the material's resistance to corrosion and degradation over time. Implanted solenoid valves must maintain their structural integrity and functionality for years, if not decades. Materials such as titanium and certain grades of stainless steel have demonstrated excellent corrosion resistance in biological environments, making them popular choices for long-term implants. However, even these materials may experience subtle changes over extended periods, potentially releasing minute amounts of ions or particles into the surrounding tissues.
The potential for material fatigue and wear is another crucial aspect of long-term implant considerations. Solenoid valves in medical applications often undergo repeated cycles of operation, which can lead to mechanical stress and wear on the materials. This wear can not only affect the valve's performance but also potentially generate particulate debris. The body's response to these wear particles can range from localized inflammation to more severe complications, emphasizing the need for materials that exhibit minimal wear characteristics and generate biocompatible wear debris.
Long-term implants must also contend with the body's natural defense mechanisms and potential encapsulation. The formation of a fibrous capsule around the implant is a common biological response, which can impact the device's functionality and potentially lead to complications. Materials and surface treatments that promote favorable tissue integration while minimizing excessive fibrous encapsulation are highly desirable for long-term implantable solenoid valves.
The potential for material-induced systemic effects over extended periods is another critical consideration. While a material may demonstrate short-term biocompatibility, long-term exposure could lead to cumulative effects or delayed reactions. This necessitates extensive pre-clinical and clinical studies to evaluate the long-term safety profile of materials used in implantable solenoid valves.
Lastly, the interaction between the implant materials and the patient's specific physiological conditions must be considered. Factors such as the patient's age, overall health, and potential comorbidities can influence the long-term performance and biocompatibility of the implanted materials. This underscores the importance of personalized medicine approaches in selecting materials for long-term implantable devices, potentially tailoring material choices to individual patient profiles for optimal long-term outcomes.
One of the primary considerations is the material's resistance to corrosion and degradation over time. Implanted solenoid valves must maintain their structural integrity and functionality for years, if not decades. Materials such as titanium and certain grades of stainless steel have demonstrated excellent corrosion resistance in biological environments, making them popular choices for long-term implants. However, even these materials may experience subtle changes over extended periods, potentially releasing minute amounts of ions or particles into the surrounding tissues.
The potential for material fatigue and wear is another crucial aspect of long-term implant considerations. Solenoid valves in medical applications often undergo repeated cycles of operation, which can lead to mechanical stress and wear on the materials. This wear can not only affect the valve's performance but also potentially generate particulate debris. The body's response to these wear particles can range from localized inflammation to more severe complications, emphasizing the need for materials that exhibit minimal wear characteristics and generate biocompatible wear debris.
Long-term implants must also contend with the body's natural defense mechanisms and potential encapsulation. The formation of a fibrous capsule around the implant is a common biological response, which can impact the device's functionality and potentially lead to complications. Materials and surface treatments that promote favorable tissue integration while minimizing excessive fibrous encapsulation are highly desirable for long-term implantable solenoid valves.
The potential for material-induced systemic effects over extended periods is another critical consideration. While a material may demonstrate short-term biocompatibility, long-term exposure could lead to cumulative effects or delayed reactions. This necessitates extensive pre-clinical and clinical studies to evaluate the long-term safety profile of materials used in implantable solenoid valves.
Lastly, the interaction between the implant materials and the patient's specific physiological conditions must be considered. Factors such as the patient's age, overall health, and potential comorbidities can influence the long-term performance and biocompatibility of the implanted materials. This underscores the importance of personalized medicine approaches in selecting materials for long-term implantable devices, potentially tailoring material choices to individual patient profiles for optimal long-term outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!