How to Build a Zeta Potential Measurement SOP for Multi-operator Labs — Checklist & Templates
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeta Potential Measurement Background and Objectives
Zeta potential measurement has evolved significantly since its conceptual development in the early 20th century. Initially a theoretical construct in colloidal science, it has transformed into an essential analytical technique for characterizing particle surface properties in various industries including pharmaceuticals, ceramics, water treatment, and nanotechnology. The measurement quantifies the electrical potential at the slipping plane of a colloidal particle, providing critical insights into suspension stability and particle-particle interactions.
The evolution of zeta potential measurement technology has been marked by several key advancements. Early methods relied on electrophoresis observations under microscopes, while modern techniques employ laser Doppler electrophoresis and electroacoustic measurements that offer higher precision and automation. Recent developments have focused on improving measurement accuracy across different sample types and concentrating conditions.
Despite technological progress, multi-operator laboratories face significant challenges in achieving consistent and comparable zeta potential measurements. Variations in sample preparation, instrument calibration, measurement parameters, and data interpretation can lead to discrepancies between operators and laboratories. These inconsistencies undermine the reliability of results and hinder cross-laboratory collaboration and data sharing.
The primary objective of developing a standardized SOP for zeta potential measurements is to establish a unified methodology that ensures reproducibility and reliability across different operators and laboratory settings. This standardization aims to minimize variability in measurement outcomes, enabling meaningful comparisons of results obtained by different personnel or at different facilities.
Additionally, the SOP development seeks to address common technical challenges in zeta potential measurements, including sample preparation protocols for diverse material types, appropriate selection of measurement parameters based on sample characteristics, and systematic approaches to data analysis and interpretation. By incorporating best practices from industry leaders and research institutions, the SOP will serve as a comprehensive guide for laboratories seeking to implement robust zeta potential measurement protocols.
The long-term goal extends beyond standardization to establishing a framework for continuous improvement in measurement techniques. As technology evolves and new applications emerge, the SOP must be adaptable while maintaining its core standardization principles. This balance between standardization and flexibility will support both current analytical needs and future technological advancements in the field of zeta potential measurement.
The evolution of zeta potential measurement technology has been marked by several key advancements. Early methods relied on electrophoresis observations under microscopes, while modern techniques employ laser Doppler electrophoresis and electroacoustic measurements that offer higher precision and automation. Recent developments have focused on improving measurement accuracy across different sample types and concentrating conditions.
Despite technological progress, multi-operator laboratories face significant challenges in achieving consistent and comparable zeta potential measurements. Variations in sample preparation, instrument calibration, measurement parameters, and data interpretation can lead to discrepancies between operators and laboratories. These inconsistencies undermine the reliability of results and hinder cross-laboratory collaboration and data sharing.
The primary objective of developing a standardized SOP for zeta potential measurements is to establish a unified methodology that ensures reproducibility and reliability across different operators and laboratory settings. This standardization aims to minimize variability in measurement outcomes, enabling meaningful comparisons of results obtained by different personnel or at different facilities.
Additionally, the SOP development seeks to address common technical challenges in zeta potential measurements, including sample preparation protocols for diverse material types, appropriate selection of measurement parameters based on sample characteristics, and systematic approaches to data analysis and interpretation. By incorporating best practices from industry leaders and research institutions, the SOP will serve as a comprehensive guide for laboratories seeking to implement robust zeta potential measurement protocols.
The long-term goal extends beyond standardization to establishing a framework for continuous improvement in measurement techniques. As technology evolves and new applications emerge, the SOP must be adaptable while maintaining its core standardization principles. This balance between standardization and flexibility will support both current analytical needs and future technological advancements in the field of zeta potential measurement.
Market Demand Analysis for Standardized Zeta Potential Measurement
The global market for zeta potential measurement standardization is experiencing significant growth, driven by increasing demands for quality control and reproducibility in various industries. The current market size for zeta potential measurement instruments is estimated at $350 million, with a compound annual growth rate of 6.8% projected through 2028. This growth is particularly pronounced in pharmaceutical, biotechnology, and advanced materials sectors where colloidal stability is critical.
Research institutions and pharmaceutical companies represent the largest market segment, accounting for approximately 45% of the total demand. These organizations require standardized measurement protocols to ensure consistency across multi-operator laboratories and facilitate regulatory compliance. The pharmaceutical industry alone has seen a 12% increase in demand for standardized zeta potential measurement procedures over the past three years.
A significant market driver is the growing emphasis on nanomedicine and drug delivery systems, where zeta potential serves as a critical quality attribute. The FDA and EMA have both increased scrutiny of colloidal stability data in regulatory submissions, creating urgent demand for validated measurement procedures. Industry surveys indicate that 78% of laboratory managers consider standardization of zeta potential measurements a high or very high priority.
Regional analysis shows North America leading the market with 38% share, followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate at 8.5% annually, primarily due to expanding pharmaceutical manufacturing and research facilities in China and India.
Contract research organizations (CROs) represent an emerging market segment with 15% annual growth in demand for standardized zeta potential measurement protocols. This trend reflects the increasing outsourcing of analytical testing and the need for consistent methodologies across different laboratory sites.
Customer pain points identified through market research include inter-laboratory variability (cited by 82% of users), operator-dependent results (76%), and difficulties in method transfer between sites (68%). These challenges directly impact product development timelines and regulatory approval processes, with an estimated cost of $120,000-$180,000 per month of delay in pharmaceutical product launches.
The market for training and certification programs related to zeta potential measurement standardization is also expanding, with an estimated value of $45 million and 14% annual growth. This indicates strong industry recognition of the need for operator competency in executing standardized procedures.
Research institutions and pharmaceutical companies represent the largest market segment, accounting for approximately 45% of the total demand. These organizations require standardized measurement protocols to ensure consistency across multi-operator laboratories and facilitate regulatory compliance. The pharmaceutical industry alone has seen a 12% increase in demand for standardized zeta potential measurement procedures over the past three years.
A significant market driver is the growing emphasis on nanomedicine and drug delivery systems, where zeta potential serves as a critical quality attribute. The FDA and EMA have both increased scrutiny of colloidal stability data in regulatory submissions, creating urgent demand for validated measurement procedures. Industry surveys indicate that 78% of laboratory managers consider standardization of zeta potential measurements a high or very high priority.
Regional analysis shows North America leading the market with 38% share, followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate at 8.5% annually, primarily due to expanding pharmaceutical manufacturing and research facilities in China and India.
Contract research organizations (CROs) represent an emerging market segment with 15% annual growth in demand for standardized zeta potential measurement protocols. This trend reflects the increasing outsourcing of analytical testing and the need for consistent methodologies across different laboratory sites.
Customer pain points identified through market research include inter-laboratory variability (cited by 82% of users), operator-dependent results (76%), and difficulties in method transfer between sites (68%). These challenges directly impact product development timelines and regulatory approval processes, with an estimated cost of $120,000-$180,000 per month of delay in pharmaceutical product launches.
The market for training and certification programs related to zeta potential measurement standardization is also expanding, with an estimated value of $45 million and 14% annual growth. This indicates strong industry recognition of the need for operator competency in executing standardized procedures.
Current Challenges in Multi-operator Zeta Potential Measurements
Zeta potential measurement in multi-operator laboratories faces significant challenges that impact data reliability and reproducibility. One of the primary issues is operator variability, where different technicians may follow slightly different procedures despite having the same written protocol. This inconsistency manifests in sample preparation techniques, handling methods, and interpretation of measurement parameters, leading to statistically significant variations in results even when measuring identical samples.
Instrument calibration represents another major challenge. Different laboratories often use varying calibration standards and schedules, creating systematic biases in measurement results. The lack of universally accepted calibration materials specifically designed for zeta potential measurements compounds this problem, making cross-laboratory comparisons particularly difficult.
Environmental factors introduce additional complexity, as zeta potential measurements are highly sensitive to temperature fluctuations, electromagnetic interference, and vibrations. Multi-operator labs frequently struggle to maintain consistent environmental conditions, especially when equipment is shared across different research groups or departments with varying facility setups.
Sample preparation inconsistencies present perhaps the most insidious challenge. Minor variations in sample concentration, pH adjustment techniques, filtration methods, and storage conditions can dramatically alter zeta potential readings. Without rigorous standardization, these preparation steps become a significant source of measurement error that is difficult to trace and correct.
Documentation practices vary widely across laboratories, with some maintaining detailed records of measurement parameters while others record only final results. This inconsistency makes troubleshooting and method validation extremely difficult, particularly when anomalous results appear and root cause analysis becomes necessary.
Training disparities among operators represent a fundamental challenge. The theoretical understanding of electrokinetic principles underlying zeta potential measurements varies significantly among laboratory personnel. Without comprehensive training programs that address both theoretical concepts and practical skills, operators may not recognize subtle issues that affect measurement quality.
Quality control procedures are often inadequately implemented in multi-operator environments. Many laboratories lack robust systems for regular verification of instrument performance, statistical analysis of measurement variability, and systematic evaluation of operator proficiency. Without these quality assurance mechanisms, measurement drift and methodological inconsistencies can go undetected for extended periods.
The absence of standardized reporting formats further complicates data interpretation and comparison. Different operators may report zeta potential values with varying units, statistical treatments, or contextual information, making it challenging to build cohesive datasets across multiple measurement sessions or research projects.
Instrument calibration represents another major challenge. Different laboratories often use varying calibration standards and schedules, creating systematic biases in measurement results. The lack of universally accepted calibration materials specifically designed for zeta potential measurements compounds this problem, making cross-laboratory comparisons particularly difficult.
Environmental factors introduce additional complexity, as zeta potential measurements are highly sensitive to temperature fluctuations, electromagnetic interference, and vibrations. Multi-operator labs frequently struggle to maintain consistent environmental conditions, especially when equipment is shared across different research groups or departments with varying facility setups.
Sample preparation inconsistencies present perhaps the most insidious challenge. Minor variations in sample concentration, pH adjustment techniques, filtration methods, and storage conditions can dramatically alter zeta potential readings. Without rigorous standardization, these preparation steps become a significant source of measurement error that is difficult to trace and correct.
Documentation practices vary widely across laboratories, with some maintaining detailed records of measurement parameters while others record only final results. This inconsistency makes troubleshooting and method validation extremely difficult, particularly when anomalous results appear and root cause analysis becomes necessary.
Training disparities among operators represent a fundamental challenge. The theoretical understanding of electrokinetic principles underlying zeta potential measurements varies significantly among laboratory personnel. Without comprehensive training programs that address both theoretical concepts and practical skills, operators may not recognize subtle issues that affect measurement quality.
Quality control procedures are often inadequately implemented in multi-operator environments. Many laboratories lack robust systems for regular verification of instrument performance, statistical analysis of measurement variability, and systematic evaluation of operator proficiency. Without these quality assurance mechanisms, measurement drift and methodological inconsistencies can go undetected for extended periods.
The absence of standardized reporting formats further complicates data interpretation and comparison. Different operators may report zeta potential values with varying units, statistical treatments, or contextual information, making it challenging to build cohesive datasets across multiple measurement sessions or research projects.
Current SOP Solutions for Zeta Potential Measurements
01 Standardized measurement protocols for zeta potential
Standardized operating procedures (SOPs) for zeta potential measurements ensure consistency and reproducibility across different laboratories and instruments. These protocols specify sample preparation methods, measurement conditions, calibration procedures, and data analysis techniques. Standardization helps in establishing reliable reference values and enables comparison of results obtained from different sources, which is crucial for quality control and regulatory compliance.- Standardized measurement protocols for zeta potential: Standardized operating procedures (SOPs) for zeta potential measurements ensure consistency and reproducibility across different laboratories and instruments. These protocols typically include detailed steps for sample preparation, instrument calibration, measurement parameters, and data analysis. Standardization helps in establishing reference methods that can be universally applied in various fields such as pharmaceuticals, colloid science, and materials characterization.
- Calibration methods for zeta potential measurement devices: Proper calibration of zeta potential measurement instruments is crucial for accurate and reliable results. Calibration methods involve the use of standard reference materials with known zeta potential values, verification of electrode conditions, and system performance checks. Regular calibration helps to identify and correct systematic errors, ensuring the validity of measurements across different samples and experimental conditions.
- Sample preparation techniques for zeta potential analysis: Effective sample preparation is essential for accurate zeta potential measurements. Techniques include proper dilution to appropriate concentration, pH adjustment, ionic strength control, and dispersion methods to prevent aggregation. The preparation process must be standardized to minimize variables that could affect the electrical double layer and subsequently the zeta potential readings. Consistent sample handling procedures ensure that measurements reflect the true surface properties of the particles.
- Automated systems for zeta potential measurement: Automated systems for zeta potential measurement enhance reproducibility and efficiency in standardized procedures. These systems incorporate automated sample handling, measurement sequencing, and data processing capabilities. By reducing human intervention, automated platforms minimize operator-dependent variations and enable high-throughput analysis. Advanced systems may include quality control checks, automated calibration routines, and integration with laboratory information management systems for comprehensive standardization.
- Data analysis and reporting standards for zeta potential: Standardized data analysis and reporting frameworks are critical for interpreting zeta potential measurements consistently. These standards define methods for statistical analysis, outlier identification, and uncertainty estimation. Reporting guidelines specify essential information to be included in documentation, such as measurement conditions, sample characteristics, and instrument parameters. Harmonized data formats facilitate comparison of results across different studies and enable meta-analysis for establishing industry benchmarks.
02 Advanced instrumentation for zeta potential measurement
Modern instruments for zeta potential measurement incorporate sophisticated technologies for enhanced accuracy and precision. These include laser Doppler electrophoresis, phase analysis light scattering, and electroacoustic techniques. Advanced systems feature automated calibration, real-time monitoring, and integrated quality control measures to ensure measurement reliability. Some instruments also offer multi-parameter analysis capabilities, allowing simultaneous measurement of particle size, concentration, and zeta potential.Expand Specific Solutions03 Sample preparation techniques for zeta potential analysis
Proper sample preparation is critical for accurate zeta potential measurements. Standardized procedures include methods for controlling sample concentration, pH adjustment, ionic strength regulation, and dispersion stability. Techniques for minimizing contamination, preventing aggregation, and ensuring representative sampling are essential components of standardized protocols. The preparation methods may vary depending on the nature of the sample (colloidal suspensions, emulsions, biological materials) and must be optimized accordingly.Expand Specific Solutions04 Calibration and validation methods for zeta potential systems
Standardized calibration and validation procedures ensure the accuracy and reliability of zeta potential measurement systems. These include the use of certified reference materials with known zeta potential values, regular performance verification tests, and system suitability checks. Statistical methods for evaluating measurement uncertainty, determining confidence intervals, and assessing method robustness are integral parts of standardization. Validation protocols typically address linearity, precision, accuracy, and the limits of detection and quantification.Expand Specific Solutions05 Data analysis and reporting standards for zeta potential measurements
Standardized approaches to data analysis and reporting ensure consistency in interpreting zeta potential measurements. These include statistical methods for processing raw data, algorithms for signal processing, and criteria for accepting or rejecting measurements. Reporting standards specify essential information such as measurement conditions, sample characteristics, instrument parameters, and uncertainty estimates that must be documented. Some standards also address data management practices, including storage, traceability, and electronic record requirements for regulatory compliance.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Zeta Potential Technology
The zeta potential measurement SOP market is in a growth phase, characterized by increasing demand for standardized procedures in multi-operator laboratories. The market size is expanding as pharmaceutical, academic, and industrial sectors recognize the importance of consistent colloidal stability measurements. Technologically, the field shows moderate maturity with established principles but ongoing refinement in standardization practices. Key players include diversified technology corporations (ABB Group, IBM), specialized instrument manufacturers (Bettersize Instruments, Sartorius BioAnalytical), pharmaceutical giants (Roche Diagnostics, F. Hoffmann-La Roche), and academic institutions (Max Planck Gesellschaft, Fuzhou University). The competitive landscape reveals collaboration between industry and academia to develop robust, reproducible measurement protocols that minimize operator variability while maintaining measurement accuracy across different laboratory environments.
Bettersize Instruments Ltd.
Technical Solution: Bettersize Instruments has developed a comprehensive Zeta Potential Measurement SOP that integrates their proprietary BeNano series instruments with standardized protocols for multi-operator environments. Their approach focuses on three key components: instrument qualification, sample preparation standardization, and measurement protocol harmonization. The BeNano system employs phase analysis light scattering (PALS) technology for high-sensitivity zeta potential measurements across various sample types. Their SOP includes detailed calibration procedures using NIST-traceable standards, automated quality control checks, and statistical analysis tools to identify operator variability. The system incorporates digital workflow management with electronic signatures and audit trails to ensure compliance with 21 CFR Part 11 regulations. Bettersize's SOP template includes mandatory training modules with competency assessments and periodic proficiency testing to minimize operator-dependent variations. Their cloud-based data management system allows for real-time monitoring of measurement quality across multiple laboratory sites.
Strengths: Specialized expertise in particle characterization technologies with purpose-built instruments for zeta potential measurement; comprehensive training programs and certification processes for operators; robust data management system for multi-site standardization. Weaknesses: Potentially higher implementation costs compared to generic solutions; may require significant adaptation for specialized sample types outside their standard applications.
Roche Diagnostics GmbH
Technical Solution: Roche Diagnostics GmbH has developed an integrated Zeta Potential Measurement SOP system as part of their broader quality management framework for diagnostic product development and manufacturing. Their approach combines rigorous analytical methodology with practical implementation tools designed specifically for multi-operator environments. The Roche SOP incorporates their proprietary electrophoretic mobility analysis technology with standardized sample preparation workflows validated across multiple laboratory sites. Their system features a tiered training program with competency verification at each level, ensuring all operators demonstrate proficiency before performing independent measurements. The SOP includes detailed environmental control specifications, as Roche's research has identified temperature fluctuation and electromagnetic interference as significant contributors to measurement variability between operators. Their digital workflow management system enforces sequential protocol steps with electronic verification at critical control points, preventing common procedural deviations. Roche's approach includes regular inter-laboratory comparison studies using reference materials to quantify and minimize site-to-site and operator-to-operator variability.
Strengths: Extensive experience in regulated diagnostic environments with robust validation methodologies; comprehensive operator training and certification program; sophisticated environmental control specifications to minimize external variables. Weaknesses: System may be overly complex for basic research applications; potentially higher implementation costs due to extensive documentation and validation requirements.
Key Technical Specifications and Parameters for Accurate Measurements
Zeta potential measurement method and measurement device
PatentActiveUS12313590B2
Innovation
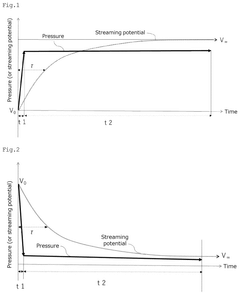
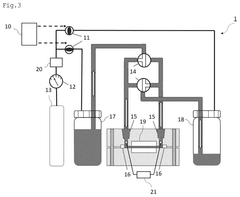
- A method and device for measuring zeta potential using a step-wise pressure change profile, where the pressure is changed in a rising or falling manner for a short time followed by a steady state phase lasting longer than the relaxation time, allowing for the calculation of zeta potential from the asymptotic value of the streaming potential.
Zirconia dispersion for use in forming NANO ceramics
PatentWO2020161451A9
Innovation
- An aqueous dispersion of zirconia nanoparticles with specific composition and polydispersity index, stabilized with additives like amino acids, is used to inhibit particle packing and reduce green density, allowing for efficient drying and minimizing cracking during the formation of ceramic articles.
Quality Control and Validation Procedures for Zeta Potential Measurements
Quality control and validation procedures are essential components of any robust zeta potential measurement protocol in multi-operator laboratory environments. Establishing standardized validation methods ensures measurement consistency and reliability across different operators and instruments.
The foundation of quality control begins with reference materials. Certified reference materials with known zeta potential values should be measured regularly to verify instrument performance. These standards should be selected to match the sample type being analyzed whenever possible, with polystyrene latex standards being commonly used for general applications due to their stability and well-characterized properties.
System suitability tests must be performed at defined intervals—typically daily before sample analysis and after major maintenance events. These tests should include verification of key parameters such as conductivity measurement accuracy, pH electrode calibration, and optical system performance. Acceptance criteria must be clearly defined, with documented procedures for actions required when results fall outside acceptable ranges.
Statistical process control charts represent a valuable tool for monitoring measurement stability over time. By plotting reference material measurements chronologically, laboratories can identify trends, shifts, or increased variability before they significantly impact results. Control limits should be established based on historical data, typically set at ±2σ (warning limits) and ±3σ (action limits).
Inter-operator reproducibility assessments are particularly critical in multi-operator environments. Periodic testing where multiple operators measure identical samples allows for quantification of operator-induced variability. Results should be statistically analyzed to determine if significant differences exist between operators, with targeted training implemented when necessary to reduce variability.
External quality assessment participation provides an additional layer of validation. Laboratories should regularly participate in proficiency testing programs or inter-laboratory comparisons specific to zeta potential measurements. These external checks help identify systematic biases that may not be apparent through internal quality control procedures alone.
Documentation requirements must be comprehensive and include calibration records, maintenance logs, control chart data, and all validation test results. Electronic laboratory information management systems can streamline this process while ensuring data integrity and traceability. Regular audits of documentation should be conducted to verify compliance with established procedures.
Measurement uncertainty calculations should follow established metrological principles, accounting for all significant sources of variability including instrument factors, sample preparation, environmental conditions, and operator technique. The expanded uncertainty should be reported alongside all zeta potential results to provide context for data interpretation and decision-making.
The foundation of quality control begins with reference materials. Certified reference materials with known zeta potential values should be measured regularly to verify instrument performance. These standards should be selected to match the sample type being analyzed whenever possible, with polystyrene latex standards being commonly used for general applications due to their stability and well-characterized properties.
System suitability tests must be performed at defined intervals—typically daily before sample analysis and after major maintenance events. These tests should include verification of key parameters such as conductivity measurement accuracy, pH electrode calibration, and optical system performance. Acceptance criteria must be clearly defined, with documented procedures for actions required when results fall outside acceptable ranges.
Statistical process control charts represent a valuable tool for monitoring measurement stability over time. By plotting reference material measurements chronologically, laboratories can identify trends, shifts, or increased variability before they significantly impact results. Control limits should be established based on historical data, typically set at ±2σ (warning limits) and ±3σ (action limits).
Inter-operator reproducibility assessments are particularly critical in multi-operator environments. Periodic testing where multiple operators measure identical samples allows for quantification of operator-induced variability. Results should be statistically analyzed to determine if significant differences exist between operators, with targeted training implemented when necessary to reduce variability.
External quality assessment participation provides an additional layer of validation. Laboratories should regularly participate in proficiency testing programs or inter-laboratory comparisons specific to zeta potential measurements. These external checks help identify systematic biases that may not be apparent through internal quality control procedures alone.
Documentation requirements must be comprehensive and include calibration records, maintenance logs, control chart data, and all validation test results. Electronic laboratory information management systems can streamline this process while ensuring data integrity and traceability. Regular audits of documentation should be conducted to verify compliance with established procedures.
Measurement uncertainty calculations should follow established metrological principles, accounting for all significant sources of variability including instrument factors, sample preparation, environmental conditions, and operator technique. The expanded uncertainty should be reported alongside all zeta potential results to provide context for data interpretation and decision-making.
Training Requirements and Competency Assessment for Laboratory Personnel
Effective training and competency assessment are critical components for ensuring reliable zeta potential measurements across multi-operator laboratories. A comprehensive training program must be established that addresses both theoretical knowledge and practical skills required for accurate zeta potential analysis.
Laboratory personnel should undergo initial training covering fundamental principles of zeta potential, including colloidal stability theory, electrical double layer concepts, and the relationship between zeta potential and particle behavior. This theoretical foundation ensures operators understand the significance of their measurements and can interpret results appropriately.
Hands-on training must include instrument operation, sample preparation techniques, data acquisition, and analysis software utilization. Special emphasis should be placed on recognizing and troubleshooting common measurement artifacts and errors that can affect zeta potential readings, such as electrode fouling, sample aggregation, and concentration effects.
A structured competency assessment framework should be implemented with clearly defined proficiency levels. New operators should demonstrate basic competency through supervised measurements of standard reference materials, with results compared against established acceptance criteria. Advanced competency should include the ability to prepare samples independently, optimize measurement parameters, and interpret complex data sets.
Regular proficiency testing is essential for maintaining measurement quality. This can be accomplished through blind sample analysis, where operators measure identical samples and results are compared for consistency. Statistical analysis of inter-operator variability provides valuable feedback on training effectiveness and highlights areas requiring additional focus.
Documentation of training completion and competency assessment results must be maintained in a centralized system. Each operator should have an individual training record that tracks initial qualification, periodic reassessments, and continuing education activities. These records serve as evidence of laboratory quality assurance and are valuable during audits or regulatory inspections.
Refresher training should be scheduled at defined intervals or triggered by specific events such as significant procedural changes, instrument modifications, or when quality control metrics indicate potential measurement drift. This ensures that all operators maintain current knowledge and skills despite potential changes in methodology or instrumentation.
Cross-training between laboratories within multi-site organizations further enhances measurement consistency and provides valuable perspective on different operational approaches. This collaborative training approach helps establish best practices and standardizes procedures across facilities.
Laboratory personnel should undergo initial training covering fundamental principles of zeta potential, including colloidal stability theory, electrical double layer concepts, and the relationship between zeta potential and particle behavior. This theoretical foundation ensures operators understand the significance of their measurements and can interpret results appropriately.
Hands-on training must include instrument operation, sample preparation techniques, data acquisition, and analysis software utilization. Special emphasis should be placed on recognizing and troubleshooting common measurement artifacts and errors that can affect zeta potential readings, such as electrode fouling, sample aggregation, and concentration effects.
A structured competency assessment framework should be implemented with clearly defined proficiency levels. New operators should demonstrate basic competency through supervised measurements of standard reference materials, with results compared against established acceptance criteria. Advanced competency should include the ability to prepare samples independently, optimize measurement parameters, and interpret complex data sets.
Regular proficiency testing is essential for maintaining measurement quality. This can be accomplished through blind sample analysis, where operators measure identical samples and results are compared for consistency. Statistical analysis of inter-operator variability provides valuable feedback on training effectiveness and highlights areas requiring additional focus.
Documentation of training completion and competency assessment results must be maintained in a centralized system. Each operator should have an individual training record that tracks initial qualification, periodic reassessments, and continuing education activities. These records serve as evidence of laboratory quality assurance and are valuable during audits or regulatory inspections.
Refresher training should be scheduled at defined intervals or triggered by specific events such as significant procedural changes, instrument modifications, or when quality control metrics indicate potential measurement drift. This ensures that all operators maintain current knowledge and skills despite potential changes in methodology or instrumentation.
Cross-training between laboratories within multi-site organizations further enhances measurement consistency and provides valuable perspective on different operational approaches. This collaborative training approach helps establish best practices and standardizes procedures across facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!