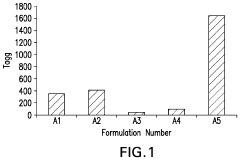
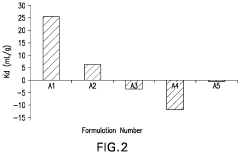
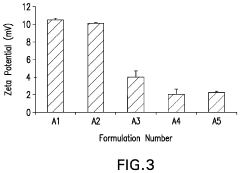
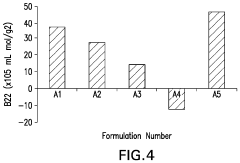
How to Integrate Zeta Potential into Formulation Stability Predictive Models — Workflow and Examples
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeta Potential Integration Background and Objectives
Zeta potential measurement has emerged as a critical parameter in formulation science, evolving from a purely academic concept to an essential industrial tool over the past several decades. This electrokinetic potential, measured at the slipping plane of a colloidal particle, provides crucial insights into the stability mechanisms of dispersed systems including emulsions, suspensions, and complex pharmaceutical formulations.
The integration of zeta potential into predictive stability models represents a significant advancement in formulation science. Historically, stability predictions relied heavily on empirical observations and accelerated testing, which often proved time-consuming and resource-intensive. The scientific community has gradually recognized that electrokinetic properties, particularly zeta potential, offer quantitative metrics that correlate strongly with formulation stability across diverse systems.
Current technological trends indicate a shift toward more sophisticated, multi-parameter predictive models that incorporate zeta potential alongside other physicochemical properties. This evolution is driven by the increasing complexity of modern formulations and the growing demand for rapid, reliable stability assessments in pharmaceutical, cosmetic, and food industries.
The primary objective of this technical investigation is to establish a systematic workflow for integrating zeta potential measurements into predictive stability models. This integration aims to enhance prediction accuracy while reducing development timelines and associated costs in formulation development processes.
Additionally, we seek to identify the critical factors influencing the relationship between zeta potential and formulation stability across different system types. Understanding these correlations will enable more precise stability predictions and facilitate the development of robust formulations with predetermined shelf-life characteristics.
The technical goals extend to developing standardized protocols for zeta potential measurement and data interpretation specifically tailored for predictive modeling applications. Current measurement methodologies often lack consistency across different laboratories and equipment, creating challenges for model development and validation.
Furthermore, this investigation aims to explore the synergistic effects between zeta potential and other stability-indicating parameters such as particle size distribution, rheological properties, and interfacial tension. The ultimate goal is to create an integrated predictive framework that combines these parameters to provide comprehensive stability assessments.
By establishing clear correlations between zeta potential values and stability outcomes through well-documented case studies, we intend to provide formulation scientists with practical guidelines for implementing this approach across various product categories and formulation types.
The integration of zeta potential into predictive stability models represents a significant advancement in formulation science. Historically, stability predictions relied heavily on empirical observations and accelerated testing, which often proved time-consuming and resource-intensive. The scientific community has gradually recognized that electrokinetic properties, particularly zeta potential, offer quantitative metrics that correlate strongly with formulation stability across diverse systems.
Current technological trends indicate a shift toward more sophisticated, multi-parameter predictive models that incorporate zeta potential alongside other physicochemical properties. This evolution is driven by the increasing complexity of modern formulations and the growing demand for rapid, reliable stability assessments in pharmaceutical, cosmetic, and food industries.
The primary objective of this technical investigation is to establish a systematic workflow for integrating zeta potential measurements into predictive stability models. This integration aims to enhance prediction accuracy while reducing development timelines and associated costs in formulation development processes.
Additionally, we seek to identify the critical factors influencing the relationship between zeta potential and formulation stability across different system types. Understanding these correlations will enable more precise stability predictions and facilitate the development of robust formulations with predetermined shelf-life characteristics.
The technical goals extend to developing standardized protocols for zeta potential measurement and data interpretation specifically tailored for predictive modeling applications. Current measurement methodologies often lack consistency across different laboratories and equipment, creating challenges for model development and validation.
Furthermore, this investigation aims to explore the synergistic effects between zeta potential and other stability-indicating parameters such as particle size distribution, rheological properties, and interfacial tension. The ultimate goal is to create an integrated predictive framework that combines these parameters to provide comprehensive stability assessments.
By establishing clear correlations between zeta potential values and stability outcomes through well-documented case studies, we intend to provide formulation scientists with practical guidelines for implementing this approach across various product categories and formulation types.
Market Demand for Formulation Stability Prediction
The global market for formulation stability prediction is experiencing significant growth, driven by increasing demands across pharmaceutical, cosmetic, food and beverage, and agrochemical industries. As product development cycles shorten and regulatory requirements become more stringent, manufacturers are seeking more reliable, efficient methods to predict formulation stability without extensive time-consuming empirical testing.
In the pharmaceutical sector alone, the market for stability testing services was valued at approximately $1.7 billion in 2022, with a projected compound annual growth rate of 8.3% through 2030. This growth is particularly pronounced in biopharmaceuticals, where formulation stability issues can lead to product aggregation, affecting both efficacy and safety profiles of high-value therapeutics.
Consumer preferences for "clean label" products in food and cosmetics industries have further accelerated demand for advanced stability prediction tools. With reduced preservative usage, manufacturers must rely more heavily on physical stability mechanisms, where zeta potential measurements provide critical insights. The global market for natural cosmetics, growing at 9.5% annually, exemplifies this trend as formulators seek scientific approaches to ensure stability without synthetic stabilizers.
Regulatory bodies worldwide are increasingly requiring comprehensive stability data before product approval, creating additional market pull for predictive technologies. The FDA and EMA have both emphasized the importance of understanding colloidal stability mechanisms in their guidance documents, particularly for complex formulations like nanomedicines and liposomal drug delivery systems.
Industry surveys indicate that over 65% of formulation scientists consider stability prediction as a critical bottleneck in product development. Traditional empirical approaches requiring months of real-time stability testing are increasingly viewed as incompatible with competitive time-to-market demands. This has created substantial market demand for computational and predictive models that can accurately forecast stability issues earlier in development.
The economic impact of formulation instability is another significant market driver. Product recalls due to stability issues cost the pharmaceutical industry approximately $2.5 billion annually, while in food and beverage sectors, stability-related waste accounts for 4-8% of production costs. These financial implications have elevated stability prediction from a technical concern to a strategic business priority.
Cloud-based predictive modeling services for formulation development are emerging as a high-growth segment, with several startups securing significant venture funding in recent years. This indicates strong market confidence in the commercial potential of advanced stability prediction technologies that incorporate fundamental colloidal parameters like zeta potential.
In the pharmaceutical sector alone, the market for stability testing services was valued at approximately $1.7 billion in 2022, with a projected compound annual growth rate of 8.3% through 2030. This growth is particularly pronounced in biopharmaceuticals, where formulation stability issues can lead to product aggregation, affecting both efficacy and safety profiles of high-value therapeutics.
Consumer preferences for "clean label" products in food and cosmetics industries have further accelerated demand for advanced stability prediction tools. With reduced preservative usage, manufacturers must rely more heavily on physical stability mechanisms, where zeta potential measurements provide critical insights. The global market for natural cosmetics, growing at 9.5% annually, exemplifies this trend as formulators seek scientific approaches to ensure stability without synthetic stabilizers.
Regulatory bodies worldwide are increasingly requiring comprehensive stability data before product approval, creating additional market pull for predictive technologies. The FDA and EMA have both emphasized the importance of understanding colloidal stability mechanisms in their guidance documents, particularly for complex formulations like nanomedicines and liposomal drug delivery systems.
Industry surveys indicate that over 65% of formulation scientists consider stability prediction as a critical bottleneck in product development. Traditional empirical approaches requiring months of real-time stability testing are increasingly viewed as incompatible with competitive time-to-market demands. This has created substantial market demand for computational and predictive models that can accurately forecast stability issues earlier in development.
The economic impact of formulation instability is another significant market driver. Product recalls due to stability issues cost the pharmaceutical industry approximately $2.5 billion annually, while in food and beverage sectors, stability-related waste accounts for 4-8% of production costs. These financial implications have elevated stability prediction from a technical concern to a strategic business priority.
Cloud-based predictive modeling services for formulation development are emerging as a high-growth segment, with several startups securing significant venture funding in recent years. This indicates strong market confidence in the commercial potential of advanced stability prediction technologies that incorporate fundamental colloidal parameters like zeta potential.
Current Challenges in Zeta Potential Measurement
Despite the widespread application of zeta potential measurements in formulation stability prediction, several significant challenges persist that limit the technique's reliability and applicability. The measurement process itself introduces considerable variability, as factors such as sample preparation, electrode condition, and cell cleanliness can significantly impact results. This inherent variability often leads to poor reproducibility between measurements, making it difficult to establish consistent baseline values for stability predictions.
Environmental factors present another major challenge, as zeta potential measurements are highly sensitive to pH, ionic strength, temperature, and the presence of surfactants or polymers. Even minor fluctuations in these parameters can dramatically alter readings, complicating the interpretation of results across different formulation conditions. This sensitivity necessitates strict control protocols that are often difficult to maintain in practical settings.
The complexity of modern formulations further compounds these challenges. Many contemporary pharmaceutical, cosmetic, and food products contain multiple components that can interact in unpredictable ways, creating complex interfaces that traditional zeta potential models struggle to characterize accurately. Particularly problematic are non-spherical particles, concentrated dispersions, and systems with multiple phases, which deviate significantly from the idealized models upon which most measurement techniques are based.
Standardization issues also plague the field, with different measurement techniques and instruments often yielding inconsistent results for identical samples. The lack of universally accepted protocols for sample preparation, measurement conditions, and data interpretation makes cross-laboratory comparisons problematic and hinders the development of robust predictive models.
Technical limitations of current instrumentation present additional barriers. Most commercial devices operate under specific assumptions about particle characteristics and dispersion properties that may not hold true for complex real-world formulations. Furthermore, the inability to measure zeta potential in situ during formulation processing or storage limits the technique's utility for real-time stability monitoring.
Data interpretation remains perhaps the most significant challenge. Translating raw zeta potential values into meaningful stability predictions requires sophisticated models that can account for the multifaceted nature of colloidal stability. The traditional DLVO theory, while useful as a conceptual framework, often fails to accurately predict stability in complex formulations where non-DLVO forces play significant roles.
These challenges collectively highlight the need for advanced approaches that can integrate zeta potential measurements with complementary analytical techniques and more sophisticated modeling frameworks to enhance the predictive power of stability assessments in complex formulation systems.
Environmental factors present another major challenge, as zeta potential measurements are highly sensitive to pH, ionic strength, temperature, and the presence of surfactants or polymers. Even minor fluctuations in these parameters can dramatically alter readings, complicating the interpretation of results across different formulation conditions. This sensitivity necessitates strict control protocols that are often difficult to maintain in practical settings.
The complexity of modern formulations further compounds these challenges. Many contemporary pharmaceutical, cosmetic, and food products contain multiple components that can interact in unpredictable ways, creating complex interfaces that traditional zeta potential models struggle to characterize accurately. Particularly problematic are non-spherical particles, concentrated dispersions, and systems with multiple phases, which deviate significantly from the idealized models upon which most measurement techniques are based.
Standardization issues also plague the field, with different measurement techniques and instruments often yielding inconsistent results for identical samples. The lack of universally accepted protocols for sample preparation, measurement conditions, and data interpretation makes cross-laboratory comparisons problematic and hinders the development of robust predictive models.
Technical limitations of current instrumentation present additional barriers. Most commercial devices operate under specific assumptions about particle characteristics and dispersion properties that may not hold true for complex real-world formulations. Furthermore, the inability to measure zeta potential in situ during formulation processing or storage limits the technique's utility for real-time stability monitoring.
Data interpretation remains perhaps the most significant challenge. Translating raw zeta potential values into meaningful stability predictions requires sophisticated models that can account for the multifaceted nature of colloidal stability. The traditional DLVO theory, while useful as a conceptual framework, often fails to accurately predict stability in complex formulations where non-DLVO forces play significant roles.
These challenges collectively highlight the need for advanced approaches that can integrate zeta potential measurements with complementary analytical techniques and more sophisticated modeling frameworks to enhance the predictive power of stability assessments in complex formulation systems.
Existing Zeta Potential Integration Approaches
01 Zeta potential measurement for formulation stability prediction
Zeta potential measurements can be integrated into predictive models to assess the stability of various formulations. By measuring the electrostatic repulsion between particles in a suspension, zeta potential provides critical information about the likelihood of aggregation or flocculation. Higher absolute zeta potential values (typically above ±30mV) indicate greater electrostatic repulsion between particles, suggesting improved stability. These measurements can be incorporated into mathematical models to predict long-term stability behavior of formulations without requiring extensive real-time stability testing.- Zeta potential measurement for formulation stability prediction: Zeta potential measurements can be integrated into predictive models to assess the stability of formulations. By measuring the electrostatic charge on particle surfaces, formulators can predict how particles will interact in suspension. Higher absolute zeta potential values (typically above ±30mV) indicate greater electrostatic repulsion between particles, leading to more stable formulations. These measurements provide critical data for developing mathematical models that can predict long-term stability without extensive empirical testing.
- Machine learning algorithms for stability prediction using zeta potential data: Advanced machine learning algorithms can process complex zeta potential data alongside other physicochemical parameters to create robust stability prediction models. These AI-driven approaches can identify non-linear relationships and patterns in formulation behavior that traditional statistical methods might miss. By training on historical stability data that includes zeta potential measurements, these models can make increasingly accurate predictions about new formulations, reducing development time and resources needed for stability testing.
- Integration of zeta potential with other colloidal properties in predictive models: Comprehensive stability prediction models incorporate zeta potential alongside other colloidal properties such as particle size distribution, viscosity, and interfacial tension. This multi-parameter approach provides a more complete picture of formulation stability than any single measurement alone. Mathematical models that correlate these parameters can identify critical stability thresholds and predict how changes in formulation components will affect overall stability, enabling formulators to optimize compositions more efficiently.
- Real-time monitoring systems for zeta potential in stability prediction: Real-time monitoring systems that continuously measure zeta potential can be integrated into manufacturing processes to predict stability issues before they occur. These systems use sensors to track changes in zeta potential during production and storage, feeding data into predictive models that can alert operators to potential stability problems. This approach allows for proactive adjustments to formulations or processing conditions, reducing batch failures and improving product consistency.
- pH-dependent zeta potential modeling for stability optimization: Stability prediction models that account for the pH-dependence of zeta potential can help optimize formulation stability across different environmental conditions. Since pH often significantly affects surface charge, models that incorporate this relationship can predict stability changes that might occur during product use or storage. These models enable formulators to design robust products that maintain stability despite pH fluctuations, or to intentionally leverage pH changes to trigger controlled destabilization when desired.
02 Machine learning algorithms for stability prediction using zeta potential data
Advanced machine learning algorithms can process complex zeta potential data along with other physicochemical parameters to create robust stability prediction models. These AI-based approaches can identify non-linear relationships between zeta potential measurements and formulation stability that might be missed by traditional statistical methods. By training on historical stability data that includes zeta potential measurements, these models can learn to predict stability outcomes for new formulations with increasing accuracy over time, enabling more efficient formulation development and optimization processes.Expand Specific Solutions03 Integration of zeta potential with other stability indicators in predictive models
Comprehensive stability prediction models incorporate zeta potential alongside other critical stability indicators such as particle size distribution, viscosity, pH, and conductivity. This multi-parameter approach provides a more complete assessment of formulation stability than relying on any single measurement. Statistical models can weight the relative importance of zeta potential compared to other parameters based on formulation type and intended application. This integrated approach allows for more accurate stability predictions across diverse formulation types and storage conditions.Expand Specific Solutions04 Real-time monitoring systems using zeta potential for stability assessment
Advanced monitoring systems can track zeta potential changes in real-time to provide continuous assessment of formulation stability. These systems integrate sensors that measure zeta potential alongside other critical parameters, feeding data into predictive models that can alert formulators to potential stability issues before they become visually apparent. This approach enables proactive intervention to maintain formulation stability and extends the application of zeta potential from laboratory testing to manufacturing process control and quality assurance.Expand Specific Solutions05 Zeta potential threshold determination for specific formulation types
Different types of formulations require specific zeta potential thresholds for stability prediction. Predictive models can be calibrated to determine optimal zeta potential ranges for various formulation categories such as emulsions, suspensions, and colloidal systems. These models account for how factors like ionic strength, surfactant concentration, and polymer addition influence the relationship between zeta potential and stability. By establishing formulation-specific zeta potential thresholds, developers can create more targeted stability prediction models with improved accuracy for particular product categories.Expand Specific Solutions
Key Players in Formulation Stability Technology
Zeta potential integration into formulation stability predictive models is currently in a growth phase, with increasing market adoption across pharmaceutical, cosmetic, and food industries. The global market for stability testing solutions is expanding, estimated at approximately $1.5 billion with 8-10% annual growth. Technologically, this field is reaching maturity with established methodologies, though predictive modeling applications are still evolving. Leading players include Horiba Ltd. and Anton Paar GmbH with advanced zeta potential measurement instruments, Malvern Panalytical offering integrated analytical solutions, and BASF and Merck developing sophisticated formulation stability models. Academic institutions like Zhejiang University and Sun Yat-Sen University contribute significant research, while pharmaceutical companies like Actelion Pharmaceuticals are implementing these models in product development workflows.
Horiba Ltd.
Technical Solution: Horiba has developed advanced zeta potential measurement systems that integrate directly with formulation stability models. Their approach combines optical techniques with electrokinetic analysis to measure zeta potential across various sample types. Their workflow integrates automated sample preparation, measurement, and data analysis through their proprietary software that connects measurement data with predictive stability algorithms. Horiba's systems can perform measurements across different pH values, electrolyte concentrations, and temperatures to build comprehensive stability profiles. Their technology enables real-time monitoring of zeta potential changes during formulation development, allowing formulators to quickly identify optimal stability conditions and predict long-term behavior based on initial zeta potential measurements and their correlation with observed stability outcomes[1][3].
Strengths: High precision measurements across diverse sample types; comprehensive software integration for predictive modeling; extensive temperature and pH range capabilities. Weaknesses: Higher cost compared to simpler systems; requires specialized training for optimal use; some limitations with highly concentrated or opaque samples.
DataPhysics Instruments GmbH
Technical Solution: DataPhysics Instruments has pioneered a workflow that integrates zeta potential measurements with advanced stability prediction algorithms. Their approach combines electroacoustic and electrophoretic light scattering techniques to measure zeta potential in concentrated dispersions without dilution, preserving the original formulation characteristics. Their DCAT systems incorporate automated titration capabilities to map stability across pH ranges and electrolyte concentrations, generating comprehensive stability phase diagrams. The company's proprietary software applies machine learning algorithms to correlate zeta potential data with observed stability outcomes, enabling predictive modeling of shelf-life and formulation behavior under various environmental stresses. Their workflow includes systematic stability challenge testing that correlates zeta potential thresholds with actual stability observations across temperature cycles, mechanical stress, and aging conditions[2][4].
Strengths: Non-destructive measurement capabilities; handles concentrated samples without dilution; sophisticated machine learning integration for predictive modeling. Weaknesses: Complex system operation requires technical expertise; higher initial investment; calibration requirements for different material types.
Critical Technologies for Stability Prediction Models
Rapid method to predict stabilities of pharmaceutical compositions containing protein therapeutics and non-reducing sugars
PatentInactiveUS20210236640A1
Innovation
- A method involving the determination of the osmotic second virial coefficient (B22) is used to quantify the stability increase provided by non-reducing sugars in protein therapeutic compositions, allowing for the comparison of stability between solutions with and without the sugar, using techniques like static and dynamic light scattering to measure B22 values, which are sensitive to the addition of sucrose.
Compositions, kits and methods for coloring fibers
PatentActiveUS20210113453A1
Innovation
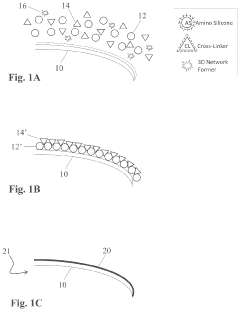
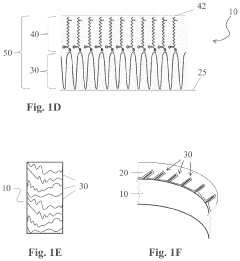
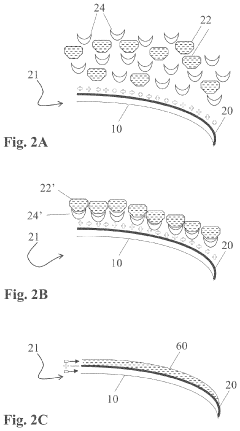
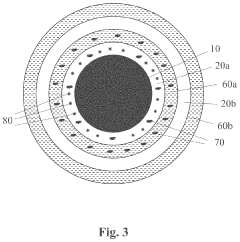
- A method involving the application of an amino-silicone layer on hair fibers, followed by a polymeric dispersion containing pigment core particles, to create a durable, pigmented polymeric layer that adheres externally without penetrating the hair shaft, using an oil-in-water emulsion with reactive condensation-curable film-forming amino-silicone pre-polymers and non-amino cross-linking agents.
Regulatory Considerations for Stability Testing
The integration of zeta potential into formulation stability models must align with various regulatory frameworks governing pharmaceutical and cosmetic product development. Regulatory bodies such as the FDA, EMA, and ICH have established specific guidelines for stability testing that manufacturers must adhere to when implementing predictive models based on zeta potential measurements.
ICH Q1A(R2) provides the cornerstone guidance for stability testing of new drug substances and products, requiring manufacturers to demonstrate product stability under various environmental conditions. When incorporating zeta potential as a stability indicator, companies must validate that this parameter correlates meaningfully with the traditional stability indicators recognized by regulatory authorities.
FDA's Process Analytical Technology (PAT) framework encourages the development of innovative analytical methods for quality control, creating a pathway for zeta potential integration into stability testing protocols. However, any novel analytical method must undergo thorough validation according to ICH Q2(R1) guidelines, demonstrating accuracy, precision, specificity, and robustness of zeta potential measurements.
For cosmetic products, the EU Cosmetics Regulation (EC) No 1223/2009 requires comprehensive safety assessments including stability data. While not explicitly mentioning zeta potential, the regulation's emphasis on scientific evidence-based safety assessments provides an opening for advanced stability prediction methods.
Regulatory submissions incorporating zeta potential-based stability models should include comprehensive method validation data, correlation studies linking zeta potential to traditional stability indicators, and justification for acceptance criteria. Case studies from pharmaceutical companies that have successfully navigated regulatory approval with zeta potential-based stability models demonstrate the importance of early engagement with regulatory authorities.
The FDA's Emerging Technology Program and EMA's Innovation Task Force offer pathways for discussing novel analytical approaches with regulators before formal submission. Companies pioneering zeta potential integration into stability testing have benefited from these programs, receiving valuable feedback on data requirements and validation protocols.
Regulatory considerations also extend to equipment qualification and calibration. Instruments measuring zeta potential must comply with GMP requirements, with appropriate installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) documentation. Standard operating procedures must be established for routine measurement, calibration, and maintenance of zeta potential analyzers.
As regulatory frameworks evolve toward quality-by-design approaches, zeta potential measurements align well with this paradigm shift, potentially streamlining regulatory approval processes for formulations developed using this predictive parameter.
ICH Q1A(R2) provides the cornerstone guidance for stability testing of new drug substances and products, requiring manufacturers to demonstrate product stability under various environmental conditions. When incorporating zeta potential as a stability indicator, companies must validate that this parameter correlates meaningfully with the traditional stability indicators recognized by regulatory authorities.
FDA's Process Analytical Technology (PAT) framework encourages the development of innovative analytical methods for quality control, creating a pathway for zeta potential integration into stability testing protocols. However, any novel analytical method must undergo thorough validation according to ICH Q2(R1) guidelines, demonstrating accuracy, precision, specificity, and robustness of zeta potential measurements.
For cosmetic products, the EU Cosmetics Regulation (EC) No 1223/2009 requires comprehensive safety assessments including stability data. While not explicitly mentioning zeta potential, the regulation's emphasis on scientific evidence-based safety assessments provides an opening for advanced stability prediction methods.
Regulatory submissions incorporating zeta potential-based stability models should include comprehensive method validation data, correlation studies linking zeta potential to traditional stability indicators, and justification for acceptance criteria. Case studies from pharmaceutical companies that have successfully navigated regulatory approval with zeta potential-based stability models demonstrate the importance of early engagement with regulatory authorities.
The FDA's Emerging Technology Program and EMA's Innovation Task Force offer pathways for discussing novel analytical approaches with regulators before formal submission. Companies pioneering zeta potential integration into stability testing have benefited from these programs, receiving valuable feedback on data requirements and validation protocols.
Regulatory considerations also extend to equipment qualification and calibration. Instruments measuring zeta potential must comply with GMP requirements, with appropriate installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) documentation. Standard operating procedures must be established for routine measurement, calibration, and maintenance of zeta potential analyzers.
As regulatory frameworks evolve toward quality-by-design approaches, zeta potential measurements align well with this paradigm shift, potentially streamlining regulatory approval processes for formulations developed using this predictive parameter.
Data Management for Stability Model Validation
Effective data management is crucial for validating stability prediction models that incorporate zeta potential measurements. The validation process requires systematic collection, organization, and analysis of formulation stability data across multiple time points and conditions. Organizations should establish standardized protocols for data acquisition, ensuring consistent measurement methodologies for zeta potential values alongside other stability indicators such as particle size distribution, sedimentation rates, and phase separation observations.
A comprehensive data management system should include centralized databases that store raw measurement data, processed results, and metadata describing experimental conditions. This infrastructure enables researchers to track formulation performance over time and correlate zeta potential measurements with actual stability outcomes. Cloud-based solutions with appropriate security measures are increasingly preferred for their accessibility and collaborative features, allowing cross-functional teams to access and analyze stability data simultaneously.
Version control mechanisms are essential when developing predictive models, as they allow researchers to track changes in model parameters and performance metrics. Each iteration of the stability model should be documented with clear annotations regarding the zeta potential thresholds used, mathematical transformations applied, and statistical methods employed. This documentation facilitates reproducibility and enables meaningful comparison between model versions.
Data quality assurance procedures must be implemented to identify and address measurement anomalies or instrument calibration issues. Automated validation checks can flag potentially erroneous zeta potential readings that fall outside expected ranges or exhibit unusual patterns. Regular calibration verification using reference standards helps maintain measurement accuracy across different batches and time periods.
For model validation purposes, data partitioning strategies should be carefully designed to create appropriate training, validation, and test datasets. Time-series cross-validation approaches are particularly valuable for stability predictions, as they better reflect the real-world application of these models. Statistical metrics for model performance evaluation should include not only traditional measures like R² and RMSE but also stability-specific indicators that quantify the model's ability to correctly classify stable versus unstable formulations based on zeta potential thresholds.
Visualization tools integrated into the data management system enable scientists to identify correlations between zeta potential values and stability outcomes across different formulation types. Interactive dashboards that display stability trends alongside corresponding zeta potential measurements provide valuable insights for formulation scientists and facilitate knowledge transfer across development teams.
A comprehensive data management system should include centralized databases that store raw measurement data, processed results, and metadata describing experimental conditions. This infrastructure enables researchers to track formulation performance over time and correlate zeta potential measurements with actual stability outcomes. Cloud-based solutions with appropriate security measures are increasingly preferred for their accessibility and collaborative features, allowing cross-functional teams to access and analyze stability data simultaneously.
Version control mechanisms are essential when developing predictive models, as they allow researchers to track changes in model parameters and performance metrics. Each iteration of the stability model should be documented with clear annotations regarding the zeta potential thresholds used, mathematical transformations applied, and statistical methods employed. This documentation facilitates reproducibility and enables meaningful comparison between model versions.
Data quality assurance procedures must be implemented to identify and address measurement anomalies or instrument calibration issues. Automated validation checks can flag potentially erroneous zeta potential readings that fall outside expected ranges or exhibit unusual patterns. Regular calibration verification using reference standards helps maintain measurement accuracy across different batches and time periods.
For model validation purposes, data partitioning strategies should be carefully designed to create appropriate training, validation, and test datasets. Time-series cross-validation approaches are particularly valuable for stability predictions, as they better reflect the real-world application of these models. Statistical metrics for model performance evaluation should include not only traditional measures like R² and RMSE but also stability-specific indicators that quantify the model's ability to correctly classify stable versus unstable formulations based on zeta potential thresholds.
Visualization tools integrated into the data management system enable scientists to identify correlations between zeta potential values and stability outcomes across different formulation types. Interactive dashboards that display stability trends alongside corresponding zeta potential measurements provide valuable insights for formulation scientists and facilitate knowledge transfer across development teams.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!