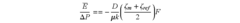How to Measure Zeta Potential in Complex Media (Serum, High Ionic Strength) — Protocol Adjustments
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeta Potential Measurement Background and Objectives
Zeta potential measurement has evolved significantly since its conceptualization in the early 20th century, emerging from fundamental electrokinetic theories developed by scientists like Smoluchowski and Hückel. This analytical technique has become increasingly critical in various fields including pharmaceuticals, nanomaterials, and biomedical applications where understanding colloidal stability is paramount.
The evolution of zeta potential measurement technology has progressed from rudimentary electrophoresis setups to sophisticated laser Doppler electrophoresis and electroacoustic systems. Modern instruments can measure particle velocities in electric fields with remarkable precision, converting these measurements into zeta potential values through established theoretical frameworks.
Traditional zeta potential measurement protocols were primarily designed for simple aqueous systems with moderate ionic strengths. However, contemporary research and industrial applications increasingly demand reliable measurements in complex biological media such as serum, plasma, cell culture media, and high ionic strength solutions that more accurately represent physiological conditions.
The fundamental challenge lies in the inherent limitations of conventional measurement approaches when applied to complex media. High protein content in serum creates multiple scattering effects and can adsorb onto particle surfaces, altering their electrophoretic behavior. Similarly, high ionic strength environments compress the electrical double layer, reducing electrophoretic mobility and challenging the sensitivity limits of standard instruments.
Current technical objectives in this field focus on developing robust protocol adjustments that can overcome these limitations without compromising measurement accuracy or reproducibility. These objectives include optimizing sample preparation techniques to minimize protein interference, modifying measurement parameters to account for high conductivity, and developing mathematical models that can accurately interpret electrophoretic data from complex media.
Recent advancements have introduced specialized cuvettes, advanced signal processing algorithms, and alternative measurement techniques such as phase analysis light scattering (PALS) that offer improved sensitivity in challenging media. However, standardized protocols for complex biological samples remain elusive, creating significant variability in reported values across different laboratories and instruments.
The ultimate goal of current research efforts is to establish universally applicable methodologies that enable reliable zeta potential measurements in complex biological media, thereby supporting critical applications in drug delivery, protein formulation stability, and nanomedicine. Such advancements would bridge the gap between controlled laboratory conditions and real-world biological environments, enhancing the predictive power of zeta potential measurements for practical applications.
The evolution of zeta potential measurement technology has progressed from rudimentary electrophoresis setups to sophisticated laser Doppler electrophoresis and electroacoustic systems. Modern instruments can measure particle velocities in electric fields with remarkable precision, converting these measurements into zeta potential values through established theoretical frameworks.
Traditional zeta potential measurement protocols were primarily designed for simple aqueous systems with moderate ionic strengths. However, contemporary research and industrial applications increasingly demand reliable measurements in complex biological media such as serum, plasma, cell culture media, and high ionic strength solutions that more accurately represent physiological conditions.
The fundamental challenge lies in the inherent limitations of conventional measurement approaches when applied to complex media. High protein content in serum creates multiple scattering effects and can adsorb onto particle surfaces, altering their electrophoretic behavior. Similarly, high ionic strength environments compress the electrical double layer, reducing electrophoretic mobility and challenging the sensitivity limits of standard instruments.
Current technical objectives in this field focus on developing robust protocol adjustments that can overcome these limitations without compromising measurement accuracy or reproducibility. These objectives include optimizing sample preparation techniques to minimize protein interference, modifying measurement parameters to account for high conductivity, and developing mathematical models that can accurately interpret electrophoretic data from complex media.
Recent advancements have introduced specialized cuvettes, advanced signal processing algorithms, and alternative measurement techniques such as phase analysis light scattering (PALS) that offer improved sensitivity in challenging media. However, standardized protocols for complex biological samples remain elusive, creating significant variability in reported values across different laboratories and instruments.
The ultimate goal of current research efforts is to establish universally applicable methodologies that enable reliable zeta potential measurements in complex biological media, thereby supporting critical applications in drug delivery, protein formulation stability, and nanomedicine. Such advancements would bridge the gap between controlled laboratory conditions and real-world biological environments, enhancing the predictive power of zeta potential measurements for practical applications.
Market Applications and Demand Analysis for Complex Media Measurements
The measurement of zeta potential in complex media represents a critical analytical need across multiple industries, with significant market demand driven by both research and commercial applications. The pharmaceutical sector stands as a primary market, where accurate zeta potential measurements in serum and high ionic strength environments are essential for drug delivery system development. Pharmaceutical companies require these measurements to predict the stability, circulation time, and biodistribution of nanoparticle-based therapeutics in physiological conditions.
Biotechnology represents another substantial market segment, with companies developing protein-based therapeutics, gene delivery systems, and diagnostic tools requiring precise characterization of biomolecular interactions in complex biological media. The ability to measure zeta potential in serum conditions directly correlates with predicting therapeutic efficacy and safety profiles, driving significant demand for advanced measurement protocols.
The medical device industry, particularly companies developing implantable materials and diagnostic sensors, constitutes a growing market for complex media zeta potential measurements. These measurements help predict protein adsorption patterns and potential inflammatory responses when materials contact biological fluids, critical factors in device biocompatibility assessment.
Environmental monitoring and water treatment sectors demonstrate increasing demand for zeta potential measurements in high ionic strength conditions, as these measurements provide crucial data on colloidal stability in wastewater, seawater, and industrial effluents. This application supports pollution control strategies and treatment efficiency optimization.
Food and beverage manufacturers utilize these measurements to assess the stability of emulsions, suspensions, and colloidal systems in complex food matrices with varying ionic compositions. The measurements help predict shelf-life stability and texture properties of formulated products.
Market analysis indicates the global zeta potential analyzer market is experiencing steady growth, with specialized instruments capable of handling complex media commanding premium pricing. The demand for protocol adjustments and specialized methodologies for complex media measurements has created a distinct consulting and method development sub-market within the analytical services sector.
Academic and government research institutions represent a significant market segment, particularly as interdisciplinary research at the bio-nano interface expands. These institutions drive fundamental research that ultimately informs industrial applications, creating a feedback loop that continuously refines measurement protocols and expands application areas.
Biotechnology represents another substantial market segment, with companies developing protein-based therapeutics, gene delivery systems, and diagnostic tools requiring precise characterization of biomolecular interactions in complex biological media. The ability to measure zeta potential in serum conditions directly correlates with predicting therapeutic efficacy and safety profiles, driving significant demand for advanced measurement protocols.
The medical device industry, particularly companies developing implantable materials and diagnostic sensors, constitutes a growing market for complex media zeta potential measurements. These measurements help predict protein adsorption patterns and potential inflammatory responses when materials contact biological fluids, critical factors in device biocompatibility assessment.
Environmental monitoring and water treatment sectors demonstrate increasing demand for zeta potential measurements in high ionic strength conditions, as these measurements provide crucial data on colloidal stability in wastewater, seawater, and industrial effluents. This application supports pollution control strategies and treatment efficiency optimization.
Food and beverage manufacturers utilize these measurements to assess the stability of emulsions, suspensions, and colloidal systems in complex food matrices with varying ionic compositions. The measurements help predict shelf-life stability and texture properties of formulated products.
Market analysis indicates the global zeta potential analyzer market is experiencing steady growth, with specialized instruments capable of handling complex media commanding premium pricing. The demand for protocol adjustments and specialized methodologies for complex media measurements has created a distinct consulting and method development sub-market within the analytical services sector.
Academic and government research institutions represent a significant market segment, particularly as interdisciplinary research at the bio-nano interface expands. These institutions drive fundamental research that ultimately informs industrial applications, creating a feedback loop that continuously refines measurement protocols and expands application areas.
Technical Challenges in Complex Media Zeta Potential Analysis
Measuring zeta potential in complex media presents significant technical challenges that require careful consideration and protocol adjustments. Traditional zeta potential measurement techniques, primarily based on electrophoretic light scattering, were developed for simple aqueous systems with low ionic strength. When applied to complex biological media such as serum or high ionic strength solutions, these conventional methods encounter numerous obstacles.
The primary challenge stems from the high conductivity of complex media, which generates excessive Joule heating during electrophoresis. This heating can denature proteins, alter sample composition, and create convection currents that interfere with accurate particle movement tracking. Additionally, the applied electric field may be significantly attenuated in high ionic strength environments, reducing the electrophoretic mobility of particles and compromising measurement sensitivity.
Protein adsorption presents another major hurdle in complex media analysis. Serum proteins readily adsorb onto particle surfaces, forming a protein corona that fundamentally alters the electric double layer structure. This dynamic protein layer changes over time and varies with protein concentration, making reproducible measurements exceptionally difficult. The protein corona also increases particle size and modifies surface charge characteristics, further complicating data interpretation.
Multiple scattering effects emerge as a critical limitation when measuring samples with high particle concentrations or in media containing various biological components. These effects distort the scattered light signal, leading to inaccurate zeta potential calculations. The heterogeneity of biological samples adds another layer of complexity, as diverse particle populations with different sizes and surface properties produce overlapping signals that are challenging to deconvolute.
Electrode polarization and degradation occur more rapidly in complex media, particularly those with high salt content. This leads to unstable measurements and reduced electrode lifespan. Furthermore, the viscosity of complex biological fluids differs significantly from water, requiring careful calibration adjustments since electrophoretic mobility calculations typically assume water-like viscosity properties.
pH stability represents another technical challenge, as complex media often have inherent buffering capacity that may resist the pH adjustments necessary for standardized measurements. The ionic composition of biological fluids also creates ion-specific effects that can alter the structure of the electric double layer in ways not accounted for by classical theories.
These technical challenges necessitate substantial protocol modifications and potentially new measurement approaches to obtain reliable zeta potential data in complex biological environments. Addressing these limitations requires interdisciplinary solutions combining principles from colloid science, biophysics, and analytical chemistry.
The primary challenge stems from the high conductivity of complex media, which generates excessive Joule heating during electrophoresis. This heating can denature proteins, alter sample composition, and create convection currents that interfere with accurate particle movement tracking. Additionally, the applied electric field may be significantly attenuated in high ionic strength environments, reducing the electrophoretic mobility of particles and compromising measurement sensitivity.
Protein adsorption presents another major hurdle in complex media analysis. Serum proteins readily adsorb onto particle surfaces, forming a protein corona that fundamentally alters the electric double layer structure. This dynamic protein layer changes over time and varies with protein concentration, making reproducible measurements exceptionally difficult. The protein corona also increases particle size and modifies surface charge characteristics, further complicating data interpretation.
Multiple scattering effects emerge as a critical limitation when measuring samples with high particle concentrations or in media containing various biological components. These effects distort the scattered light signal, leading to inaccurate zeta potential calculations. The heterogeneity of biological samples adds another layer of complexity, as diverse particle populations with different sizes and surface properties produce overlapping signals that are challenging to deconvolute.
Electrode polarization and degradation occur more rapidly in complex media, particularly those with high salt content. This leads to unstable measurements and reduced electrode lifespan. Furthermore, the viscosity of complex biological fluids differs significantly from water, requiring careful calibration adjustments since electrophoretic mobility calculations typically assume water-like viscosity properties.
pH stability represents another technical challenge, as complex media often have inherent buffering capacity that may resist the pH adjustments necessary for standardized measurements. The ionic composition of biological fluids also creates ion-specific effects that can alter the structure of the electric double layer in ways not accounted for by classical theories.
These technical challenges necessitate substantial protocol modifications and potentially new measurement approaches to obtain reliable zeta potential data in complex biological environments. Addressing these limitations requires interdisciplinary solutions combining principles from colloid science, biophysics, and analytical chemistry.
Current Protocol Solutions for Complex Media Measurements
01 Sample preparation techniques for zeta potential measurement
Proper sample preparation is crucial for accurate zeta potential measurements. This includes techniques for dispersing particles in appropriate media, controlling concentration levels, and ensuring sample homogeneity. Specialized preparation protocols may be required for different types of materials such as nanoparticles, colloids, or biological samples. The preparation process often involves sonication, filtration, or dilution steps to achieve optimal measurement conditions.- Sample preparation techniques for zeta potential measurement: Proper sample preparation is crucial for accurate zeta potential measurements. This includes adjusting the concentration of particles, selecting appropriate dispersants, and controlling the pH of the suspension. The preparation process may involve sonication to break up aggregates, filtration to remove large particles, and stabilization of the suspension to prevent sedimentation during measurement. These techniques help ensure that the measured zeta potential accurately represents the surface charge of the particles in the suspension.
- Environmental parameter control during zeta potential measurement: Environmental parameters significantly affect zeta potential measurements and require careful control. Temperature regulation is essential as it influences particle mobility and electrolyte conductivity. Ionic strength and pH of the medium must be monitored and adjusted as they directly impact the electrical double layer around particles. Controlling these parameters ensures reproducibility and accuracy in zeta potential measurements across different experimental conditions and sample types.
- Advanced instrumentation for zeta potential measurement: Modern zeta potential measurement systems incorporate advanced technologies to improve accuracy and reliability. These include laser Doppler electrophoresis, phase analysis light scattering, and electroacoustic techniques. Some instruments feature automated calibration, real-time monitoring, and multi-angle detection capabilities. These advancements allow for measurements across a wider range of sample concentrations and particle sizes, reducing measurement errors and increasing data quality.
- Data analysis and interpretation methods for zeta potential measurements: Effective data analysis is essential for extracting meaningful information from zeta potential measurements. This includes statistical processing of raw data, application of appropriate theoretical models, and correction factors for various experimental conditions. Advanced software algorithms can identify and filter outliers, perform trend analysis, and generate comprehensive reports. Proper interpretation considers the relationship between zeta potential values and colloidal stability, taking into account the specific characteristics of the sample being analyzed.
- Protocol optimization for specific sample types: Zeta potential measurement protocols often require customization based on the specific properties of the sample being analyzed. For biological samples, adjustments may include using physiological buffers and controlling protein adsorption. For nanomaterials, protocols might address agglomeration issues and surface coating effects. Industrial suspensions may require dilution strategies and contaminant removal procedures. These tailored approaches ensure that the measurement conditions are appropriate for the unique characteristics of each sample type, leading to more reliable and relevant zeta potential data.
02 Environmental parameter adjustments for zeta potential measurements
Environmental parameters significantly affect zeta potential measurements and require careful adjustment. These parameters include pH, ionic strength, temperature, and conductivity of the measurement medium. Protocols often specify precise control of these variables to ensure reproducibility and accuracy. Adjustments may involve titration methods for pH control, temperature regulation systems, or specific buffer compositions to maintain consistent ionic environments during measurement.Expand Specific Solutions03 Instrumentation modifications and calibration procedures
Specialized modifications to zeta potential measurement instruments can improve accuracy for specific sample types. These modifications may include electrode designs, cell configurations, or optical components. Regular calibration procedures using standard reference materials are essential for maintaining measurement accuracy. Protocols often detail specific calibration sequences, verification steps, and maintenance procedures to ensure optimal instrument performance and reliable results.Expand Specific Solutions04 Data analysis and interpretation methodologies
Advanced data analysis techniques are crucial for interpreting zeta potential measurements accurately. These methodologies include statistical approaches for handling measurement variability, algorithms for processing raw electrophoretic mobility data, and models for converting measurements to zeta potential values. Protocols may specify particular mathematical treatments, filtering methods, or software settings to ensure consistent interpretation across different sample types and measurement conditions.Expand Specific Solutions05 Protocol adaptations for challenging sample types
Special protocol adaptations are necessary for measuring zeta potential in challenging samples such as highly concentrated suspensions, non-aqueous systems, or samples with extreme properties. These adaptations may include dilution strategies, alternative measurement modes, or specialized cell designs. For biological or sensitive samples, protocols often incorporate gentle handling techniques, specific buffer systems, or modified measurement parameters to preserve sample integrity while obtaining accurate zeta potential values.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Zeta Potential Technology
Zeta potential measurement in complex media represents a challenging frontier in colloidal science, currently in a growth phase with expanding applications across biomedical and industrial sectors. The market is experiencing significant expansion as researchers tackle the limitations of traditional measurement techniques in high ionic strength environments and biological media. Leading companies like Malvern Panalytical, Anton Paar, and Bettersize Instruments are developing specialized instrumentation, while academic institutions including MIT, University of Tokyo, and Nanjing University are advancing fundamental research. The technology is approaching maturity for standard applications but remains in development for complex media, with recent innovations focusing on protocol modifications, alternative measurement techniques, and specialized sample preparation methods to overcome the challenges of serum and high ionic strength environments.
Bettersize Instruments Ltd.
Technical Solution: Bettersize Instruments has developed the BeNano series specifically addressing zeta potential measurements in complex media through innovative hardware and protocol modifications. Their approach combines traditional electrophoretic light scattering with a proprietary cross-correlation optical system that effectively filters out multiple scattering effects common in complex biological samples. For high ionic strength measurements, they've implemented specialized platinum-iridium alloy electrodes with enhanced resistance to polarization and degradation. Their protocol adjustments include a systematic dilution approach with conductivity matching to maintain sample integrity while enabling accurate measurements. The BeNano system features automatic field strength modulation that adjusts measurement parameters based on real-time sample conductivity feedback, preventing electrode damage while optimizing signal quality. For serum applications, they've developed a specialized sample preparation protocol involving gentle filtration and buffer exchange techniques that preserve the electric double layer characteristics of particles. Their analysis software incorporates advanced algorithms that can extract meaningful mobility data even from samples with significant background interference.
Strengths: Cost-effective solution compared to premium market offerings; robust performance across diverse sample types; simplified workflow designed for routine analysis environments. Weaknesses: Less extensive validation in pharmaceutical applications; more limited resolution for complex multimodal distributions; fewer specialized accessories for extreme measurement conditions.
Izon Science Ltd.
Technical Solution: Izon Science has pioneered an alternative approach to zeta potential measurement in complex media through their Tunable Resistive Pulse Sensing (TRPS) technology. Unlike traditional light scattering methods, TRPS measures the electrical properties of individual particles as they pass through a nanopore, enabling direct particle-by-particle zeta potential determination even in challenging media. Their protocol adjustments for complex samples include specialized nanopore coatings that resist protein fouling and maintain pore stability in high ionic strength solutions. For serum applications, they've developed a gradient buffer system that creates a stable measurement environment while preserving the native particle surface characteristics. The company's qNano instrument incorporates pressure control systems that can counteract electroosmotic flow effects common in complex media measurements. Their analysis software features machine learning algorithms trained on extensive reference datasets to identify and correct for media-specific interferences. Additionally, Izon has established standardized calibration procedures using reference particles specifically designed for high-conductivity environments.
Strengths: Unique particle-by-particle measurement capability provides detailed distribution data; less affected by sample opacity than optical methods; direct measurement approach reduces theoretical assumptions. Weaknesses: Lower throughput compared to ensemble techniques; more complex sample preparation requirements; limited to particles within specific size ranges determined by nanopore dimensions.
Key Innovations in Electrophoretic Light Scattering Methods
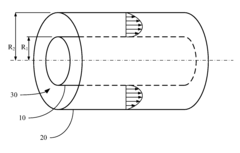
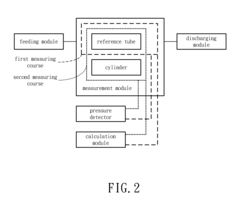
Method and system for measuring the zeta potential of the cylinder's outer surface
PatentActiveUS7662268B2
Innovation
- A method and system using an annular pipe design where a reference tube and a cylinder are coaxially aligned, with solution flow through an annular channel, measuring the streaming potential difference to determine the zeta potential of the cylinder's outer surface using an electrokinetic relationship.
Zeta potential measurement method and measurement device
PatentWO2022196533A1
Innovation
- A method and device that utilize a stepwise pressure change profile to measure zeta potential, allowing for the calculation of an equilibrium value from the transient response of streaming potential, thereby reducing hysteresis and improving reproducibility and accuracy by considering the relaxation time of the streaming potential.
Sample Preparation Optimization Strategies for High Ionic Strength Media
Measuring zeta potential in high ionic strength media presents significant challenges due to the electrical double layer compression effect. To overcome these obstacles, several sample preparation optimization strategies can be implemented to enhance measurement accuracy and reliability.
Dilution techniques represent a primary approach for managing high ionic strength samples. By systematically diluting the sample with appropriate diluents, researchers can reduce ionic concentration while maintaining sample integrity. The optimal dilution ratio must be determined experimentally for each specific sample type, typically ranging from 1:10 to 1:100 depending on the initial ionic strength. This approach helps minimize electrical double layer compression while preserving the essential characteristics of the particles under investigation.
Buffer selection and modification play crucial roles in optimizing sample preparation. Phosphate buffers at concentrations between 1-10 mM have demonstrated effectiveness in maintaining pH stability while minimizing ionic interference. For biological samples, HEPES buffer (5-20 mM) offers superior performance due to its minimal interaction with biomolecules and moderate ionic contribution. Custom buffer formulations incorporating zwitterionic components can further reduce ionic effects while maintaining physiological relevance.
Surfactant addition represents another valuable strategy for high ionic strength media. Non-ionic surfactants such as Tween-20 or Triton X-100 at concentrations of 0.01-0.05% can stabilize particle dispersions without significantly altering surface charge properties. These additives prevent particle aggregation caused by the screening of electrostatic repulsions in high ionic environments, thereby improving measurement reproducibility.
Filtration and centrifugation protocols must be optimized specifically for high ionic strength conditions. Sequential filtration using decreasing pore sizes (starting at 0.45 μm and progressing to 0.22 μm) effectively removes larger aggregates while preserving the target particles. Differential centrifugation at carefully calibrated speeds (typically 5,000-15,000 g) can separate particles of interest from ionic components, though care must be taken to avoid altering the particle surface properties during this process.
Temperature control during sample preparation and measurement represents a critical yet often overlooked parameter. Maintaining consistent temperature (±0.5°C) throughout the preparation process helps stabilize ionic interactions and prevents temperature-induced aggregation. Most high ionic strength samples demonstrate optimal stability between 20-25°C, with measurements ideally conducted at the same temperature used during preparation to ensure data consistency.
Dilution techniques represent a primary approach for managing high ionic strength samples. By systematically diluting the sample with appropriate diluents, researchers can reduce ionic concentration while maintaining sample integrity. The optimal dilution ratio must be determined experimentally for each specific sample type, typically ranging from 1:10 to 1:100 depending on the initial ionic strength. This approach helps minimize electrical double layer compression while preserving the essential characteristics of the particles under investigation.
Buffer selection and modification play crucial roles in optimizing sample preparation. Phosphate buffers at concentrations between 1-10 mM have demonstrated effectiveness in maintaining pH stability while minimizing ionic interference. For biological samples, HEPES buffer (5-20 mM) offers superior performance due to its minimal interaction with biomolecules and moderate ionic contribution. Custom buffer formulations incorporating zwitterionic components can further reduce ionic effects while maintaining physiological relevance.
Surfactant addition represents another valuable strategy for high ionic strength media. Non-ionic surfactants such as Tween-20 or Triton X-100 at concentrations of 0.01-0.05% can stabilize particle dispersions without significantly altering surface charge properties. These additives prevent particle aggregation caused by the screening of electrostatic repulsions in high ionic environments, thereby improving measurement reproducibility.
Filtration and centrifugation protocols must be optimized specifically for high ionic strength conditions. Sequential filtration using decreasing pore sizes (starting at 0.45 μm and progressing to 0.22 μm) effectively removes larger aggregates while preserving the target particles. Differential centrifugation at carefully calibrated speeds (typically 5,000-15,000 g) can separate particles of interest from ionic components, though care must be taken to avoid altering the particle surface properties during this process.
Temperature control during sample preparation and measurement represents a critical yet often overlooked parameter. Maintaining consistent temperature (±0.5°C) throughout the preparation process helps stabilize ionic interactions and prevents temperature-induced aggregation. Most high ionic strength samples demonstrate optimal stability between 20-25°C, with measurements ideally conducted at the same temperature used during preparation to ensure data consistency.
Validation and Reproducibility Standards for Complex Media Measurements
Establishing robust validation and reproducibility standards is critical for zeta potential measurements in complex media such as serum or high ionic strength solutions. These standards must address the unique challenges posed by these environments while ensuring consistent and reliable results across different laboratories and experimental conditions.
The foundation of validation standards begins with reference materials specifically designed for complex media. Unlike traditional calibration standards used in simple aqueous solutions, these reference materials must maintain stability in protein-rich or high-salt environments. Polystyrene latex particles with well-characterized surface modifications have emerged as promising candidates, demonstrating reasonable stability in serum conditions when properly functionalized with appropriate polymers.
Interlaboratory comparison studies represent another crucial component of validation protocols. These studies should involve multiple facilities measuring identical samples under standardized conditions, with results analyzed for consistency and variability. For complex media measurements, these comparisons must account for sample preparation variations, equipment differences, and environmental factors that may influence zeta potential readings.
Statistical analysis frameworks specifically adapted for complex media measurements are essential. These frameworks should incorporate uncertainty calculations that account for the inherent variability in biological samples and high ionic strength solutions. Recommended approaches include multiple replicate measurements (minimum n=5), outlier identification protocols, and appropriate statistical tests to determine measurement confidence intervals in non-ideal conditions.
Sample handling and preparation standardization is particularly critical for reproducibility in complex media. Detailed protocols must specify sample storage conditions, temperature equilibration procedures, and precise dilution methodologies. For serum samples, standardized protocols should address protein concentration normalization and potential pre-filtration requirements to remove large aggregates that may interfere with measurements.
Equipment qualification procedures must be more rigorous for complex media applications. This includes regular verification using both standard and complex media reference materials, with acceptance criteria specifically defined for measurements in challenging environments. Performance verification should assess stability, drift, and signal-to-noise ratios under conditions that mimic actual experimental samples.
Documentation requirements for complex media measurements should exceed standard practices, including comprehensive reporting of sample preparation details, measurement parameters, data processing methods, and environmental conditions. This enhanced documentation facilitates troubleshooting and enables meaningful comparison between studies conducted in different laboratories or at different times.
The foundation of validation standards begins with reference materials specifically designed for complex media. Unlike traditional calibration standards used in simple aqueous solutions, these reference materials must maintain stability in protein-rich or high-salt environments. Polystyrene latex particles with well-characterized surface modifications have emerged as promising candidates, demonstrating reasonable stability in serum conditions when properly functionalized with appropriate polymers.
Interlaboratory comparison studies represent another crucial component of validation protocols. These studies should involve multiple facilities measuring identical samples under standardized conditions, with results analyzed for consistency and variability. For complex media measurements, these comparisons must account for sample preparation variations, equipment differences, and environmental factors that may influence zeta potential readings.
Statistical analysis frameworks specifically adapted for complex media measurements are essential. These frameworks should incorporate uncertainty calculations that account for the inherent variability in biological samples and high ionic strength solutions. Recommended approaches include multiple replicate measurements (minimum n=5), outlier identification protocols, and appropriate statistical tests to determine measurement confidence intervals in non-ideal conditions.
Sample handling and preparation standardization is particularly critical for reproducibility in complex media. Detailed protocols must specify sample storage conditions, temperature equilibration procedures, and precise dilution methodologies. For serum samples, standardized protocols should address protein concentration normalization and potential pre-filtration requirements to remove large aggregates that may interfere with measurements.
Equipment qualification procedures must be more rigorous for complex media applications. This includes regular verification using both standard and complex media reference materials, with acceptance criteria specifically defined for measurements in challenging environments. Performance verification should assess stability, drift, and signal-to-noise ratios under conditions that mimic actual experimental samples.
Documentation requirements for complex media measurements should exceed standard practices, including comprehensive reporting of sample preparation details, measurement parameters, data processing methods, and environmental conditions. This enhanced documentation facilitates troubleshooting and enables meaningful comparison between studies conducted in different laboratories or at different times.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!