How to Test Luteolin Antioxidant Capacity in Vivo
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luteolin Antioxidant Research Background and Objectives
Luteolin, a natural flavonoid found in various fruits, vegetables, and medicinal herbs, has gained significant attention in the scientific community due to its potent antioxidant properties. The exploration of antioxidant compounds has evolved substantially over the past few decades, transitioning from basic chemical characterization to sophisticated in vivo testing methodologies that better reflect biological relevance.
The historical development of antioxidant research began in the 1950s with the free radical theory of aging, which established the fundamental relationship between oxidative stress and various pathological conditions. By the 1990s, researchers had identified numerous plant-derived compounds, including luteolin, as potential therapeutic agents against oxidative damage. The 21st century has witnessed an exponential growth in publications focusing on the biological activities of luteolin, with particular emphasis on its antioxidant capacity.
Current technological trends in antioxidant assessment have shifted from traditional in vitro chemical assays toward more physiologically relevant in vivo models. This evolution reflects the growing recognition that antioxidant efficacy observed in test tubes often fails to translate directly to living systems due to complex factors including bioavailability, metabolism, and tissue distribution.
The primary objective of in vivo luteolin antioxidant testing is to establish reliable, reproducible methodologies that accurately reflect the compound's ability to mitigate oxidative stress in living organisms. This includes determining effective dosages, optimal administration routes, and identifying specific biomarkers that can quantitatively measure antioxidant activity in target tissues.
Secondary objectives include elucidating the molecular mechanisms underlying luteolin's antioxidant effects, such as its interaction with endogenous antioxidant defense systems, influence on redox-sensitive signaling pathways, and potential synergistic effects with other dietary components. Understanding these mechanisms is crucial for developing targeted therapeutic applications.
Long-term research goals in this field aim to establish standardized protocols for in vivo antioxidant assessment that can facilitate comparison across different studies and compounds. Additionally, there is growing interest in developing personalized approaches to antioxidant therapy, recognizing that individual variations in metabolism and oxidative stress profiles may significantly impact treatment efficacy.
The technological trajectory suggests an increasing integration of advanced analytical techniques, including metabolomics, proteomics, and real-time imaging, to provide comprehensive insights into the dynamic interactions between luteolin and biological systems. These developments promise to bridge the gap between laboratory findings and clinical applications, potentially leading to novel therapeutic strategies for oxidative stress-related disorders.
The historical development of antioxidant research began in the 1950s with the free radical theory of aging, which established the fundamental relationship between oxidative stress and various pathological conditions. By the 1990s, researchers had identified numerous plant-derived compounds, including luteolin, as potential therapeutic agents against oxidative damage. The 21st century has witnessed an exponential growth in publications focusing on the biological activities of luteolin, with particular emphasis on its antioxidant capacity.
Current technological trends in antioxidant assessment have shifted from traditional in vitro chemical assays toward more physiologically relevant in vivo models. This evolution reflects the growing recognition that antioxidant efficacy observed in test tubes often fails to translate directly to living systems due to complex factors including bioavailability, metabolism, and tissue distribution.
The primary objective of in vivo luteolin antioxidant testing is to establish reliable, reproducible methodologies that accurately reflect the compound's ability to mitigate oxidative stress in living organisms. This includes determining effective dosages, optimal administration routes, and identifying specific biomarkers that can quantitatively measure antioxidant activity in target tissues.
Secondary objectives include elucidating the molecular mechanisms underlying luteolin's antioxidant effects, such as its interaction with endogenous antioxidant defense systems, influence on redox-sensitive signaling pathways, and potential synergistic effects with other dietary components. Understanding these mechanisms is crucial for developing targeted therapeutic applications.
Long-term research goals in this field aim to establish standardized protocols for in vivo antioxidant assessment that can facilitate comparison across different studies and compounds. Additionally, there is growing interest in developing personalized approaches to antioxidant therapy, recognizing that individual variations in metabolism and oxidative stress profiles may significantly impact treatment efficacy.
The technological trajectory suggests an increasing integration of advanced analytical techniques, including metabolomics, proteomics, and real-time imaging, to provide comprehensive insights into the dynamic interactions between luteolin and biological systems. These developments promise to bridge the gap between laboratory findings and clinical applications, potentially leading to novel therapeutic strategies for oxidative stress-related disorders.
Market Demand for Natural Antioxidant Compounds
The global market for natural antioxidant compounds has experienced significant growth over the past decade, driven primarily by increasing consumer awareness of health benefits and a shift toward preventive healthcare. Luteolin, a flavonoid found in various fruits, vegetables, and medicinal herbs, has emerged as a particularly promising antioxidant compound with substantial market potential.
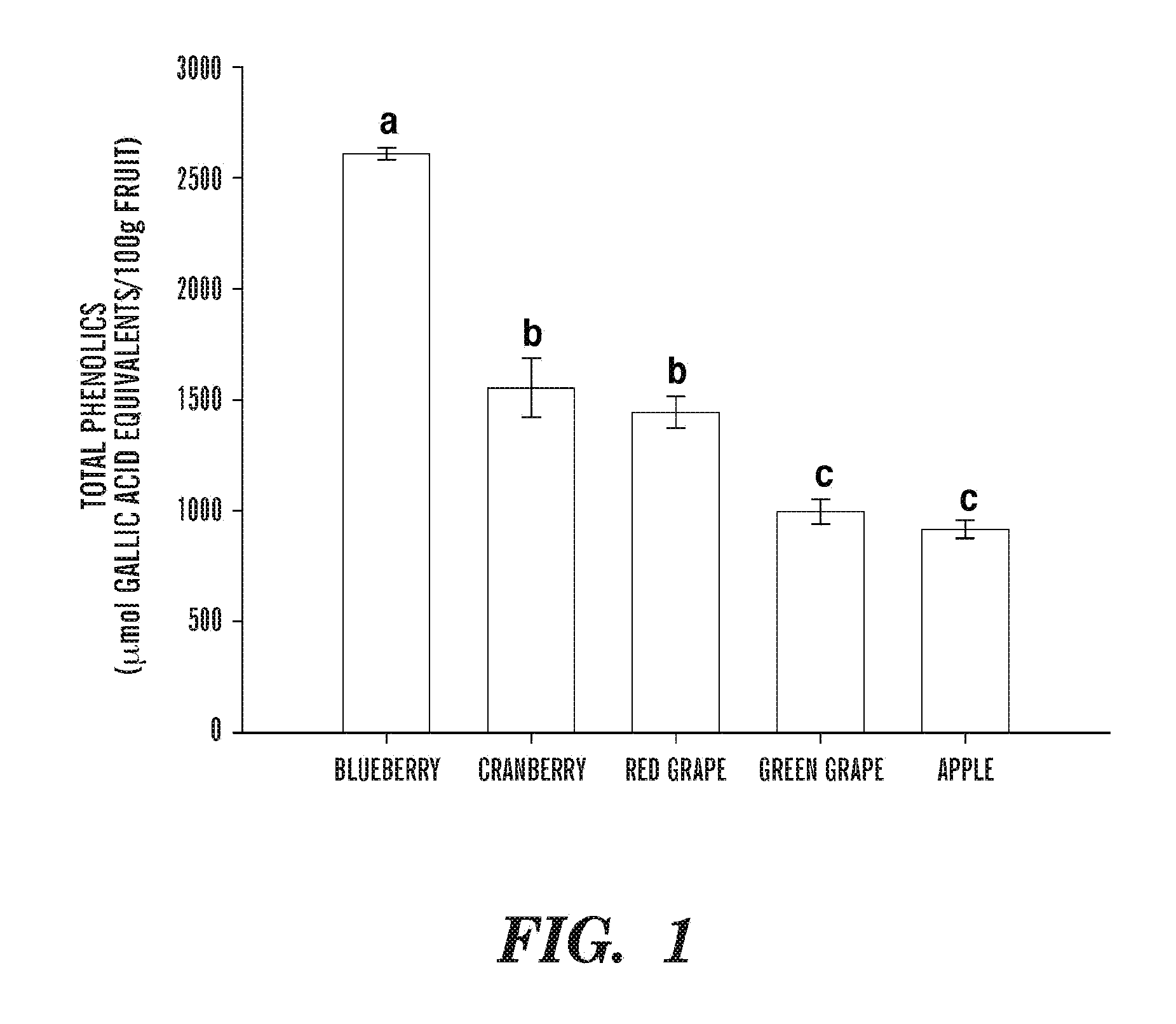
Consumer demand for natural antioxidants like luteolin has been fueled by growing concerns about oxidative stress-related conditions, including cardiovascular diseases, neurodegenerative disorders, and cancer. Market research indicates that the global antioxidant market reached approximately $5.5 billion in 2022, with natural antioxidants accounting for over 60% of this value. The compound annual growth rate (CAGR) for natural antioxidants is projected at 6.8% through 2028.
The nutraceutical and dietary supplement sectors represent the largest market segments for luteolin and similar flavonoids. These industries have witnessed double-digit growth rates in regions like North America, Europe, and Asia-Pacific. Particularly in Japan, South Korea, and China, traditional medicine-inspired supplements containing luteolin have gained significant market traction.
Pharmaceutical companies have also shown increasing interest in luteolin's antioxidant properties, investing in research to develop drug formulations that leverage its therapeutic potential. This pharmaceutical application represents a high-value segment, though it faces more regulatory hurdles than the supplement market.
The cosmetics and personal care industry constitutes another rapidly expanding market for natural antioxidants. Anti-aging products featuring luteolin and other flavonoids have seen particularly strong consumer demand, with premium brands increasingly highlighting these ingredients in their marketing campaigns.
Food and beverage manufacturers have begun incorporating luteolin-rich extracts into functional foods, creating a new market segment with substantial growth potential. Products marketed as "antioxidant-enhanced" command premium pricing, with consumers willing to pay 15-30% more for foods with proven health benefits.
Market analysis reveals regional variations in demand patterns. North American and European markets show preference for clinically validated antioxidant compounds with substantial scientific backing, while Asian markets place higher value on traditional usage history and natural sourcing. This regional differentiation necessitates tailored approaches to in vivo testing methodologies that can address specific market requirements.
The growing demand for transparency and scientific validation presents both a challenge and an opportunity for luteolin market development. Consumers increasingly expect robust evidence of efficacy, particularly from in vivo studies that demonstrate real physiological benefits rather than merely theoretical antioxidant capacity.
Consumer demand for natural antioxidants like luteolin has been fueled by growing concerns about oxidative stress-related conditions, including cardiovascular diseases, neurodegenerative disorders, and cancer. Market research indicates that the global antioxidant market reached approximately $5.5 billion in 2022, with natural antioxidants accounting for over 60% of this value. The compound annual growth rate (CAGR) for natural antioxidants is projected at 6.8% through 2028.
The nutraceutical and dietary supplement sectors represent the largest market segments for luteolin and similar flavonoids. These industries have witnessed double-digit growth rates in regions like North America, Europe, and Asia-Pacific. Particularly in Japan, South Korea, and China, traditional medicine-inspired supplements containing luteolin have gained significant market traction.
Pharmaceutical companies have also shown increasing interest in luteolin's antioxidant properties, investing in research to develop drug formulations that leverage its therapeutic potential. This pharmaceutical application represents a high-value segment, though it faces more regulatory hurdles than the supplement market.
The cosmetics and personal care industry constitutes another rapidly expanding market for natural antioxidants. Anti-aging products featuring luteolin and other flavonoids have seen particularly strong consumer demand, with premium brands increasingly highlighting these ingredients in their marketing campaigns.
Food and beverage manufacturers have begun incorporating luteolin-rich extracts into functional foods, creating a new market segment with substantial growth potential. Products marketed as "antioxidant-enhanced" command premium pricing, with consumers willing to pay 15-30% more for foods with proven health benefits.
Market analysis reveals regional variations in demand patterns. North American and European markets show preference for clinically validated antioxidant compounds with substantial scientific backing, while Asian markets place higher value on traditional usage history and natural sourcing. This regional differentiation necessitates tailored approaches to in vivo testing methodologies that can address specific market requirements.
The growing demand for transparency and scientific validation presents both a challenge and an opportunity for luteolin market development. Consumers increasingly expect robust evidence of efficacy, particularly from in vivo studies that demonstrate real physiological benefits rather than merely theoretical antioxidant capacity.
Current In Vivo Testing Methodologies and Limitations
Current in vivo methodologies for testing luteolin's antioxidant capacity encompass several established approaches, each with distinct advantages and limitations. The oxidative stress biomarker measurement technique stands as a primary method, wherein researchers quantify malondialdehyde (MDA), 8-isoprostane, and protein carbonyl levels in biological samples from animal models. While this approach provides direct evidence of oxidative damage reduction, it often suffers from inconsistent sampling protocols and variable baseline measurements across different animal strains.
Antioxidant enzyme activity assessment represents another widely employed methodology, measuring superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities in tissue homogenates after luteolin administration. This method effectively demonstrates the compound's ability to enhance endogenous antioxidant defense systems but faces challenges in standardization of tissue preparation techniques and enzyme activity measurement conditions.
Gene expression analysis of antioxidant response elements (AREs) and nuclear factor erythroid 2-related factor 2 (Nrf2) pathway components has gained prominence in recent years. This approach reveals luteolin's molecular mechanisms of action through quantification of mRNA and protein levels of antioxidant genes. However, the correlation between gene expression changes and functional antioxidant capacity remains incompletely understood, limiting definitive conclusions.
Reactive oxygen species (ROS) direct measurement using fluorescent probes like 2',7'-dichlorofluorescein diacetate (DCFDA) in tissue samples provides immediate visualization of oxidative status. Nevertheless, these probes exhibit limited specificity for different ROS types and suffer from potential artifacts during sample processing, reducing reliability.
Functional outcome assessments measuring physiological parameters like blood pressure, vascular function, or cognitive performance after oxidative challenge represent more holistic approaches. While these methods demonstrate real-world relevance, they introduce complexity through multiple confounding variables that can obscure luteolin's specific antioxidant effects.
Significant methodological limitations persist across these techniques. The translation gap between controlled laboratory conditions and complex in vivo environments remains substantial. Dosage optimization presents ongoing challenges, as pharmacokinetic profiles of luteolin vary considerably between species and administration routes. Bioavailability issues further complicate interpretation, as luteolin undergoes extensive metabolism, potentially altering its antioxidant properties before reaching target tissues.
Standardization deficiencies across laboratories impede reliable comparison between studies, with variations in animal models, experimental durations, and analytical techniques yielding inconsistent results. Additionally, most current methodologies provide only snapshot measurements rather than continuous monitoring of antioxidant activity, failing to capture the dynamic nature of oxidative stress responses in living systems.
Antioxidant enzyme activity assessment represents another widely employed methodology, measuring superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities in tissue homogenates after luteolin administration. This method effectively demonstrates the compound's ability to enhance endogenous antioxidant defense systems but faces challenges in standardization of tissue preparation techniques and enzyme activity measurement conditions.
Gene expression analysis of antioxidant response elements (AREs) and nuclear factor erythroid 2-related factor 2 (Nrf2) pathway components has gained prominence in recent years. This approach reveals luteolin's molecular mechanisms of action through quantification of mRNA and protein levels of antioxidant genes. However, the correlation between gene expression changes and functional antioxidant capacity remains incompletely understood, limiting definitive conclusions.
Reactive oxygen species (ROS) direct measurement using fluorescent probes like 2',7'-dichlorofluorescein diacetate (DCFDA) in tissue samples provides immediate visualization of oxidative status. Nevertheless, these probes exhibit limited specificity for different ROS types and suffer from potential artifacts during sample processing, reducing reliability.
Functional outcome assessments measuring physiological parameters like blood pressure, vascular function, or cognitive performance after oxidative challenge represent more holistic approaches. While these methods demonstrate real-world relevance, they introduce complexity through multiple confounding variables that can obscure luteolin's specific antioxidant effects.
Significant methodological limitations persist across these techniques. The translation gap between controlled laboratory conditions and complex in vivo environments remains substantial. Dosage optimization presents ongoing challenges, as pharmacokinetic profiles of luteolin vary considerably between species and administration routes. Bioavailability issues further complicate interpretation, as luteolin undergoes extensive metabolism, potentially altering its antioxidant properties before reaching target tissues.
Standardization deficiencies across laboratories impede reliable comparison between studies, with variations in animal models, experimental durations, and analytical techniques yielding inconsistent results. Additionally, most current methodologies provide only snapshot measurements rather than continuous monitoring of antioxidant activity, failing to capture the dynamic nature of oxidative stress responses in living systems.
Established In Vivo Luteolin Testing Protocols
01 Antioxidant properties of luteolin
Luteolin exhibits strong antioxidant properties by scavenging free radicals and reactive oxygen species. Its chemical structure, particularly the presence of hydroxyl groups, contributes to its ability to neutralize oxidative stress. Studies have demonstrated that luteolin can effectively inhibit lipid peroxidation and protect cells from oxidative damage, making it valuable for various health applications.- Antioxidant properties of luteolin: Luteolin exhibits strong antioxidant properties due to its ability to scavenge free radicals and reactive oxygen species. Its chemical structure, particularly the presence of hydroxyl groups, contributes to its antioxidant capacity. Studies have shown that luteolin can effectively neutralize various types of free radicals, including superoxide anions and hydroxyl radicals, thereby protecting cells from oxidative damage.
- Methods for measuring luteolin's antioxidant capacity: Various analytical methods have been developed to measure the antioxidant capacity of luteolin. These include DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, ORAC (Oxygen Radical Absorbance Capacity) assay, FRAP (Ferric Reducing Antioxidant Power) assay, and ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay. These methods help quantify luteolin's ability to neutralize free radicals and provide comparative data on its antioxidant efficiency relative to other antioxidants.
- Luteolin extraction and purification for antioxidant applications: Techniques for extracting and purifying luteolin from natural sources have been developed to harness its antioxidant properties. These methods include solvent extraction, chromatographic separation, and enzymatic processes. The purity and concentration of extracted luteolin significantly impact its antioxidant capacity. Optimized extraction methods ensure maximum retention of luteolin's antioxidant properties for various applications in food, pharmaceutical, and cosmetic industries.
- Formulations enhancing luteolin's antioxidant efficacy: Various formulations have been developed to enhance luteolin's antioxidant efficacy and bioavailability. These include nanoencapsulation, liposomal delivery systems, and combination with other antioxidants for synergistic effects. Such formulations protect luteolin from degradation, improve its stability, and enhance its cellular uptake, thereby maximizing its antioxidant capacity in biological systems. These advanced delivery systems have applications in nutraceuticals, functional foods, and pharmaceutical products.
- Applications of luteolin as an antioxidant in various industries: Luteolin's potent antioxidant capacity has led to its application across various industries. In the food industry, it serves as a natural preservative to prevent oxidation and extend shelf life. In cosmetics, it protects skin from oxidative stress and aging. In pharmaceuticals, it's being investigated for preventing and treating diseases associated with oxidative stress, such as cardiovascular diseases, neurodegenerative disorders, and cancer. The versatility of luteolin as an antioxidant continues to expand its commercial applications.
02 Methods for measuring luteolin's antioxidant capacity
Various analytical methods have been developed to measure and quantify the antioxidant capacity of luteolin. These include spectrophotometric assays, HPLC analysis, and other in vitro and in vivo testing protocols. These methods allow for the evaluation of luteolin's free radical scavenging activity, its ability to inhibit oxidative processes, and comparison with other antioxidant compounds.Expand Specific Solutions03 Extraction and purification of luteolin for antioxidant applications
Techniques for extracting and purifying luteolin from natural sources have been developed to maximize its antioxidant potential. These methods include solvent extraction, chromatographic separation, and other purification processes that aim to obtain high-purity luteolin with enhanced antioxidant capacity. The source material and extraction conditions significantly influence the yield and antioxidant activity of the obtained luteolin.Expand Specific Solutions04 Formulations incorporating luteolin for enhanced antioxidant effects
Various formulations have been developed to enhance the bioavailability and antioxidant efficacy of luteolin. These include nanoparticles, liposomes, and other delivery systems that protect luteolin from degradation and improve its cellular uptake. Additionally, combinations of luteolin with other antioxidants or bioactive compounds have been formulated to achieve synergistic antioxidant effects for applications in pharmaceuticals, cosmetics, and functional foods.Expand Specific Solutions05 Applications of luteolin's antioxidant capacity in health and disease prevention
The antioxidant capacity of luteolin has been applied in various health contexts, including prevention and management of oxidative stress-related diseases. Research has explored luteolin's potential in addressing inflammation, cardiovascular diseases, neurodegenerative disorders, and cancer. Its ability to neutralize free radicals and modulate cellular antioxidant defense systems makes it a promising compound for therapeutic and preventive applications.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The antioxidant capacity testing of luteolin in vivo represents an emerging field at the intersection of nutraceutical and pharmaceutical research. The market is in its growth phase, with increasing demand driven by rising interest in natural antioxidants for health applications. Key players include academic institutions (Cornell University, Zhejiang University, Louisiana State University) conducting foundational research, alongside specialized biotechnology companies like Bioquochem SL offering testing services and Golden Biotechnology Corp developing applications. Pharmaceutical corporations such as Bayer and Bausch & Lomb are exploring luteolin's therapeutic potential, while natural ingredient suppliers like Layn Natural Ingredients and Mannatech provide raw materials. Research centers including CNRS and DKFZ are advancing methodological innovations, creating a competitive landscape balanced between academic research and commercial applications.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed comprehensive in vivo methodologies for testing luteolin's antioxidant capacity using both murine and zebrafish models. Their approach combines traditional oxidative stress biomarkers with advanced imaging techniques. CNRS researchers utilize DCFH-DA fluorescent probes to visualize reactive oxygen species (ROS) in living tissues after luteolin administration. They've established protocols measuring glutathione (GSH) levels, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities in various tissues. Additionally, they employ lipid peroxidation assays (MDA and 4-HNE) and protein carbonylation measurements to assess oxidative damage reduction. Their methodology includes transcriptomic analysis to evaluate Nrf2 pathway activation, a key regulator of antioxidant response elements, providing mechanistic insights into luteolin's antioxidant effects.
Strengths: Comprehensive multi-parameter approach combining traditional biomarkers with advanced imaging techniques provides robust validation. Their established zebrafish model offers rapid, cost-effective screening with good translational value. Weaknesses: Some methodologies require specialized equipment and expertise, potentially limiting accessibility for smaller research groups. The translation of findings from animal models to human applications requires additional validation steps.
Louisiana State University
Technical Solution: Louisiana State University has pioneered an integrated approach for evaluating luteolin's antioxidant capacity in vivo using both acute and chronic rodent models of oxidative stress. Their methodology employs a multi-tissue analysis strategy, examining luteolin's effects in liver, kidney, brain, and vascular tissues. LSU researchers utilize a combination of direct ROS measurement techniques including electron paramagnetic resonance (EPR) spectroscopy with spin trapping to detect free radicals in living tissues. They've developed protocols for measuring oxidative stress biomarkers including F2-isoprostanes, 8-OHdG for DNA damage, and protein-bound 3-nitrotyrosine for peroxynitrite-mediated damage. Their approach includes functional assessments correlating antioxidant capacity with physiological outcomes such as endothelial function, cognitive performance, and metabolic parameters. LSU has also established methods for tracking the bioavailability and tissue distribution of luteolin using LC-MS/MS, providing crucial pharmacokinetic data to interpret antioxidant effects.
Strengths: Comprehensive physiological assessment correlating biochemical markers with functional outcomes provides clinically relevant insights. Their pharmacokinetic analysis offers valuable information on bioavailability and metabolism. Weaknesses: The complex multi-parameter approach requires significant resources and specialized equipment. Rodent models may not fully replicate human metabolism of flavonoids like luteolin, potentially limiting translational value.
Key Biomarkers and Mechanisms for Luteolin Efficacy
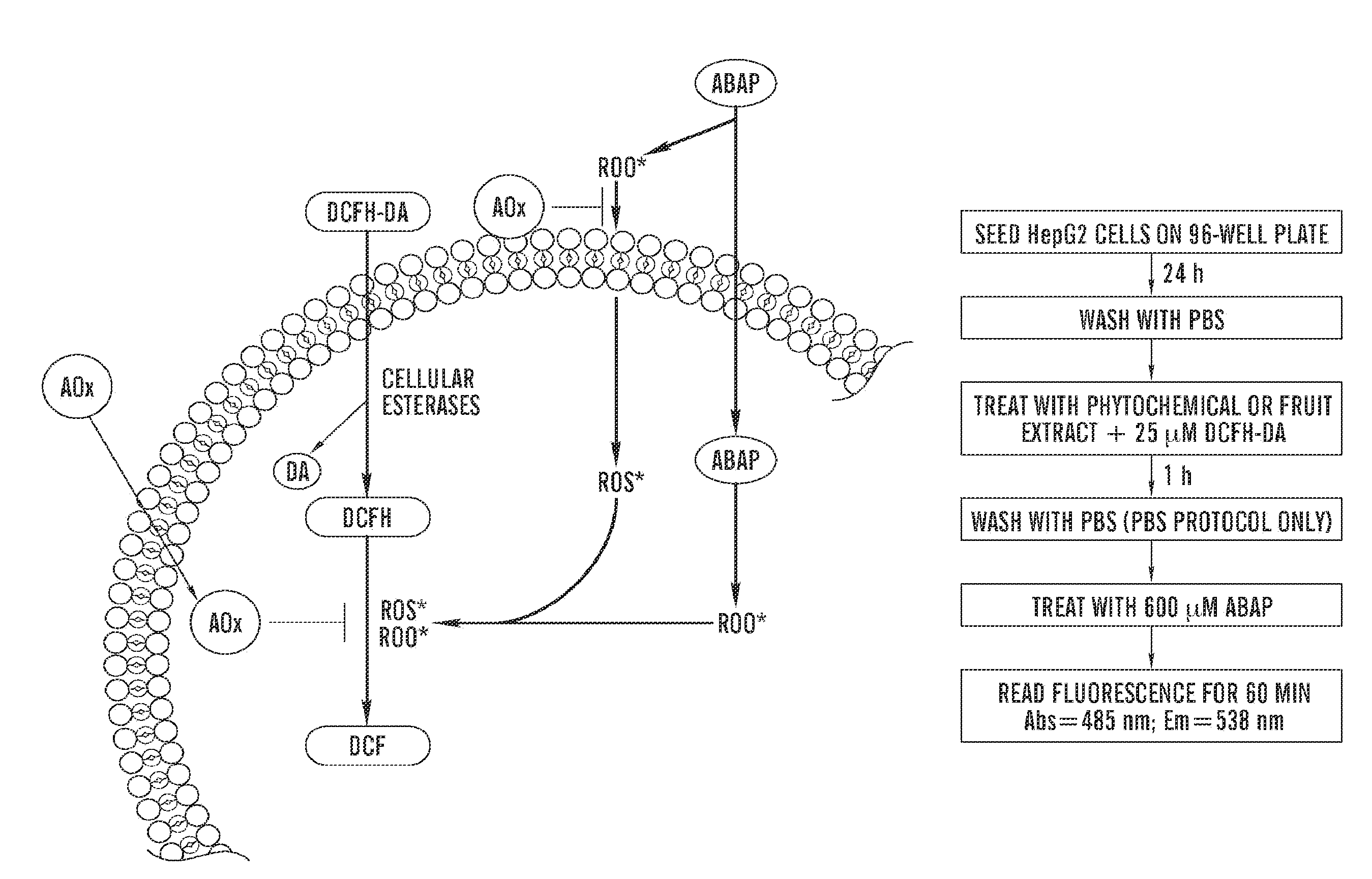
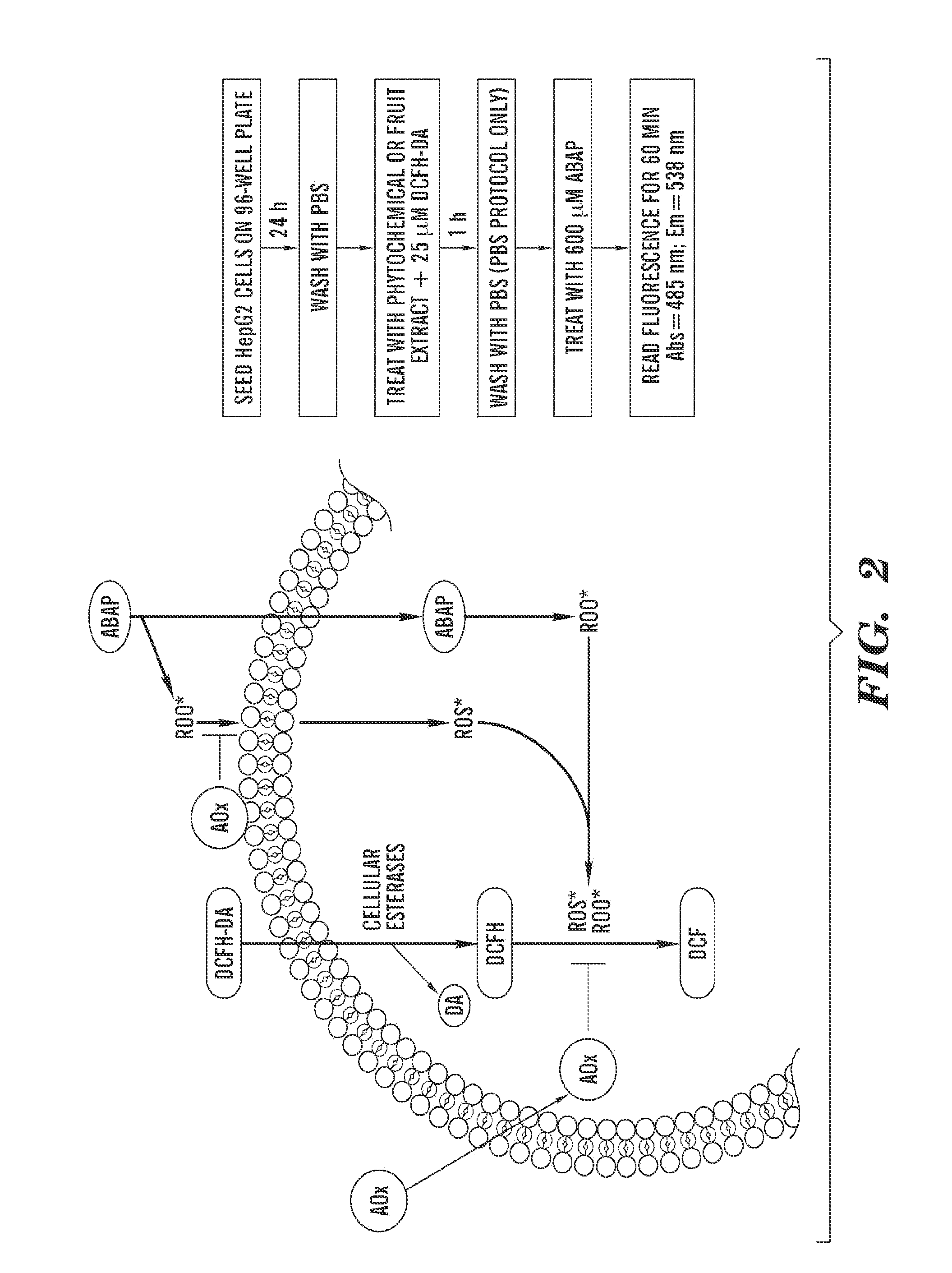
Cellular antioxidant activity (CAA) assay
PatentInactiveUS20110313672A1
Innovation
- A cell-based antioxidant activity assay using 2′,7′-dichlorofluorescin diacetate and a peroxyl radical initiator to measure fluorescence changes, allowing for the determination of antioxidant capacity by comparing area-under-the-curve values in the presence and absence of a test compound, and normalizing to a standard compound for standardized results.
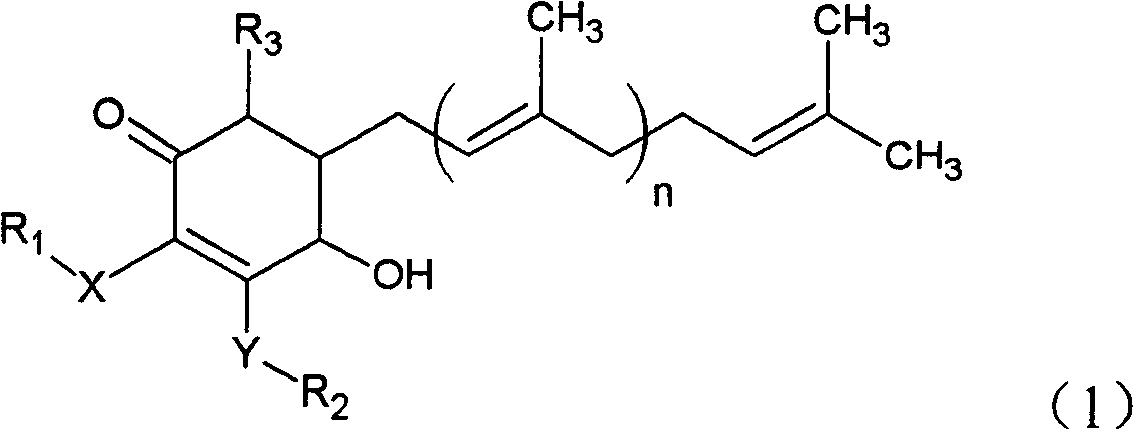
Cyclohexenone extract of antrodia camphorata
PatentActiveCN101225066B
Innovation
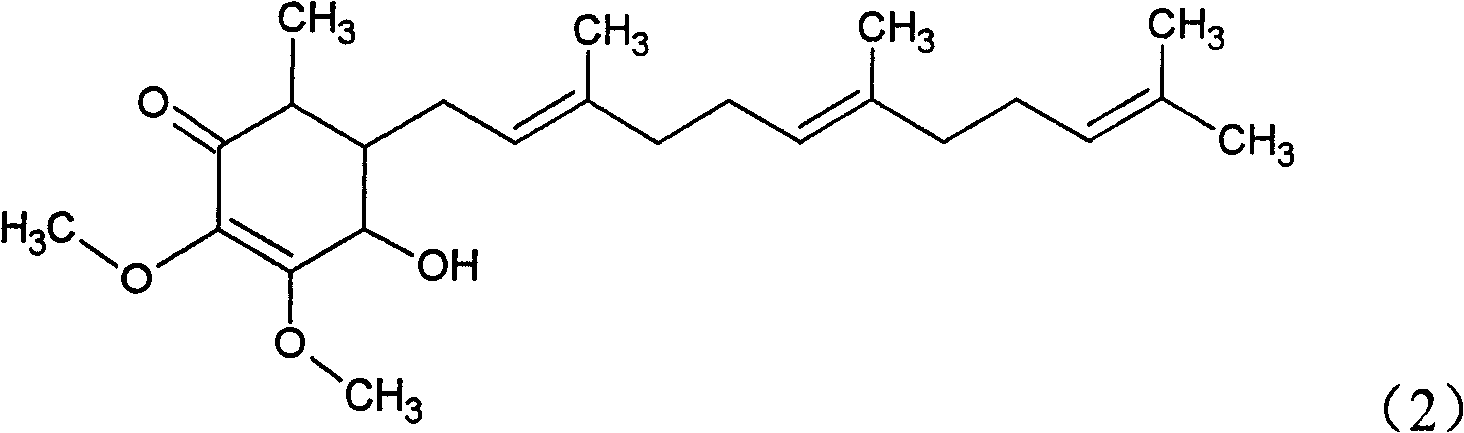
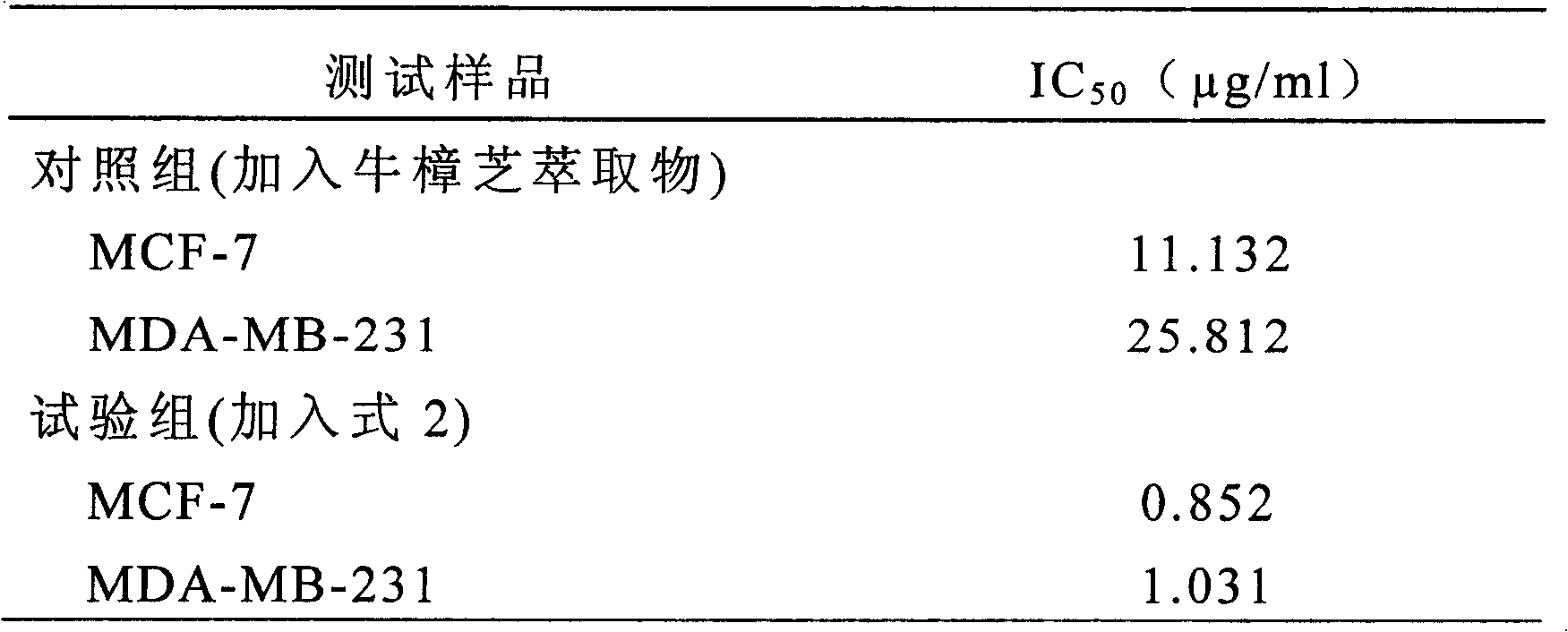
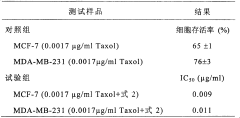
- A novel compound was isolated and purified from Antrodia camphorata, chemically named 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-2,6,10- Dodecatriene)-2-cyclohexenone is extracted by water or organic solvent and purified by high performance liquid chromatography. It is used to inhibit the growth of tumor cells such as breast cancer, liver cancer and prostate cancer.
Regulatory Framework for Nutraceutical Testing
The regulatory landscape for testing luteolin's antioxidant capacity in vivo is complex and multifaceted, requiring careful navigation across various jurisdictional frameworks. In the United States, the FDA regulates nutraceutical testing under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which establishes specific guidelines for safety assessment but does not mandate efficacy testing for antioxidant properties. This creates a significant regulatory gap for compounds like luteolin, where antioxidant claims require substantial scientific evidence without clear testing protocols.
The European Food Safety Authority (EFSA) implements more stringent requirements through Regulation (EC) No 1924/2006, demanding comprehensive scientific dossiers that include in vivo evidence before permitting antioxidant-related health claims. For luteolin testing specifically, EFSA guidelines recommend multiple biomarkers of oxidative stress and standardized animal models to demonstrate physiological relevance.
In Asia, regulatory approaches vary considerably. Japan's FOSHU (Foods for Specified Health Uses) system requires clinical evidence of antioxidant efficacy, while China's regulatory framework under the China Food and Drug Administration emphasizes traditional use history alongside modern scientific validation for botanical antioxidants like luteolin.
International harmonization efforts through the International Conference on Harmonisation (ICH) have established Good Laboratory Practice (GLP) standards that apply to in vivo antioxidant testing. These standards ensure test reliability and reproducibility across different laboratories and jurisdictions, though specific protocols for luteolin remain undefined.
Ethical considerations form another critical regulatory dimension, with animal welfare regulations imposing strict requirements on in vivo testing protocols. The 3Rs principle (Replacement, Reduction, Refinement) must be incorporated into study designs, often necessitating preliminary in vitro screening before proceeding to animal models for luteolin antioxidant assessment.
Data quality and reporting standards constitute a final regulatory consideration, with most jurisdictions requiring adherence to OECD (Organisation for Economic Co-operation and Development) guidelines for toxicity testing and the ARRIVE guidelines for reporting animal research. These frameworks ensure transparency and scientific rigor in documenting luteolin's antioxidant effects.
Researchers must navigate this complex regulatory landscape while designing in vivo studies that can withstand scientific scrutiny and support potential health claims. The absence of harmonized international standards specifically for antioxidant testing creates additional challenges, requiring careful study design that satisfies the most stringent applicable regulations while generating meaningful scientific data on luteolin's antioxidant capacity.
The European Food Safety Authority (EFSA) implements more stringent requirements through Regulation (EC) No 1924/2006, demanding comprehensive scientific dossiers that include in vivo evidence before permitting antioxidant-related health claims. For luteolin testing specifically, EFSA guidelines recommend multiple biomarkers of oxidative stress and standardized animal models to demonstrate physiological relevance.
In Asia, regulatory approaches vary considerably. Japan's FOSHU (Foods for Specified Health Uses) system requires clinical evidence of antioxidant efficacy, while China's regulatory framework under the China Food and Drug Administration emphasizes traditional use history alongside modern scientific validation for botanical antioxidants like luteolin.
International harmonization efforts through the International Conference on Harmonisation (ICH) have established Good Laboratory Practice (GLP) standards that apply to in vivo antioxidant testing. These standards ensure test reliability and reproducibility across different laboratories and jurisdictions, though specific protocols for luteolin remain undefined.
Ethical considerations form another critical regulatory dimension, with animal welfare regulations imposing strict requirements on in vivo testing protocols. The 3Rs principle (Replacement, Reduction, Refinement) must be incorporated into study designs, often necessitating preliminary in vitro screening before proceeding to animal models for luteolin antioxidant assessment.
Data quality and reporting standards constitute a final regulatory consideration, with most jurisdictions requiring adherence to OECD (Organisation for Economic Co-operation and Development) guidelines for toxicity testing and the ARRIVE guidelines for reporting animal research. These frameworks ensure transparency and scientific rigor in documenting luteolin's antioxidant effects.
Researchers must navigate this complex regulatory landscape while designing in vivo studies that can withstand scientific scrutiny and support potential health claims. The absence of harmonized international standards specifically for antioxidant testing creates additional challenges, requiring careful study design that satisfies the most stringent applicable regulations while generating meaningful scientific data on luteolin's antioxidant capacity.
Bioavailability Challenges in Flavonoid Research
Flavonoids, including luteolin, face significant bioavailability challenges that directly impact their in vivo antioxidant capacity testing. The primary obstacle lies in their poor absorption rates, with studies indicating that only 5-10% of consumed flavonoids typically enter systemic circulation intact. This limited absorption stems from their chemical structure, particularly their hydrophilic nature and relatively large molecular size, which hinders passive diffusion across intestinal membranes.
Extensive first-pass metabolism represents another major challenge. Upon ingestion, flavonoids undergo substantial biotransformation in the intestinal epithelium and liver, where they are subjected to phase II metabolism, including glucuronidation, sulfation, and methylation. These metabolic processes significantly alter the original compound structure, potentially modifying or diminishing the antioxidant properties observed in vitro. For luteolin specifically, research has shown that its primary circulating forms are glucuronide and sulfate conjugates rather than the parent compound.
The gut microbiota further complicates bioavailability assessment by metabolizing flavonoids into various degradation products. These bacterial transformations can produce compounds with different bioactivities than the parent molecule, creating a complex mixture of metabolites that must be accounted for in antioxidant capacity evaluations. Recent studies suggest that these microbial metabolites may contribute significantly to the overall health benefits attributed to flavonoids.
Tissue distribution patterns present additional challenges for in vivo testing. Flavonoids and their metabolites demonstrate selective accumulation in specific tissues, with varying concentrations across organs. This uneven distribution means that blood plasma levels may not accurately reflect concentrations at target tissues where antioxidant activity is most relevant. Advanced imaging techniques and tissue-specific sampling are necessary to properly assess local antioxidant effects.
Inter-individual variability further complicates standardized testing approaches. Genetic polymorphisms in metabolizing enzymes, differences in gut microbiota composition, and variations in dietary patterns can all significantly influence flavonoid bioavailability. Studies have documented up to 15-fold differences in plasma concentrations of flavonoid metabolites among individuals consuming identical doses, highlighting the need for personalized approaches in bioavailability assessment.
Methodological limitations in detection and quantification also present significant hurdles. Many conventional analytical techniques lack the sensitivity to detect the low concentrations of flavonoid metabolites present in biological samples. Additionally, the diverse array of metabolites requires sophisticated analytical platforms capable of identifying and quantifying multiple compounds simultaneously.
Extensive first-pass metabolism represents another major challenge. Upon ingestion, flavonoids undergo substantial biotransformation in the intestinal epithelium and liver, where they are subjected to phase II metabolism, including glucuronidation, sulfation, and methylation. These metabolic processes significantly alter the original compound structure, potentially modifying or diminishing the antioxidant properties observed in vitro. For luteolin specifically, research has shown that its primary circulating forms are glucuronide and sulfate conjugates rather than the parent compound.
The gut microbiota further complicates bioavailability assessment by metabolizing flavonoids into various degradation products. These bacterial transformations can produce compounds with different bioactivities than the parent molecule, creating a complex mixture of metabolites that must be accounted for in antioxidant capacity evaluations. Recent studies suggest that these microbial metabolites may contribute significantly to the overall health benefits attributed to flavonoids.
Tissue distribution patterns present additional challenges for in vivo testing. Flavonoids and their metabolites demonstrate selective accumulation in specific tissues, with varying concentrations across organs. This uneven distribution means that blood plasma levels may not accurately reflect concentrations at target tissues where antioxidant activity is most relevant. Advanced imaging techniques and tissue-specific sampling are necessary to properly assess local antioxidant effects.
Inter-individual variability further complicates standardized testing approaches. Genetic polymorphisms in metabolizing enzymes, differences in gut microbiota composition, and variations in dietary patterns can all significantly influence flavonoid bioavailability. Studies have documented up to 15-fold differences in plasma concentrations of flavonoid metabolites among individuals consuming identical doses, highlighting the need for personalized approaches in bioavailability assessment.
Methodological limitations in detection and quantification also present significant hurdles. Many conventional analytical techniques lack the sensitivity to detect the low concentrations of flavonoid metabolites present in biological samples. Additionally, the diverse array of metabolites requires sophisticated analytical platforms capable of identifying and quantifying multiple compounds simultaneously.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!