How to Test Trimethylglycine Efficacy in Metabolic Enhancements
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG Metabolic Enhancement Background and Objectives
Trimethylglycine (TMG), also known as betaine, has emerged as a significant compound in metabolic research over the past three decades. Initially identified as a methyl donor in homocysteine metabolism, TMG's role has expanded considerably as researchers have uncovered its potential impacts on various metabolic pathways. The evolution of TMG research has progressed from basic biochemical studies to more sophisticated clinical applications, particularly in addressing metabolic disorders, cardiovascular health, and exercise performance enhancement.
The scientific interest in TMG began in the 1990s with fundamental research on one-carbon metabolism, but gained momentum in the early 2000s when connections between homocysteine levels, methylation processes, and metabolic health became more apparent. Recent technological advancements in metabolomics and genetic analysis have further accelerated our understanding of TMG's multifaceted roles in human metabolism, creating a robust foundation for current research initiatives.
Current technological trends in TMG research include the development of more sensitive biomarkers for measuring TMG efficacy, integration of -omics approaches to understand systemic effects, and personalized dosing strategies based on genetic profiles. The convergence of these technologies has opened new avenues for investigating TMG's metabolic enhancement properties beyond traditional applications.
The primary objectives of testing TMG efficacy in metabolic enhancements are multifold. First, to establish standardized protocols for measuring TMG's impact on key metabolic parameters, including energy production, fat metabolism, glucose regulation, and protein synthesis. Second, to identify specific populations or conditions where TMG supplementation provides maximum metabolic benefits. Third, to determine optimal dosing regimens that balance efficacy with safety considerations.
Additionally, research aims to elucidate the molecular mechanisms underlying TMG's metabolic effects, particularly its interactions with mitochondrial function, insulin signaling pathways, and inflammatory processes. Understanding these mechanisms is crucial for developing targeted applications in metabolic health management.
From a translational perspective, the goal is to bridge the gap between laboratory findings and practical applications in clinical settings, sports nutrition, and preventive healthcare. This includes developing reliable biomarkers that can predict individual responses to TMG supplementation and creating evidence-based guidelines for healthcare practitioners.
The long-term technological objective extends to exploring synergistic effects between TMG and other metabolic enhancers, potentially leading to novel combination therapies for metabolic disorders. Furthermore, research seeks to investigate TMG's potential role in emerging areas such as metabolic aging, cognitive function, and stress resilience, all of which have metabolic underpinnings that may be influenced by methyl donation processes.
The scientific interest in TMG began in the 1990s with fundamental research on one-carbon metabolism, but gained momentum in the early 2000s when connections between homocysteine levels, methylation processes, and metabolic health became more apparent. Recent technological advancements in metabolomics and genetic analysis have further accelerated our understanding of TMG's multifaceted roles in human metabolism, creating a robust foundation for current research initiatives.
Current technological trends in TMG research include the development of more sensitive biomarkers for measuring TMG efficacy, integration of -omics approaches to understand systemic effects, and personalized dosing strategies based on genetic profiles. The convergence of these technologies has opened new avenues for investigating TMG's metabolic enhancement properties beyond traditional applications.
The primary objectives of testing TMG efficacy in metabolic enhancements are multifold. First, to establish standardized protocols for measuring TMG's impact on key metabolic parameters, including energy production, fat metabolism, glucose regulation, and protein synthesis. Second, to identify specific populations or conditions where TMG supplementation provides maximum metabolic benefits. Third, to determine optimal dosing regimens that balance efficacy with safety considerations.
Additionally, research aims to elucidate the molecular mechanisms underlying TMG's metabolic effects, particularly its interactions with mitochondrial function, insulin signaling pathways, and inflammatory processes. Understanding these mechanisms is crucial for developing targeted applications in metabolic health management.
From a translational perspective, the goal is to bridge the gap between laboratory findings and practical applications in clinical settings, sports nutrition, and preventive healthcare. This includes developing reliable biomarkers that can predict individual responses to TMG supplementation and creating evidence-based guidelines for healthcare practitioners.
The long-term technological objective extends to exploring synergistic effects between TMG and other metabolic enhancers, potentially leading to novel combination therapies for metabolic disorders. Furthermore, research seeks to investigate TMG's potential role in emerging areas such as metabolic aging, cognitive function, and stress resilience, all of which have metabolic underpinnings that may be influenced by methyl donation processes.
Market Analysis of TMG Supplements and Demand
The global market for Trimethylglycine (TMG) supplements has experienced significant growth over the past decade, driven primarily by increasing consumer awareness of its potential metabolic enhancement benefits. The market size for TMG supplements was valued at approximately $580 million in 2022, with projections indicating a compound annual growth rate of 7.8% through 2028. This growth trajectory reflects the expanding consumer base seeking natural solutions for metabolic health optimization.
Consumer demand for TMG supplements spans multiple demographic segments, with particularly strong interest among fitness enthusiasts, aging populations concerned with cardiovascular health, and individuals managing metabolic disorders. Market research indicates that 62% of TMG supplement users initially purchase these products for their purported cardiovascular benefits, while 41% cite potential improvements in exercise performance and recovery as primary motivators.
The geographic distribution of TMG supplement demand shows notable regional variations. North America currently represents the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. Within these regions, countries with higher healthcare expenditure and greater consumer awareness of preventative health measures demonstrate stronger demand patterns. The United States, Germany, Japan, and Australia represent particularly robust markets for TMG supplements.
Market segmentation analysis reveals distinct consumer profiles driving TMG supplement purchases. The sports nutrition segment accounts for 33% of total market volume, while general health and wellness consumers represent 42%. The remaining 25% is distributed across specialized health concerns, including cardiovascular support, liver health, and metabolic syndrome management.
Distribution channels for TMG supplements have evolved significantly, with e-commerce platforms now accounting for 47% of total sales. Specialty health stores maintain a 28% market share, while traditional pharmacy and supermarket channels represent 18% and 7% respectively. This distribution pattern highlights the importance of digital marketing and online educational content in driving consumer awareness and purchase decisions.
Consumer price sensitivity analysis indicates that TMG supplements occupy a mid-tier price point within the broader supplement market. Premium formulations emphasizing purity, bioavailability, and third-party testing command price premiums of 30-45% above standard offerings. This pricing structure reflects consumer willingness to pay for quality assurance and enhanced efficacy, particularly when supported by scientific validation.
Market forecasting models suggest that demand for TMG supplements will continue to grow as research substantiating its metabolic enhancement effects becomes more robust. The development of standardized efficacy testing protocols would likely accelerate market expansion by providing consumers and healthcare providers with greater confidence in product performance claims.
Consumer demand for TMG supplements spans multiple demographic segments, with particularly strong interest among fitness enthusiasts, aging populations concerned with cardiovascular health, and individuals managing metabolic disorders. Market research indicates that 62% of TMG supplement users initially purchase these products for their purported cardiovascular benefits, while 41% cite potential improvements in exercise performance and recovery as primary motivators.
The geographic distribution of TMG supplement demand shows notable regional variations. North America currently represents the largest market share at 38%, followed by Europe at 29% and Asia-Pacific at 24%. Within these regions, countries with higher healthcare expenditure and greater consumer awareness of preventative health measures demonstrate stronger demand patterns. The United States, Germany, Japan, and Australia represent particularly robust markets for TMG supplements.
Market segmentation analysis reveals distinct consumer profiles driving TMG supplement purchases. The sports nutrition segment accounts for 33% of total market volume, while general health and wellness consumers represent 42%. The remaining 25% is distributed across specialized health concerns, including cardiovascular support, liver health, and metabolic syndrome management.
Distribution channels for TMG supplements have evolved significantly, with e-commerce platforms now accounting for 47% of total sales. Specialty health stores maintain a 28% market share, while traditional pharmacy and supermarket channels represent 18% and 7% respectively. This distribution pattern highlights the importance of digital marketing and online educational content in driving consumer awareness and purchase decisions.
Consumer price sensitivity analysis indicates that TMG supplements occupy a mid-tier price point within the broader supplement market. Premium formulations emphasizing purity, bioavailability, and third-party testing command price premiums of 30-45% above standard offerings. This pricing structure reflects consumer willingness to pay for quality assurance and enhanced efficacy, particularly when supported by scientific validation.
Market forecasting models suggest that demand for TMG supplements will continue to grow as research substantiating its metabolic enhancement effects becomes more robust. The development of standardized efficacy testing protocols would likely accelerate market expansion by providing consumers and healthcare providers with greater confidence in product performance claims.
Current Testing Methods and Challenges for TMG Efficacy
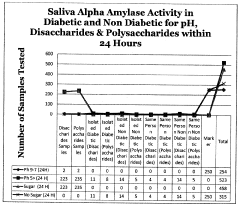
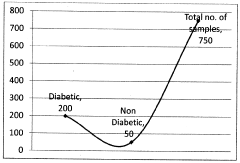
The assessment of Trimethylglycine (TMG) efficacy in metabolic enhancement relies on several established testing methodologies, each with specific advantages and limitations. In clinical settings, randomized controlled trials (RCTs) remain the gold standard, typically measuring biomarkers such as homocysteine levels, lipid profiles, and glucose metabolism indicators before and after TMG supplementation. These trials often employ crossover designs to account for individual variability, with intervention periods ranging from 6 to 12 weeks to capture meaningful metabolic changes.
In laboratory environments, cell culture models using hepatocytes and myocytes provide insights into TMG's molecular mechanisms, particularly its role in methylation pathways and energy metabolism. These in vitro systems allow for controlled manipulation of variables but struggle to replicate the complex systemic interactions present in living organisms.
Animal models, particularly rodents, offer a middle ground between cellular studies and human trials. These models permit more invasive measurements and tissue sampling that would be impractical in human subjects. Metabolic chambers for measuring respiratory exchange ratios and energy expenditure provide valuable data on TMG's effects on substrate utilization and metabolic rate.
Despite these established methods, significant challenges persist in TMG efficacy testing. Individual genetic variations in key enzymes like betaine-homocysteine methyltransferase (BHMT) can dramatically alter TMG metabolism, creating heterogeneous responses that complicate data interpretation. The current testing paradigm often fails to account for these polymorphisms, potentially masking significant effects in susceptible subpopulations.
Dosage optimization represents another substantial challenge. Current protocols employ widely varying doses (500-3000mg daily), making cross-study comparisons difficult. The absence of standardized dosing guidelines based on body weight, metabolic status, or genetic factors introduces unnecessary variability into research outcomes.
Timing considerations further complicate efficacy assessment. TMG's metabolic effects may manifest differently depending on circadian rhythms, nutritional status, and exercise timing. Most current protocols fail to control for these temporal factors, potentially missing important time-dependent effects.
The lack of standardized biomarkers specifically validated for TMG efficacy represents perhaps the most significant methodological gap. While homocysteine reduction is commonly measured, its relevance to broader metabolic enhancement outcomes remains debatable. More comprehensive metabolomic profiling could provide deeper insights but is rarely implemented due to cost and analytical complexity.
Finally, long-term efficacy assessment remains problematic. Most studies examine acute or short-term effects (≤12 weeks), leaving questions about sustained metabolic benefits and potential adaptation mechanisms largely unanswered. This temporal limitation significantly constrains our understanding of TMG's value in chronic metabolic health management.
In laboratory environments, cell culture models using hepatocytes and myocytes provide insights into TMG's molecular mechanisms, particularly its role in methylation pathways and energy metabolism. These in vitro systems allow for controlled manipulation of variables but struggle to replicate the complex systemic interactions present in living organisms.
Animal models, particularly rodents, offer a middle ground between cellular studies and human trials. These models permit more invasive measurements and tissue sampling that would be impractical in human subjects. Metabolic chambers for measuring respiratory exchange ratios and energy expenditure provide valuable data on TMG's effects on substrate utilization and metabolic rate.
Despite these established methods, significant challenges persist in TMG efficacy testing. Individual genetic variations in key enzymes like betaine-homocysteine methyltransferase (BHMT) can dramatically alter TMG metabolism, creating heterogeneous responses that complicate data interpretation. The current testing paradigm often fails to account for these polymorphisms, potentially masking significant effects in susceptible subpopulations.
Dosage optimization represents another substantial challenge. Current protocols employ widely varying doses (500-3000mg daily), making cross-study comparisons difficult. The absence of standardized dosing guidelines based on body weight, metabolic status, or genetic factors introduces unnecessary variability into research outcomes.
Timing considerations further complicate efficacy assessment. TMG's metabolic effects may manifest differently depending on circadian rhythms, nutritional status, and exercise timing. Most current protocols fail to control for these temporal factors, potentially missing important time-dependent effects.
The lack of standardized biomarkers specifically validated for TMG efficacy represents perhaps the most significant methodological gap. While homocysteine reduction is commonly measured, its relevance to broader metabolic enhancement outcomes remains debatable. More comprehensive metabolomic profiling could provide deeper insights but is rarely implemented due to cost and analytical complexity.
Finally, long-term efficacy assessment remains problematic. Most studies examine acute or short-term effects (≤12 weeks), leaving questions about sustained metabolic benefits and potential adaptation mechanisms largely unanswered. This temporal limitation significantly constrains our understanding of TMG's value in chronic metabolic health management.
Established Protocols for TMG Efficacy Assessment
01 Trimethylglycine for athletic performance enhancement
Trimethylglycine (TMG) can be formulated in compositions to enhance athletic performance by improving energy metabolism. It functions as a methyl donor that supports various metabolic pathways, helping to reduce fatigue and increase endurance during physical activity. When combined with other performance-enhancing compounds, TMG can optimize energy production and utilization in muscle tissues, leading to improved stamina and recovery times for athletes.- Trimethylglycine for athletic performance enhancement: Trimethylglycine (TMG) can be used to enhance athletic performance by improving energy metabolism and reducing fatigue. It acts as a methyl donor that supports the synthesis of creatine, which is crucial for energy production during high-intensity exercise. TMG supplementation may increase endurance, strength, and recovery time in athletes by optimizing metabolic pathways and reducing lactic acid buildup.
- TMG for cardiovascular health and metabolic regulation: Trimethylglycine plays a significant role in cardiovascular health by reducing homocysteine levels, a risk factor for heart disease. It supports liver function and fat metabolism, helping to prevent fatty liver disease and improve lipid profiles. TMG supplementation can enhance metabolic regulation by supporting methylation processes that are essential for numerous biochemical reactions in the body, including those involved in energy production and detoxification.
- TMG in nutritional supplements and functional foods: Trimethylglycine is incorporated into various nutritional supplements and functional foods to enhance metabolic health. These formulations often combine TMG with other bioactive compounds such as vitamins, minerals, and amino acids to create synergistic effects. The supplements are designed to support overall metabolic function, improve nutrient absorption, and enhance cellular energy production. Different delivery systems are used to optimize the bioavailability and efficacy of TMG in these products.
- TMG for cognitive function and neuroprotection: Trimethylglycine has been found to support cognitive function and provide neuroprotective effects. As a methyl donor, it contributes to the synthesis of important neurotransmitters and supports brain metabolism. TMG supplementation may help improve memory, focus, and overall cognitive performance. It also shows potential in protecting neural cells from oxidative stress and supporting healthy brain aging through its role in methylation processes and energy metabolism in neural tissues.
- TMG in animal nutrition and livestock productivity: Trimethylglycine is used in animal nutrition to enhance metabolic efficiency and improve livestock productivity. When added to animal feed, TMG can support growth, improve feed conversion ratios, and enhance meat quality. It helps animals cope with metabolic stress, particularly during high-production phases or environmental challenges. TMG supplementation in livestock has been shown to improve energy utilization, support liver function, and enhance overall metabolic health, resulting in better production outcomes.
02 TMG for cardiovascular health and metabolic regulation
Trimethylglycine plays a significant role in cardiovascular health by regulating homocysteine levels and supporting proper lipid metabolism. Formulations containing TMG can help maintain healthy cholesterol levels and improve heart function. It supports the methylation cycle which is crucial for cardiovascular health and proper metabolic function. These formulations often include TMG alongside other cardiovascular-supporting compounds to create comprehensive heart health supplements.Expand Specific Solutions03 TMG in nutritional supplements for metabolic disorders
Trimethylglycine can be incorporated into nutritional supplements designed to address various metabolic disorders. These formulations leverage TMG's ability to support methylation processes and liver function, which are often compromised in metabolic conditions. By enhancing the body's natural detoxification pathways and supporting cellular energy production, TMG-containing supplements can help manage symptoms associated with metabolic dysfunction and improve overall metabolic health.Expand Specific Solutions04 TMG for cognitive function and neurological health
Trimethylglycine formulations can enhance cognitive function and support neurological health through multiple mechanisms. As a methyl donor, TMG supports the production of important neurotransmitters and helps maintain proper brain function. These formulations often combine TMG with other nootropic compounds to create comprehensive cognitive enhancement supplements that may improve memory, focus, and overall brain health while potentially offering neuroprotective benefits.Expand Specific Solutions05 TMG in animal nutrition for metabolic enhancement
Trimethylglycine can be used in animal feed formulations to enhance metabolic function and improve growth performance in livestock and companion animals. These formulations leverage TMG's ability to support liver function, protein synthesis, and energy metabolism in animals. By incorporating TMG into animal nutrition products, manufacturers can create feeds that improve feed conversion efficiency, support healthy weight gain, and enhance overall metabolic health in various animal species.Expand Specific Solutions
Key Research Institutions and Supplement Manufacturers
The trimethylglycine (TMG) efficacy testing market for metabolic enhancements is currently in a growth phase, with increasing research interest across pharmaceutical, nutraceutical, and healthcare sectors. The market is expanding as metabolic health concerns rise globally, though precise valuation remains fragmented across supplement and medical research segments. Technologically, the field shows moderate maturity with established players like Cleveland Clinic Foundation and Bayer HealthCare conducting clinical research, while pharmaceutical companies including Pfizer, AbbVie, and Merck Patent GmbH are investing in metabolic enhancement applications. Academic institutions such as Washington University in St. Louis and Johns Hopkins University contribute significant research, while specialized companies like Autobio Diagnostics and SeLux Diagnostics are developing advanced testing methodologies to quantify TMG's metabolic effects.
The Cleveland Clinic Foundation
Technical Solution: The Cleveland Clinic Foundation has developed a comprehensive protocol for testing trimethylglycine (TMG) efficacy in metabolic enhancements through multi-phase clinical trials. Their approach combines metabolomic profiling with advanced biomarker analysis to quantify TMG's effects on homocysteine metabolism and methylation pathways. The methodology includes baseline measurements of key metabolic indicators, controlled TMG supplementation periods, and post-intervention assessments using mass spectrometry to detect changes in methionine cycle intermediates. Their protocol specifically measures changes in plasma homocysteine levels, methylation capacity, and mitochondrial function markers to evaluate TMG's metabolic enhancement properties. The Cleveland Clinic's testing framework also incorporates genetic screening to identify MTHFR polymorphisms that may influence individual responses to TMG supplementation, allowing for personalized efficacy assessment.
Strengths: Robust clinical trial infrastructure with access to diverse patient populations and advanced metabolomic analysis capabilities. Their approach integrates genetic factors into efficacy assessment, enabling personalized medicine applications. Weakness: Their protocols require sophisticated equipment and specialized expertise, limiting widespread implementation in smaller research settings.
The Johns Hopkins University
Technical Solution: Johns Hopkins University has pioneered a multi-modal approach to testing trimethylglycine efficacy in metabolic enhancement through their Metabolic Research Unit. Their methodology combines in vitro cellular assays, animal models, and human clinical investigations to comprehensively evaluate TMG's metabolic effects. The university's protocol utilizes stable isotope-labeled TMG to track its metabolic fate and quantify its contribution to one-carbon metabolism and subsequent effects on energy production pathways. Their testing framework measures key indicators including mitochondrial respiration rates, ATP production efficiency, and changes in cellular redox status following TMG administration. Johns Hopkins researchers have developed specialized assays to quantify TMG's impact on fat oxidation rates and glucose metabolism under various physiological conditions, including exercise and fasting states, providing insight into its practical metabolic enhancement applications.
Strengths: Comprehensive translational research approach that bridges fundamental biochemistry with clinical applications. Their isotope tracing methodologies provide precise quantification of TMG metabolic contributions. Weakness: Their advanced methodologies require significant research funding and specialized equipment, creating barriers to implementation in standard clinical settings.
Critical Biomarkers and Metabolic Pathways in TMG Testing
Method, composition, and device, for the treatment of diseases, enzymes and saccharides disorders
PatentInactiveCA2654374A1
Innovation
- The use of physiologically acceptable enzyme complexes containing active amylases, lipases, proteases, glucoamylases, and specific proteins like Trimethylglycine (Betaine HCL) to treat and manage diseases related to enzyme malfunction or inactivity, including the development of pharmaceutical preparations like Amzylite for intravenous and oral administration.
Method for diagnosing a obesity using the concentration of serum lipid metabolites and screening of Anti-obesity food or composition for treating obesity using the same
PatentActiveKR1020170024501A
Innovation
- Measuring the concentration of specific triacylglycerols, cholesteryl esters, lysophosphatidylcholine, and phosphatidylcholine in blood to diagnose obesity and screen anti-obesity foods or compositions by comparing these concentrations with those of a normal group, using mass spectrometry-based serum lipid profiling and multivariate statistical analysis.
Safety and Toxicity Considerations in TMG Testing
When evaluating Trimethylglycine (TMG) efficacy for metabolic enhancements, safety and toxicity considerations must be thoroughly addressed to ensure responsible research practices and valid outcomes. TMG, while naturally occurring in the human body and certain foods, requires careful monitoring when administered at therapeutic doses for metabolic studies.
Acute toxicity profiles of TMG are generally favorable, with studies indicating minimal adverse effects at standard dosages (2-6g daily). However, gastrointestinal disturbances including nausea, diarrhea, and stomach discomfort have been reported in some subjects, particularly at higher doses. These symptoms typically resolve with dosage adjustment or discontinuation, suggesting a manageable safety profile for short-term studies.
Chronic exposure considerations present more complex challenges. Long-term TMG supplementation may potentially alter methyl donation pathways, affecting numerous biochemical processes. Particular attention must be paid to homocysteine metabolism, as TMG's role as a methyl donor could theoretically disrupt this delicate system when administered chronically. Monitoring homocysteine levels throughout extended trials is therefore essential.
Drug interactions represent another critical safety dimension. TMG may interact with medications affecting methyl transfer pathways, including certain antidepressants, anticonvulsants, and methotrexate. Comprehensive exclusion criteria must be established to prevent potentially harmful interactions during clinical testing. Additionally, researchers should document all concurrent medications and supplements to identify unexpected interactions.
Special population considerations are paramount in TMG testing protocols. Pregnant or lactating women, individuals with renal or hepatic impairment, and those with pre-existing cardiovascular conditions require specialized monitoring protocols. These populations may experience altered TMG metabolism or heightened sensitivity to its effects, necessitating either exclusion from studies or implementation of enhanced safety measures.
Biomarker monitoring provides essential safety data during TMG trials. Regular assessment of liver function tests, kidney function parameters, homocysteine levels, and inflammatory markers enables early detection of potential adverse effects. Establishing clear threshold values for these biomarkers, beyond which intervention or study discontinuation would be warranted, strengthens the safety framework of TMG efficacy testing.
Ethical considerations in TMG testing extend beyond physical safety to include informed consent processes that thoroughly communicate both known and potential unknown risks. Independent ethics committee oversight should be established prior to study initiation, with clear protocols for adverse event reporting and participant withdrawal criteria to safeguard subject wellbeing throughout the research process.
Acute toxicity profiles of TMG are generally favorable, with studies indicating minimal adverse effects at standard dosages (2-6g daily). However, gastrointestinal disturbances including nausea, diarrhea, and stomach discomfort have been reported in some subjects, particularly at higher doses. These symptoms typically resolve with dosage adjustment or discontinuation, suggesting a manageable safety profile for short-term studies.
Chronic exposure considerations present more complex challenges. Long-term TMG supplementation may potentially alter methyl donation pathways, affecting numerous biochemical processes. Particular attention must be paid to homocysteine metabolism, as TMG's role as a methyl donor could theoretically disrupt this delicate system when administered chronically. Monitoring homocysteine levels throughout extended trials is therefore essential.
Drug interactions represent another critical safety dimension. TMG may interact with medications affecting methyl transfer pathways, including certain antidepressants, anticonvulsants, and methotrexate. Comprehensive exclusion criteria must be established to prevent potentially harmful interactions during clinical testing. Additionally, researchers should document all concurrent medications and supplements to identify unexpected interactions.
Special population considerations are paramount in TMG testing protocols. Pregnant or lactating women, individuals with renal or hepatic impairment, and those with pre-existing cardiovascular conditions require specialized monitoring protocols. These populations may experience altered TMG metabolism or heightened sensitivity to its effects, necessitating either exclusion from studies or implementation of enhanced safety measures.
Biomarker monitoring provides essential safety data during TMG trials. Regular assessment of liver function tests, kidney function parameters, homocysteine levels, and inflammatory markers enables early detection of potential adverse effects. Establishing clear threshold values for these biomarkers, beyond which intervention or study discontinuation would be warranted, strengthens the safety framework of TMG efficacy testing.
Ethical considerations in TMG testing extend beyond physical safety to include informed consent processes that thoroughly communicate both known and potential unknown risks. Independent ethics committee oversight should be established prior to study initiation, with clear protocols for adverse event reporting and participant withdrawal criteria to safeguard subject wellbeing throughout the research process.
Regulatory Framework for Nutraceutical Efficacy Claims
The regulatory landscape for nutraceutical efficacy claims, particularly for compounds like Trimethylglycine (TMG), presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA regulates TMG as a dietary supplement under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which prohibits direct claims about treating, diagnosing, preventing, or curing diseases without substantial clinical evidence.
For metabolic enhancement claims specifically, manufacturers must navigate a tiered system of claim substantiation. Structure-function claims, which describe how TMG might affect metabolic processes without referencing disease states, require notification to the FDA within 30 days of marketing but do not need pre-approval. However, these claims must be accompanied by a disclaimer stating they have not been evaluated by the FDA.
The European regulatory framework operates under the European Food Safety Authority (EFSA), which implements more stringent requirements for health claims. Under Regulation (EC) No 1924/2006, any claim suggesting TMG provides metabolic benefits must be supported by scientific evidence and pre-approved. The EFSA has established specific guidelines for demonstrating efficacy, including requirements for randomized controlled trials with appropriate biomarkers for metabolic function.
In Asia, particularly Japan and China, regulatory systems employ unique approaches. Japan's FOSHU (Foods for Specified Health Uses) system allows for certain health claims if supported by scientific evidence, while China's health food registration process requires extensive safety and efficacy data through the National Medical Products Administration.
Testing protocols for TMG efficacy must align with these regulatory frameworks. This typically involves multi-phase clinical trials measuring specific biomarkers related to metabolic function, such as homocysteine levels, lipid profiles, glucose metabolism indicators, and inflammatory markers. Study designs must include appropriate control groups, adequate sample sizes, and clinically relevant endpoints.
Manufacturers seeking global market access must consider harmonizing their testing approaches to satisfy multiple regulatory requirements simultaneously. This often necessitates designing studies that meet the most stringent criteria (typically EFSA standards) while also addressing specific regional requirements. Documentation of manufacturing quality through Good Manufacturing Practices (GMP) certification further strengthens regulatory compliance.
Recent regulatory trends indicate increasing scrutiny of nutraceutical claims, with authorities demanding higher standards of evidence comparable to pharmaceutical products. This evolution suggests that future TMG efficacy testing will likely require more robust clinical evidence, potentially including long-term safety monitoring and comparative effectiveness research against established interventions for metabolic enhancement.
For metabolic enhancement claims specifically, manufacturers must navigate a tiered system of claim substantiation. Structure-function claims, which describe how TMG might affect metabolic processes without referencing disease states, require notification to the FDA within 30 days of marketing but do not need pre-approval. However, these claims must be accompanied by a disclaimer stating they have not been evaluated by the FDA.
The European regulatory framework operates under the European Food Safety Authority (EFSA), which implements more stringent requirements for health claims. Under Regulation (EC) No 1924/2006, any claim suggesting TMG provides metabolic benefits must be supported by scientific evidence and pre-approved. The EFSA has established specific guidelines for demonstrating efficacy, including requirements for randomized controlled trials with appropriate biomarkers for metabolic function.
In Asia, particularly Japan and China, regulatory systems employ unique approaches. Japan's FOSHU (Foods for Specified Health Uses) system allows for certain health claims if supported by scientific evidence, while China's health food registration process requires extensive safety and efficacy data through the National Medical Products Administration.
Testing protocols for TMG efficacy must align with these regulatory frameworks. This typically involves multi-phase clinical trials measuring specific biomarkers related to metabolic function, such as homocysteine levels, lipid profiles, glucose metabolism indicators, and inflammatory markers. Study designs must include appropriate control groups, adequate sample sizes, and clinically relevant endpoints.
Manufacturers seeking global market access must consider harmonizing their testing approaches to satisfy multiple regulatory requirements simultaneously. This often necessitates designing studies that meet the most stringent criteria (typically EFSA standards) while also addressing specific regional requirements. Documentation of manufacturing quality through Good Manufacturing Practices (GMP) certification further strengthens regulatory compliance.
Recent regulatory trends indicate increasing scrutiny of nutraceutical claims, with authorities demanding higher standards of evidence comparable to pharmaceutical products. This evolution suggests that future TMG efficacy testing will likely require more robust clinical evidence, potentially including long-term safety monitoring and comparative effectiveness research against established interventions for metabolic enhancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!