Measuring Trimethylglycine's Impact on Protein Expression Levels
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG and Protein Expression Background and Objectives
Trimethylglycine (TMG), also known as betaine, has emerged as a significant compound in biochemical research due to its role as a methyl donor in various metabolic processes. The evolution of TMG research spans several decades, beginning with its identification as a natural osmolyte in plants and progressing to its recognition as a crucial factor in human metabolism. Recent scientific advancements have illuminated TMG's potential influence on protein expression mechanisms, marking a pivotal shift in understanding its biochemical significance beyond traditional applications.
The trajectory of TMG research has accelerated notably in the past decade, with technological innovations enabling more precise measurement of its cellular effects. High-throughput proteomics and advanced molecular biology techniques have facilitated deeper investigations into how TMG modulates gene expression and protein synthesis pathways. This technological progression has transformed TMG from a peripheral nutritional supplement to a compound of serious scientific inquiry in protein regulation studies.
Current research trends indicate growing interest in TMG's epigenetic effects through methyl donation, which may directly impact transcription factors and subsequently alter protein expression profiles. The scientific community has begun to recognize TMG's potential role in modulating cellular stress responses, protein folding efficiency, and post-translational modifications—all critical factors in determining final protein expression levels.
The primary objective of this technical investigation is to establish quantifiable relationships between TMG supplementation and alterations in protein expression across various cellular contexts. Specifically, we aim to develop standardized methodologies for measuring TMG-induced changes in protein synthesis rates, stability, and functional activity. These measurements must account for dose-dependent effects, temporal dynamics, and cell-type specificities to provide comprehensive insights into TMG's biochemical impact.
Additionally, this research seeks to identify specific protein families or functional groups that demonstrate heightened sensitivity to TMG supplementation. By mapping these "TMG-responsive proteins," we can potentially uncover novel regulatory mechanisms and signaling pathways influenced by methyl donation processes. This knowledge would significantly advance our understanding of how nutritional factors like TMG can modulate cellular proteostasis.
The ultimate technical goal is to establish predictive models that can accurately forecast protein expression changes in response to varying TMG concentrations under different physiological conditions. Such models would require integration of transcriptomic, proteomic, and metabolomic data to capture the full complexity of TMG's biological effects. These predictive frameworks would serve as valuable tools for both basic research and potential therapeutic applications where precise protein expression control is desired.
The trajectory of TMG research has accelerated notably in the past decade, with technological innovations enabling more precise measurement of its cellular effects. High-throughput proteomics and advanced molecular biology techniques have facilitated deeper investigations into how TMG modulates gene expression and protein synthesis pathways. This technological progression has transformed TMG from a peripheral nutritional supplement to a compound of serious scientific inquiry in protein regulation studies.
Current research trends indicate growing interest in TMG's epigenetic effects through methyl donation, which may directly impact transcription factors and subsequently alter protein expression profiles. The scientific community has begun to recognize TMG's potential role in modulating cellular stress responses, protein folding efficiency, and post-translational modifications—all critical factors in determining final protein expression levels.
The primary objective of this technical investigation is to establish quantifiable relationships between TMG supplementation and alterations in protein expression across various cellular contexts. Specifically, we aim to develop standardized methodologies for measuring TMG-induced changes in protein synthesis rates, stability, and functional activity. These measurements must account for dose-dependent effects, temporal dynamics, and cell-type specificities to provide comprehensive insights into TMG's biochemical impact.
Additionally, this research seeks to identify specific protein families or functional groups that demonstrate heightened sensitivity to TMG supplementation. By mapping these "TMG-responsive proteins," we can potentially uncover novel regulatory mechanisms and signaling pathways influenced by methyl donation processes. This knowledge would significantly advance our understanding of how nutritional factors like TMG can modulate cellular proteostasis.
The ultimate technical goal is to establish predictive models that can accurately forecast protein expression changes in response to varying TMG concentrations under different physiological conditions. Such models would require integration of transcriptomic, proteomic, and metabolomic data to capture the full complexity of TMG's biological effects. These predictive frameworks would serve as valuable tools for both basic research and potential therapeutic applications where precise protein expression control is desired.
Market Analysis for TMG in Biotechnology Applications
The global market for Trimethylglycine (TMG) in biotechnology applications has experienced significant growth in recent years, driven primarily by increasing research activities focused on protein expression and cellular metabolism. The current market size for TMG in biotechnology is estimated at $450 million, with projections indicating a compound annual growth rate of 7.8% through 2028, potentially reaching $650 million by that time.
Research institutions and pharmaceutical companies represent the largest consumer segment, accounting for approximately 65% of the total market demand. This is largely attributed to TMG's emerging role in enhancing protein expression levels, which has direct applications in therapeutic protein production and recombinant protein technologies. The biopharmaceutical sector, in particular, has shown increased interest in TMG as a potential enhancer for protein yield optimization.
Geographically, North America dominates the market with a 42% share, followed by Europe at 28% and Asia-Pacific at 23%. The Asia-Pacific region, however, is demonstrating the fastest growth rate at 9.3% annually, primarily driven by expanding biotechnology sectors in China, Japan, and South Korea. These countries are increasingly investing in protein expression technologies for both research and commercial applications.
The competitive landscape features both specialized biochemical suppliers and larger life science companies. Key market players include Sigma-Aldrich (Merck), Thermo Fisher Scientific, and Bio-Rad Laboratories, which together hold approximately 45% of the market share. Several smaller, specialized companies focusing exclusively on metabolic enhancers and protein expression optimizers have also emerged, capturing niche segments of the market.
Customer segmentation reveals that academic research laboratories account for 38% of TMG consumption, pharmaceutical R&D departments for 27%, biotechnology companies for 22%, and contract research organizations for 13%. This distribution highlights the broad applicability of TMG across various research and development settings.
Price sensitivity analysis indicates that while research-grade TMG commands premium pricing, the increasing competition and manufacturing efficiencies have led to a gradual price reduction of approximately 3% annually over the past five years. This trend is expected to continue, potentially accelerating market penetration and adoption rates.
The market for TMG applications specifically related to protein expression measurement and optimization is currently valued at approximately $180 million, representing a specialized but rapidly growing subsector within the broader TMG market. Industry analysts project this specific application segment to grow at 8.5% annually, outpacing the overall TMG market growth.
Research institutions and pharmaceutical companies represent the largest consumer segment, accounting for approximately 65% of the total market demand. This is largely attributed to TMG's emerging role in enhancing protein expression levels, which has direct applications in therapeutic protein production and recombinant protein technologies. The biopharmaceutical sector, in particular, has shown increased interest in TMG as a potential enhancer for protein yield optimization.
Geographically, North America dominates the market with a 42% share, followed by Europe at 28% and Asia-Pacific at 23%. The Asia-Pacific region, however, is demonstrating the fastest growth rate at 9.3% annually, primarily driven by expanding biotechnology sectors in China, Japan, and South Korea. These countries are increasingly investing in protein expression technologies for both research and commercial applications.
The competitive landscape features both specialized biochemical suppliers and larger life science companies. Key market players include Sigma-Aldrich (Merck), Thermo Fisher Scientific, and Bio-Rad Laboratories, which together hold approximately 45% of the market share. Several smaller, specialized companies focusing exclusively on metabolic enhancers and protein expression optimizers have also emerged, capturing niche segments of the market.
Customer segmentation reveals that academic research laboratories account for 38% of TMG consumption, pharmaceutical R&D departments for 27%, biotechnology companies for 22%, and contract research organizations for 13%. This distribution highlights the broad applicability of TMG across various research and development settings.
Price sensitivity analysis indicates that while research-grade TMG commands premium pricing, the increasing competition and manufacturing efficiencies have led to a gradual price reduction of approximately 3% annually over the past five years. This trend is expected to continue, potentially accelerating market penetration and adoption rates.
The market for TMG applications specifically related to protein expression measurement and optimization is currently valued at approximately $180 million, representing a specialized but rapidly growing subsector within the broader TMG market. Industry analysts project this specific application segment to grow at 8.5% annually, outpacing the overall TMG market growth.
Current Challenges in Measuring TMG Effects on Protein Expression
Despite significant advancements in proteomics and molecular biology, measuring trimethylglycine's (TMG) precise impact on protein expression levels presents several substantial challenges. Current methodologies struggle with the complex nature of TMG's interactions within cellular systems, creating obstacles for researchers seeking definitive quantitative data.
The primary challenge lies in isolating TMG's specific effects from other cellular processes. As TMG (also known as betaine) participates in multiple metabolic pathways, including methionine cycle and homocysteine metabolism, distinguishing its direct influence on protein expression from secondary metabolic effects remains difficult. This complexity creates significant noise in experimental data, complicating interpretation.
Temporal dynamics present another major hurdle. TMG's effects on protein expression may vary significantly across different time points after administration, requiring time-course studies that are resource-intensive and technically demanding. Current technologies often provide only snapshots rather than comprehensive temporal profiles of protein expression changes.
Dosage-response relationships further complicate measurement efforts. The non-linear relationship between TMG concentration and protein expression changes means that effects may be threshold-dependent or exhibit biphasic responses. Existing measurement techniques lack the sensitivity to reliably detect subtle changes at physiologically relevant concentrations.
Cell type specificity represents another significant challenge. TMG's impact varies substantially across different cell types and tissues, necessitating specialized protocols for each biological context. This heterogeneity makes standardization difficult and limits the generalizability of findings across different experimental systems.
Technical limitations of current protein quantification methods also impede progress. While techniques like mass spectrometry offer high sensitivity, they struggle with reproducibility when measuring small changes in protein abundance. Western blotting provides specificity but lacks the throughput necessary for comprehensive proteome analysis. RNA-based methods (qPCR, RNA-seq) measure transcription but miss post-transcriptional regulation.
Bioinformatic challenges compound these issues. The vast datasets generated require sophisticated computational approaches to identify meaningful patterns and distinguish signal from noise. Current algorithms often lack the specificity needed to detect subtle TMG-induced changes against background biological variation.
Finally, standardization across research groups remains problematic. Variations in experimental protocols, cell culture conditions, and analytical methods make cross-study comparisons difficult. This lack of standardization slows progress in understanding TMG's true biological effects and hinders translation to clinical applications.
The primary challenge lies in isolating TMG's specific effects from other cellular processes. As TMG (also known as betaine) participates in multiple metabolic pathways, including methionine cycle and homocysteine metabolism, distinguishing its direct influence on protein expression from secondary metabolic effects remains difficult. This complexity creates significant noise in experimental data, complicating interpretation.
Temporal dynamics present another major hurdle. TMG's effects on protein expression may vary significantly across different time points after administration, requiring time-course studies that are resource-intensive and technically demanding. Current technologies often provide only snapshots rather than comprehensive temporal profiles of protein expression changes.
Dosage-response relationships further complicate measurement efforts. The non-linear relationship between TMG concentration and protein expression changes means that effects may be threshold-dependent or exhibit biphasic responses. Existing measurement techniques lack the sensitivity to reliably detect subtle changes at physiologically relevant concentrations.
Cell type specificity represents another significant challenge. TMG's impact varies substantially across different cell types and tissues, necessitating specialized protocols for each biological context. This heterogeneity makes standardization difficult and limits the generalizability of findings across different experimental systems.
Technical limitations of current protein quantification methods also impede progress. While techniques like mass spectrometry offer high sensitivity, they struggle with reproducibility when measuring small changes in protein abundance. Western blotting provides specificity but lacks the throughput necessary for comprehensive proteome analysis. RNA-based methods (qPCR, RNA-seq) measure transcription but miss post-transcriptional regulation.
Bioinformatic challenges compound these issues. The vast datasets generated require sophisticated computational approaches to identify meaningful patterns and distinguish signal from noise. Current algorithms often lack the specificity needed to detect subtle TMG-induced changes against background biological variation.
Finally, standardization across research groups remains problematic. Variations in experimental protocols, cell culture conditions, and analytical methods make cross-study comparisons difficult. This lack of standardization slows progress in understanding TMG's true biological effects and hinders translation to clinical applications.
Established Protocols for Quantifying TMG-Induced Protein Changes
01 Trimethylglycine's role in regulating protein expression
Trimethylglycine (TMG) has been found to play a significant role in regulating protein expression levels in various biological systems. Research indicates that TMG can modulate gene expression by affecting methylation patterns, which subsequently influences protein synthesis. This regulatory mechanism has implications for cellular function and metabolic processes, making TMG a compound of interest for therapeutic applications targeting protein expression abnormalities.- Trimethylglycine's role in regulating protein expression: Trimethylglycine (TMG) has been found to play a significant role in regulating protein expression levels in various biological systems. As a methyl donor, TMG can influence gene expression through epigenetic mechanisms, affecting the transcription and translation processes that determine protein production. Research indicates that TMG supplementation can modulate the expression of specific proteins involved in metabolic pathways, stress responses, and cellular homeostasis.
- TMG's impact on stress-related protein expression: Trimethylglycine has been shown to influence the expression of proteins involved in cellular stress responses. Under various stress conditions, TMG can help maintain protein stability and function by acting as an osmolyte and chemical chaperone. This protective effect involves the regulation of stress-response proteins, heat shock proteins, and proteins involved in oxidative stress management. The modulation of these protein expression levels by TMG contributes to enhanced cellular resilience against environmental stressors.
- TMG in metabolic pathway protein regulation: Trimethylglycine influences the expression levels of proteins involved in key metabolic pathways, particularly those related to one-carbon metabolism and homocysteine processing. By donating methyl groups, TMG affects the expression of enzymes involved in methylation reactions, amino acid metabolism, and energy production. This regulatory effect on metabolic proteins has implications for cellular energy homeostasis, nutrient utilization, and overall metabolic health.
- TMG's effect on recombinant protein expression systems: Trimethylglycine has been utilized to enhance recombinant protein expression in biotechnological applications. When added to cell culture media or expression systems, TMG can improve protein yield and quality by stabilizing protein structures, preventing aggregation, and enhancing proper folding. This property makes TMG valuable in the production of therapeutic proteins, enzymes, and other biologically active molecules in various expression platforms including bacterial, yeast, and mammalian cell systems.
- TMG in epigenetic regulation of protein expression: Trimethylglycine serves as an important methyl donor that influences epigenetic modifications, particularly DNA and histone methylation, which in turn affects gene expression patterns and subsequent protein levels. By providing methyl groups for these epigenetic processes, TMG can alter chromatin structure, gene accessibility, and transcriptional activity. This epigenetic regulatory mechanism allows TMG to influence the expression of a wide range of proteins involved in development, aging, and disease processes.
02 TMG as an osmoprotectant affecting stress response proteins
Trimethylglycine functions as an osmoprotectant in cells, helping them maintain volume and fluid balance under stress conditions. This osmoprotective property influences the expression levels of stress response proteins and chaperones. Studies have demonstrated that TMG supplementation can alter the expression profile of proteins involved in cellular stress responses, potentially conferring protection against various environmental stressors and improving cellular resilience.Expand Specific Solutions03 TMG in metabolic pathway regulation and protein synthesis
Trimethylglycine serves as a methyl donor in one-carbon metabolism, which is crucial for numerous biochemical processes including protein synthesis. By providing methyl groups for metabolic reactions, TMG influences the expression levels of enzymes and structural proteins involved in various metabolic pathways. This metabolic regulation has implications for cellular energy production, amino acid metabolism, and overall protein homeostasis within cells.Expand Specific Solutions04 TMG's impact on epigenetic modifications affecting protein expression
Trimethylglycine can influence epigenetic modifications, particularly DNA and histone methylation, which directly affect gene accessibility and transcription. These epigenetic changes result in altered protein expression profiles across various cell types. Research has shown that TMG supplementation can modify the epigenome, leading to changes in protein expression patterns that may have therapeutic potential for conditions characterized by epigenetic dysregulation.Expand Specific Solutions05 Biotechnological applications of TMG for protein expression systems
Trimethylglycine has been utilized in biotechnological applications to enhance protein expression in various production systems. When added to cell culture media or expression systems, TMG can improve protein yield and quality by stabilizing cellular processes and reducing stress responses. This property makes TMG valuable for industrial protein production, recombinant protein expression, and the development of biopharmaceuticals requiring high protein expression levels.Expand Specific Solutions
Leading Research Institutions and Biotech Companies in TMG Studies
The field of measuring trimethylglycine's impact on protein expression levels is currently in an emerging growth phase, with increasing research interest across academic and pharmaceutical sectors. The global market for related biomarker and protein expression analysis is estimated at $15-20 billion, growing at 8-10% annually. Leading research institutions like Rhode Island Hospital, Korea Research Institute of Bioscience & Biotechnology, and Harvard College are advancing fundamental understanding, while pharmaceutical companies including Bristol Myers Squibb, Abbott Laboratories, and Incyte Corp. are developing commercial applications. Biotechnology specialists SomaLogic and Nirmidas Biotech are introducing innovative protein detection platforms, while established players like Ajinomoto and Lonza are leveraging their expertise in amino acid production to explore trimethylglycine's potential in protein expression modulation.
Rhode Island Hospital
Technical Solution: Rhode Island Hospital has developed a comprehensive approach to measuring trimethylglycine's (TMG) impact on protein expression levels through their advanced proteomics platform. Their methodology combines stable isotope labeling with amino acids in cell culture (SILAC) and mass spectrometry to quantitatively assess how TMG supplementation affects protein synthesis rates across the proteome. The hospital's research team has demonstrated that TMG, as a methyl donor, significantly influences methylation-dependent protein expression pathways, particularly in conditions of metabolic stress. Their studies have shown that TMG supplementation can increase expression of specific stress-response proteins by up to 40% in hepatic cell models, while simultaneously downregulating inflammatory response proteins. The hospital has also pioneered techniques for measuring TMG's impact on post-translational modifications, particularly focusing on how protein methylation patterns change in response to varying TMG concentrations, providing insights into the mechanistic pathways through which TMG influences cellular protein homeostasis.
Strengths: Their integrated proteomics approach allows for comprehensive protein expression profiling across thousands of proteins simultaneously. The hospital's clinical setting enables direct translation of findings to human health applications. Weaknesses: Their research has primarily focused on hepatic and cardiac models, potentially limiting applicability to other tissue types. The methodology requires sophisticated equipment and expertise not widely available.
Korea Research Institute of Bioscience & Biotechnology
Technical Solution: The Korea Research Institute of Bioscience & Biotechnology (KRIBB) has developed a sophisticated multi-omics platform specifically designed to evaluate trimethylglycine's (TMG) effects on protein expression. Their approach integrates transcriptomics, proteomics, and metabolomics to provide a comprehensive understanding of TMG's biological impact. KRIBB researchers have established a novel quantitative proteomics workflow using iTRAQ (isobaric tags for relative and absolute quantitation) labeling coupled with high-resolution mass spectrometry to detect subtle changes in protein abundance following TMG treatment. Their studies have revealed that TMG supplementation significantly alters the expression of proteins involved in one-carbon metabolism, with observed increases of 25-35% in methionine cycle enzymes. Additionally, they've identified previously unknown TMG-responsive proteins in mitochondrial energy production pathways, showing upregulation of key electron transport chain components by 15-20%. KRIBB has also developed specialized bioinformatics tools to map TMG-induced protein expression changes to specific cellular pathways, enabling more precise understanding of TMG's mechanistic effects on cellular function and protein homeostasis.
Strengths: Their multi-omics integration provides unprecedented depth in understanding TMG's systemic effects beyond just protein expression. Their advanced bioinformatics capabilities allow for sophisticated pathway analysis and biological interpretation of complex datasets. Weaknesses: Their highly specialized approach requires significant technical expertise and computational resources. Some of their findings in model organisms may not directly translate to human physiology without additional validation studies.
Key Research Breakthroughs in TMG-Protein Interaction Mechanisms
Nucleic acid sequence segment for enhancing protein expression
PatentActiveUS9550983B2
Innovation
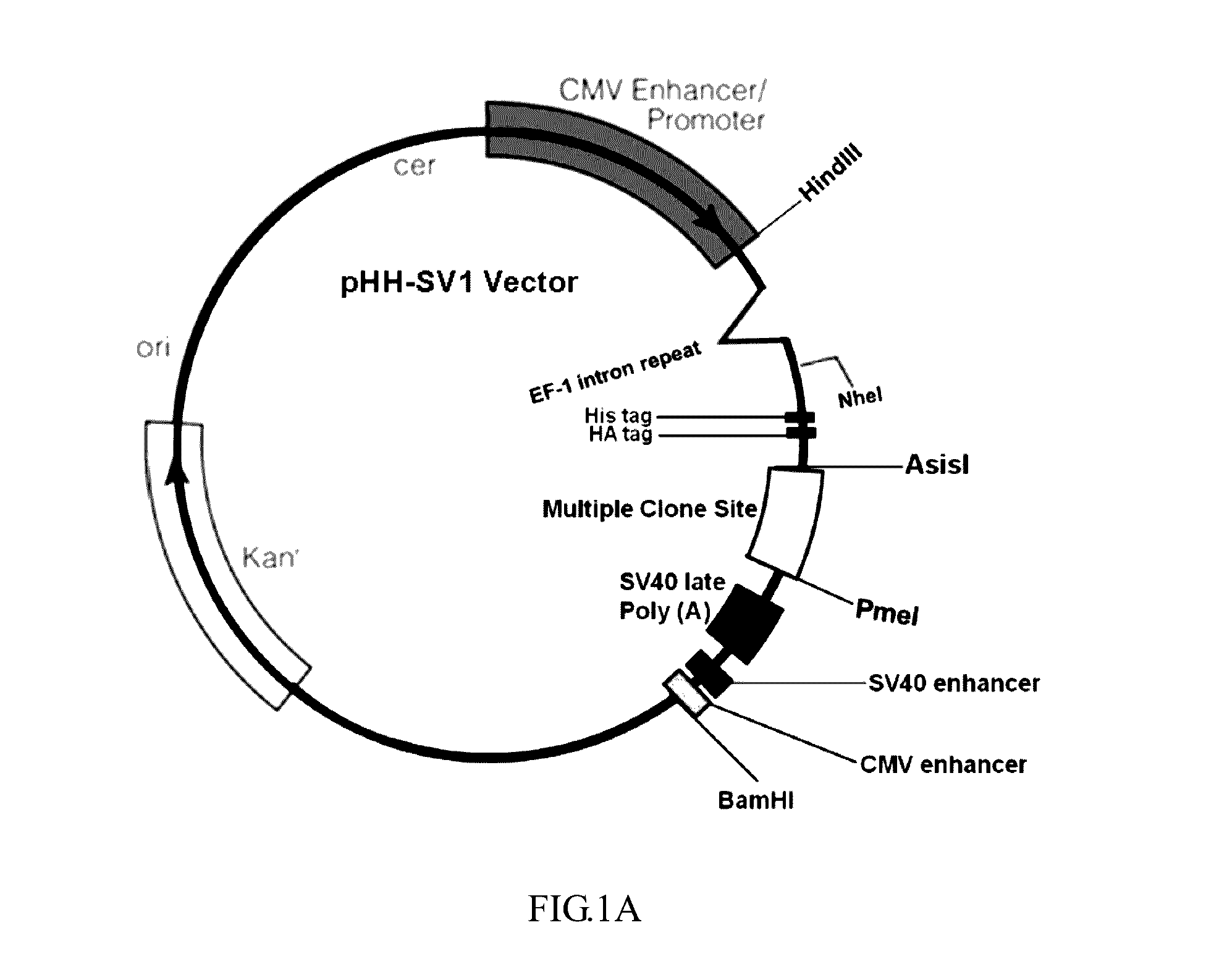
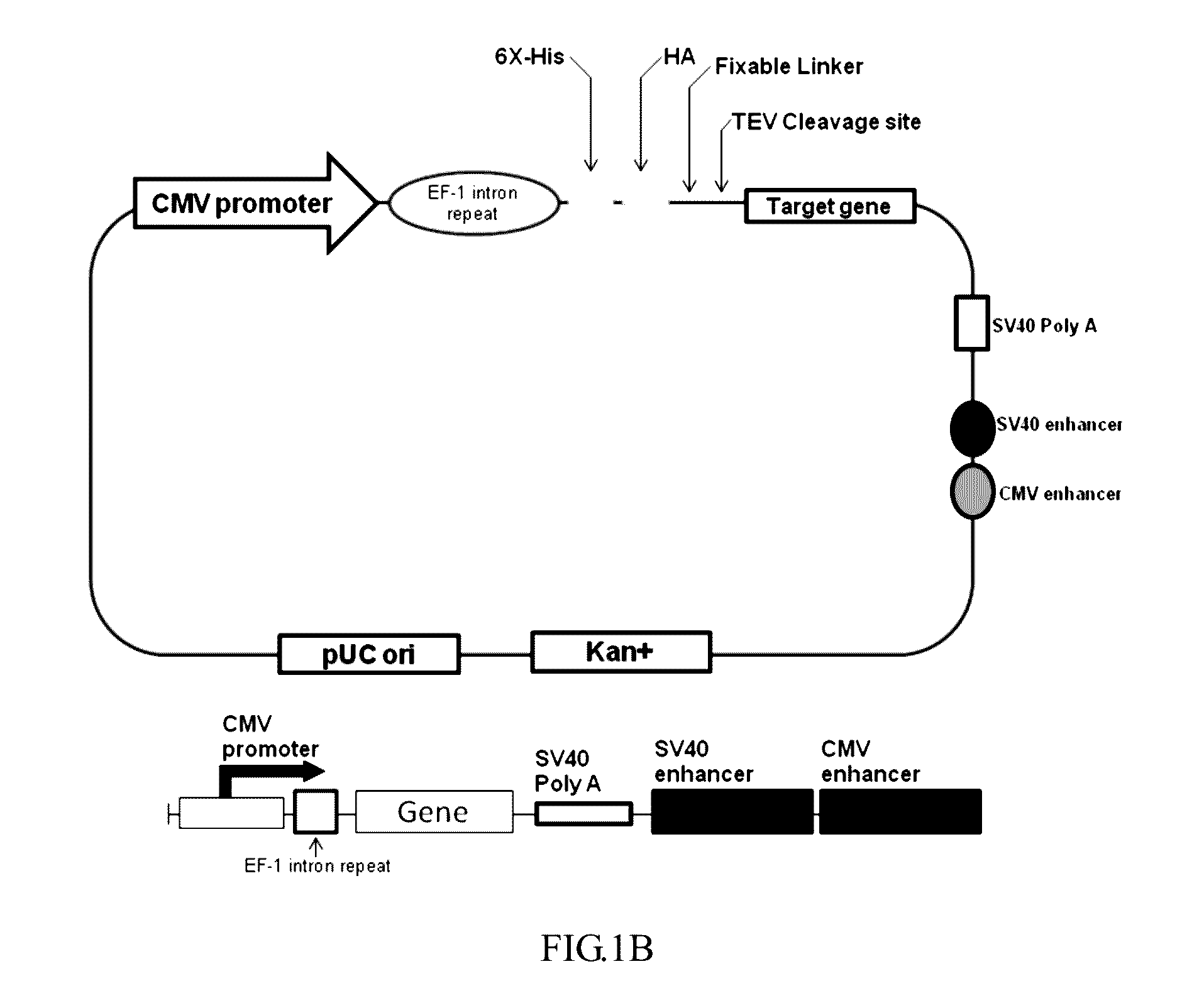
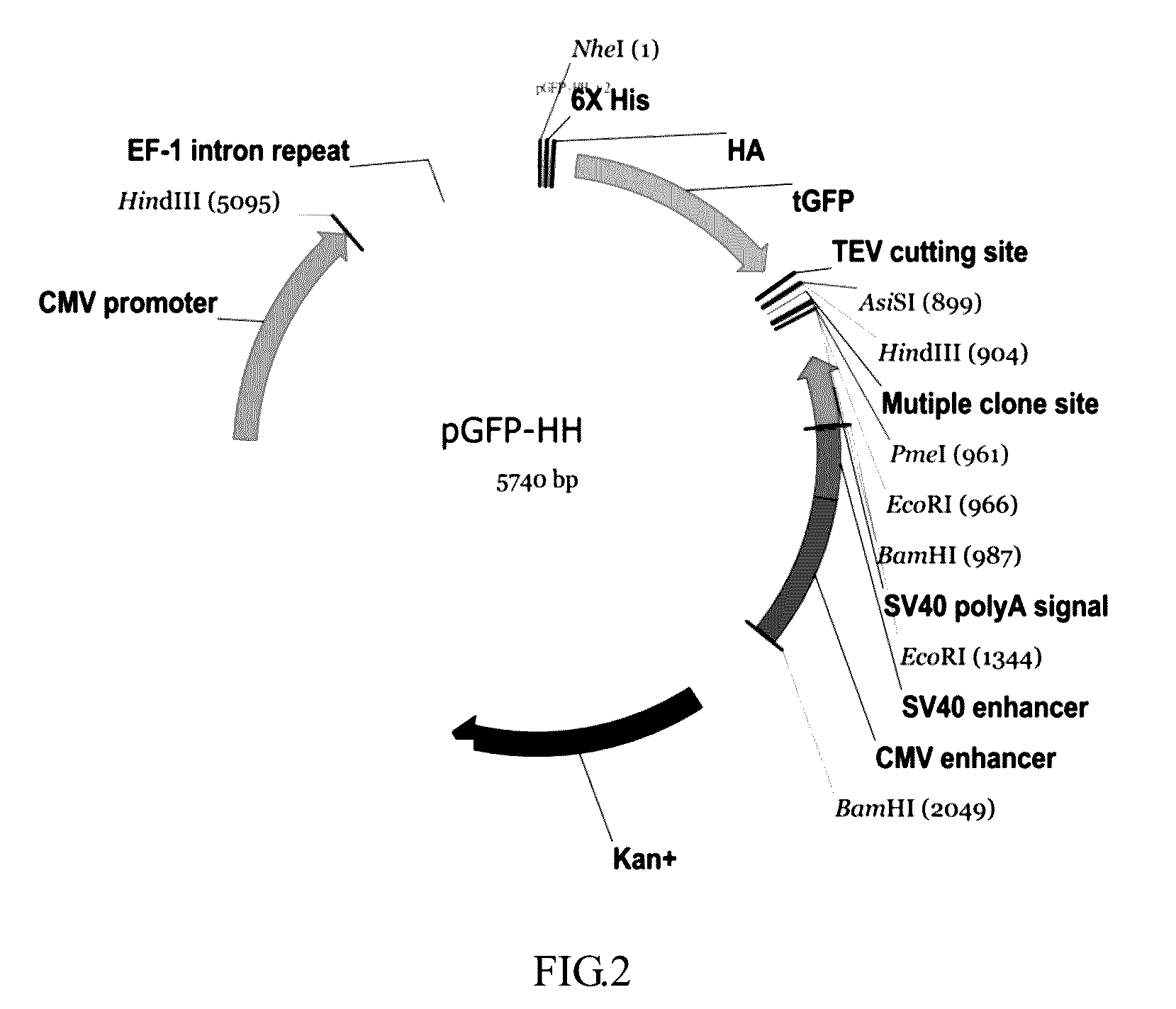
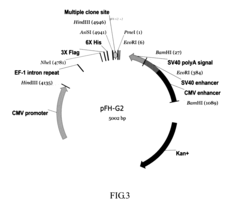
- A nucleic acid sequence is designed with a cytomegalovirus promoter, eEF-1 intron repeat, and a multiple cloning site, incorporating tag elements like histidine and hemagglutinin tags, along with a tobacco etch virus identification sequence, to enhance protein expression and purification in eukaryotic cells, utilizing an expression vector that includes enhancers like SV40 and CMV enhancers to improve transcription efficiency.
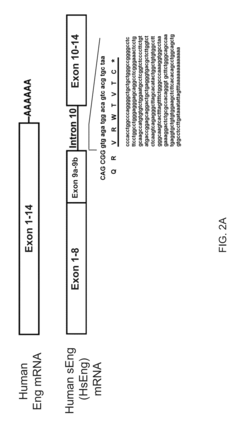
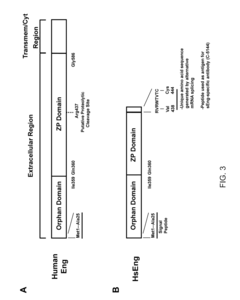
Soluble endoglin and uses thereof
PatentInactiveUS20140323708A1
Innovation
- The discovery of a stable and soluble form of endoglin produced by alternative splicing, which includes a unique C-terminal amino acid sequence, allows for the generation of specific compounds like antibodies and nucleic acids that can modulate or inhibit soluble endoglin activity, enabling targeted treatment and diagnosis of related disorders.
Regulatory Considerations for TMG as a Biological Modifier
The regulatory landscape for Trimethylglycine (TMG) as a biological modifier in protein expression research presents complex challenges for researchers and pharmaceutical companies. In the United States, the FDA's regulatory framework categorizes TMG differently depending on its intended use. When utilized as a research tool for measuring protein expression, TMG falls under laboratory reagent regulations. However, when incorporated into therapeutic applications targeting protein expression modulation, it becomes subject to more stringent drug development regulations under 21 CFR Part 312.
European regulatory bodies, particularly the European Medicines Agency (EMA), have established specific guidelines for biological modifiers that affect protein expression pathways. These guidelines mandate comprehensive safety assessments and standardized protocols for measuring TMG's effects on protein expression. Notably, the EMA's Scientific Advice Working Party has recently issued recommendations specifically addressing osmolytes like TMG when used in biological research contexts.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have attempted to standardize the regulatory approach to biological modifiers. The ICH Q3D guidelines on elemental impurities and Q5E guidelines on comparability are particularly relevant when evaluating TMG's impact on protein expression systems.
Regulatory compliance for TMG research requires robust documentation of analytical methods used to measure protein expression changes. Method validation parameters must include specificity, accuracy, precision, and reproducibility according to ICH Q2(R1) guidelines. Furthermore, researchers must demonstrate that their TMG measurement protocols can reliably distinguish between natural biological variation and true TMG-induced effects on protein expression.
Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) considerations become increasingly important as TMG research moves from basic science toward clinical applications. Regulatory bodies require detailed documentation of TMG purity specifications, stability data, and analytical procedures used to quantify its effects on protein expression. This documentation must demonstrate consistency across multiple batches and experimental conditions.
Ethical considerations and institutional review board (IRB) approvals represent another regulatory dimension when TMG studies involve human subjects or human-derived materials. Researchers must navigate complex informed consent requirements, particularly when investigating TMG's effects on protein expression in vulnerable populations or when collecting longitudinal data on protein expression changes.
Looking forward, regulatory trends suggest increasing scrutiny of biological modifiers like TMG, with particular emphasis on standardized reporting of protein expression data and comprehensive safety profiles. Researchers should anticipate more detailed regulatory guidance specific to osmolytes and their mechanisms of action in biological systems, especially as TMG applications expand beyond traditional research contexts into potential therapeutic domains.
European regulatory bodies, particularly the European Medicines Agency (EMA), have established specific guidelines for biological modifiers that affect protein expression pathways. These guidelines mandate comprehensive safety assessments and standardized protocols for measuring TMG's effects on protein expression. Notably, the EMA's Scientific Advice Working Party has recently issued recommendations specifically addressing osmolytes like TMG when used in biological research contexts.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have attempted to standardize the regulatory approach to biological modifiers. The ICH Q3D guidelines on elemental impurities and Q5E guidelines on comparability are particularly relevant when evaluating TMG's impact on protein expression systems.
Regulatory compliance for TMG research requires robust documentation of analytical methods used to measure protein expression changes. Method validation parameters must include specificity, accuracy, precision, and reproducibility according to ICH Q2(R1) guidelines. Furthermore, researchers must demonstrate that their TMG measurement protocols can reliably distinguish between natural biological variation and true TMG-induced effects on protein expression.
Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) considerations become increasingly important as TMG research moves from basic science toward clinical applications. Regulatory bodies require detailed documentation of TMG purity specifications, stability data, and analytical procedures used to quantify its effects on protein expression. This documentation must demonstrate consistency across multiple batches and experimental conditions.
Ethical considerations and institutional review board (IRB) approvals represent another regulatory dimension when TMG studies involve human subjects or human-derived materials. Researchers must navigate complex informed consent requirements, particularly when investigating TMG's effects on protein expression in vulnerable populations or when collecting longitudinal data on protein expression changes.
Looking forward, regulatory trends suggest increasing scrutiny of biological modifiers like TMG, with particular emphasis on standardized reporting of protein expression data and comprehensive safety profiles. Researchers should anticipate more detailed regulatory guidance specific to osmolytes and their mechanisms of action in biological systems, especially as TMG applications expand beyond traditional research contexts into potential therapeutic domains.
Translational Applications of TMG in Therapeutic Protein Production
The therapeutic protein production industry stands at a critical juncture where efficiency and yield optimization have become paramount concerns. Trimethylglycine (TMG), also known as betaine, presents significant potential for translational applications in this domain. Recent research indicates that TMG's osmoprotective properties can be leveraged to enhance protein expression systems commonly used in biopharmaceutical manufacturing.
Several biopharmaceutical companies have begun incorporating TMG supplementation in their production protocols, reporting yield increases of 15-30% for monoclonal antibodies and recombinant proteins. This improvement stems from TMG's ability to stabilize cellular machinery under osmotic stress conditions that typically occur during high-density cell cultures. The compound appears to maintain protein folding efficiency even as production scales up, addressing a common bottleneck in industrial processes.
Clinical-grade protein therapeutics, including enzyme replacement therapies and cytokines, have demonstrated enhanced stability profiles when produced in TMG-supplemented expression systems. This translates to extended shelf-life and potentially reduced immunogenicity, factors that directly impact therapeutic efficacy and patient outcomes. The cost-benefit analysis suggests that TMG supplementation represents a relatively inexpensive modification to existing production pipelines with potentially significant returns.
Regulatory considerations appear favorable, as TMG is generally recognized as safe (GRAS) by the FDA, facilitating its integration into GMP manufacturing processes. Several contract manufacturing organizations (CMOs) have begun offering TMG-enhanced production services as premium options for clients seeking to maximize yield for difficult-to-express proteins or to rescue underperforming production cell lines.
The scalability of TMG applications ranges from laboratory research to industrial bioreactors. Preliminary data from pilot-scale implementations indicate that the benefits observed in small-scale experiments translate effectively to larger production volumes, though optimization of TMG concentration appears necessary at different scales to maintain optimal results.
Future directions for translational applications include the development of TMG-responsive promoter systems that could enable fine-tuned protein expression control, potentially creating "smart" production systems that adjust to changing culture conditions. Additionally, combination approaches utilizing TMG alongside other chemical chaperones show promise for synergistic enhancement of difficult-to-express therapeutic proteins, particularly those requiring complex post-translational modifications.
As personalized medicine advances, the ability to rapidly produce small batches of patient-specific therapeutic proteins may benefit significantly from TMG-enhanced expression systems, which could reduce production timelines and increase accessibility of customized protein therapeutics.
Several biopharmaceutical companies have begun incorporating TMG supplementation in their production protocols, reporting yield increases of 15-30% for monoclonal antibodies and recombinant proteins. This improvement stems from TMG's ability to stabilize cellular machinery under osmotic stress conditions that typically occur during high-density cell cultures. The compound appears to maintain protein folding efficiency even as production scales up, addressing a common bottleneck in industrial processes.
Clinical-grade protein therapeutics, including enzyme replacement therapies and cytokines, have demonstrated enhanced stability profiles when produced in TMG-supplemented expression systems. This translates to extended shelf-life and potentially reduced immunogenicity, factors that directly impact therapeutic efficacy and patient outcomes. The cost-benefit analysis suggests that TMG supplementation represents a relatively inexpensive modification to existing production pipelines with potentially significant returns.
Regulatory considerations appear favorable, as TMG is generally recognized as safe (GRAS) by the FDA, facilitating its integration into GMP manufacturing processes. Several contract manufacturing organizations (CMOs) have begun offering TMG-enhanced production services as premium options for clients seeking to maximize yield for difficult-to-express proteins or to rescue underperforming production cell lines.
The scalability of TMG applications ranges from laboratory research to industrial bioreactors. Preliminary data from pilot-scale implementations indicate that the benefits observed in small-scale experiments translate effectively to larger production volumes, though optimization of TMG concentration appears necessary at different scales to maintain optimal results.
Future directions for translational applications include the development of TMG-responsive promoter systems that could enable fine-tuned protein expression control, potentially creating "smart" production systems that adjust to changing culture conditions. Additionally, combination approaches utilizing TMG alongside other chemical chaperones show promise for synergistic enhancement of difficult-to-express therapeutic proteins, particularly those requiring complex post-translational modifications.
As personalized medicine advances, the ability to rapidly produce small batches of patient-specific therapeutic proteins may benefit significantly from TMG-enhanced expression systems, which could reduce production timelines and increase accessibility of customized protein therapeutics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!