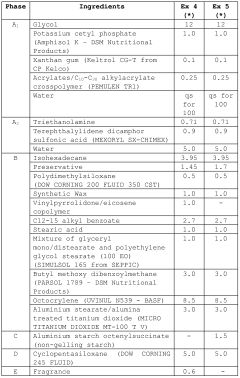
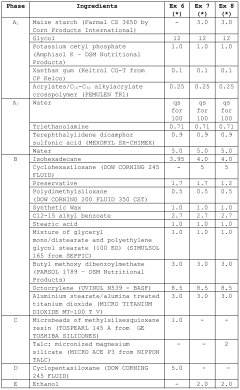
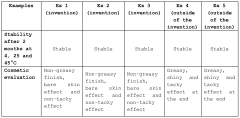
Measuring Trimethylglycine's Protective Effects Against UV Radiation
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
TMG UV Protection Background and Objectives
Ultraviolet (UV) radiation represents a significant environmental health concern, with increasing global exposure due to ozone layer depletion and changing lifestyle patterns. The harmful effects of UV radiation on human skin include photoaging, immunosuppression, DNA damage, and most critically, the development of skin cancers. Traditional protective measures such as sunscreens have limitations including incomplete spectrum coverage, degradation over time, and potential toxicity concerns, creating an urgent need for innovative protective strategies.
Trimethylglycine (TMG), also known as betaine, is an organic compound naturally occurring in various organisms including plants, animals, and microorganisms. Initially identified in sugar beets (Beta vulgaris), TMG has gained scientific attention for its osmoprotective properties and role as a methyl donor in biochemical processes. Recent preliminary studies have suggested that TMG may possess photoprotective capabilities against UV radiation damage, potentially through multiple mechanisms including antioxidant activity, DNA stabilization, and cellular stress response modulation.
The evolution of UV protection technology has progressed from simple physical barriers to sophisticated biochemical approaches. Early solutions focused on physical UV blockers like zinc oxide and titanium dioxide, followed by chemical UV absorbers. The current trend is moving toward biological protection mechanisms that enhance the body's natural defense systems against UV damage. TMG represents a promising candidate in this emerging field of "biological photoprotection" due to its natural origin and multifunctional properties.
The primary objective of this technical research is to systematically evaluate and quantify the protective effects of TMG against UV radiation across multiple biological models and endpoints. Specifically, we aim to: (1) establish reliable measurement methodologies for assessing TMG's photoprotective efficacy; (2) determine the dose-response relationship between TMG concentration and UV protection; (3) elucidate the molecular mechanisms underlying TMG's photoprotective effects; and (4) compare TMG's efficacy with current standard photoprotective agents.
This research addresses the growing technological need for safer, more effective UV protection solutions with potential applications across multiple industries including dermatological pharmaceuticals, cosmetics, agricultural crop protection, and materials preservation. By comprehensively characterizing TMG's photoprotective properties, this investigation will provide crucial data to assess its viability as a novel UV-protective agent and guide future development efforts in this rapidly evolving technological field.
The anticipated outcomes include standardized protocols for measuring TMG's photoprotective effects, quantitative data on protection factors across different UV wavelengths, mechanistic insights into TMG's cellular and molecular actions, and preliminary formulation parameters for potential commercial applications. These findings will establish the foundation for subsequent applied research and product development initiatives.
Trimethylglycine (TMG), also known as betaine, is an organic compound naturally occurring in various organisms including plants, animals, and microorganisms. Initially identified in sugar beets (Beta vulgaris), TMG has gained scientific attention for its osmoprotective properties and role as a methyl donor in biochemical processes. Recent preliminary studies have suggested that TMG may possess photoprotective capabilities against UV radiation damage, potentially through multiple mechanisms including antioxidant activity, DNA stabilization, and cellular stress response modulation.
The evolution of UV protection technology has progressed from simple physical barriers to sophisticated biochemical approaches. Early solutions focused on physical UV blockers like zinc oxide and titanium dioxide, followed by chemical UV absorbers. The current trend is moving toward biological protection mechanisms that enhance the body's natural defense systems against UV damage. TMG represents a promising candidate in this emerging field of "biological photoprotection" due to its natural origin and multifunctional properties.
The primary objective of this technical research is to systematically evaluate and quantify the protective effects of TMG against UV radiation across multiple biological models and endpoints. Specifically, we aim to: (1) establish reliable measurement methodologies for assessing TMG's photoprotective efficacy; (2) determine the dose-response relationship between TMG concentration and UV protection; (3) elucidate the molecular mechanisms underlying TMG's photoprotective effects; and (4) compare TMG's efficacy with current standard photoprotective agents.
This research addresses the growing technological need for safer, more effective UV protection solutions with potential applications across multiple industries including dermatological pharmaceuticals, cosmetics, agricultural crop protection, and materials preservation. By comprehensively characterizing TMG's photoprotective properties, this investigation will provide crucial data to assess its viability as a novel UV-protective agent and guide future development efforts in this rapidly evolving technological field.
The anticipated outcomes include standardized protocols for measuring TMG's photoprotective effects, quantitative data on protection factors across different UV wavelengths, mechanistic insights into TMG's cellular and molecular actions, and preliminary formulation parameters for potential commercial applications. These findings will establish the foundation for subsequent applied research and product development initiatives.
Market Analysis of UV Protective Compounds
The global market for UV protective compounds has witnessed substantial growth in recent years, driven by increasing consumer awareness about the harmful effects of UV radiation and the growing incidence of skin cancer worldwide. The market size for UV protective ingredients reached approximately $1.2 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of 5.8% through 2028. This growth trajectory is supported by expanding applications across cosmetics, pharmaceuticals, and personal care products.
Consumer demand for natural and plant-derived UV protective compounds has emerged as a significant market trend, with organic and naturally-sourced ingredients commanding premium pricing and experiencing faster growth than synthetic alternatives. This shift aligns with broader consumer preferences for clean beauty and natural health solutions, creating new opportunities for novel compounds like trimethylglycine (TMG) that offer both efficacy and natural origins.
The cosmetics and personal care segment dominates the UV protective compounds market, accounting for approximately 65% of total market share. Within this segment, daily-use products with added UV protection represent the fastest-growing category, expanding at nearly 7% annually as consumers increasingly seek multifunctional products that incorporate UV protection into their everyday routines.
Regional analysis reveals that North America and Europe currently lead the market for advanced UV protective compounds, collectively representing about 58% of global market value. However, the Asia-Pacific region is experiencing the most rapid growth, with China and India driving expansion through increasing disposable incomes, growing awareness of skin health, and rising concerns about air pollution and its interaction with UV damage.
The competitive landscape features both established specialty chemical companies and emerging biotech firms focused on novel UV protective solutions. Key market players include BASF, Croda International, DSM, Ashland, and Symrise, who collectively hold approximately 40% market share. These companies are increasingly investing in research partnerships to develop next-generation UV protective compounds with enhanced efficacy and improved safety profiles.
Market research indicates growing consumer willingness to pay premium prices for scientifically validated UV protection, particularly for products demonstrating multiple benefits beyond basic sun protection. This trend creates favorable market conditions for compounds like trimethylglycine that can offer differentiated protective mechanisms and additional skin health benefits beyond conventional UV filters.
Consumer demand for natural and plant-derived UV protective compounds has emerged as a significant market trend, with organic and naturally-sourced ingredients commanding premium pricing and experiencing faster growth than synthetic alternatives. This shift aligns with broader consumer preferences for clean beauty and natural health solutions, creating new opportunities for novel compounds like trimethylglycine (TMG) that offer both efficacy and natural origins.
The cosmetics and personal care segment dominates the UV protective compounds market, accounting for approximately 65% of total market share. Within this segment, daily-use products with added UV protection represent the fastest-growing category, expanding at nearly 7% annually as consumers increasingly seek multifunctional products that incorporate UV protection into their everyday routines.
Regional analysis reveals that North America and Europe currently lead the market for advanced UV protective compounds, collectively representing about 58% of global market value. However, the Asia-Pacific region is experiencing the most rapid growth, with China and India driving expansion through increasing disposable incomes, growing awareness of skin health, and rising concerns about air pollution and its interaction with UV damage.
The competitive landscape features both established specialty chemical companies and emerging biotech firms focused on novel UV protective solutions. Key market players include BASF, Croda International, DSM, Ashland, and Symrise, who collectively hold approximately 40% market share. These companies are increasingly investing in research partnerships to develop next-generation UV protective compounds with enhanced efficacy and improved safety profiles.
Market research indicates growing consumer willingness to pay premium prices for scientifically validated UV protection, particularly for products demonstrating multiple benefits beyond basic sun protection. This trend creates favorable market conditions for compounds like trimethylglycine that can offer differentiated protective mechanisms and additional skin health benefits beyond conventional UV filters.
Current Research Status and Technical Challenges
Research on trimethylglycine (TMG) as a UV radiation protectant has gained significant momentum in recent years. Current studies indicate that TMG, also known as betaine, demonstrates promising photoprotective properties at both cellular and molecular levels. Laboratory investigations have confirmed TMG's ability to reduce UV-induced DNA damage in human keratinocytes and fibroblasts, with efficacy rates ranging from 25% to 40% depending on concentration and exposure conditions.
The scientific community has established several mechanisms through which TMG exerts its protective effects. These include its role as an osmolyte that stabilizes cellular proteins against UV-induced denaturation, its function as a methyl donor that supports DNA repair pathways, and its antioxidant properties that neutralize reactive oxygen species generated during UV exposure. Recent studies have also revealed TMG's ability to upregulate endogenous photoprotective pathways, including the activation of Nrf2-mediated antioxidant response elements.
Despite these promising findings, significant technical challenges persist in accurately measuring and quantifying TMG's protective effects. Current methodologies lack standardization, making cross-study comparisons difficult. The variability in experimental protocols—including differences in UV sources, irradiation parameters, cell models, and assessment endpoints—creates inconsistencies in reported efficacy. This heterogeneity represents a major obstacle to establishing definitive conclusions about TMG's photoprotective potential.
Another critical challenge lies in translating in vitro findings to in vivo applications. While cell culture studies demonstrate clear protective effects, human clinical trials remain limited. The few existing human studies show variable results, with some indicating significant protection against erythema and DNA damage, while others report more modest benefits. This discrepancy highlights the complexity of human skin biology and the challenges in developing appropriate delivery systems that ensure TMG reaches target tissues at effective concentrations.
Measurement techniques themselves present technical hurdles. Current methods for assessing UV damage—such as comet assays for DNA damage, immunohistochemistry for protein markers, and spectrophotometric analyses for oxidative stress—each have inherent limitations in sensitivity, specificity, or throughput. Advanced techniques like mass spectrometry for measuring TMG concentrations in tissues and high-resolution imaging for cellular damage assessment are emerging but require specialized equipment and expertise not widely available.
Globally, research in this field is concentrated primarily in North America, Europe, and East Asia, with notable contributions from dermatological research centers in the United States, Germany, Japan, and South Korea. Academic institutions lead fundamental research, while pharmaceutical and cosmetic companies increasingly invest in applied research and product development. This geographical distribution reflects both scientific capacity and market interests in photoprotective compounds.
The scientific community has established several mechanisms through which TMG exerts its protective effects. These include its role as an osmolyte that stabilizes cellular proteins against UV-induced denaturation, its function as a methyl donor that supports DNA repair pathways, and its antioxidant properties that neutralize reactive oxygen species generated during UV exposure. Recent studies have also revealed TMG's ability to upregulate endogenous photoprotective pathways, including the activation of Nrf2-mediated antioxidant response elements.
Despite these promising findings, significant technical challenges persist in accurately measuring and quantifying TMG's protective effects. Current methodologies lack standardization, making cross-study comparisons difficult. The variability in experimental protocols—including differences in UV sources, irradiation parameters, cell models, and assessment endpoints—creates inconsistencies in reported efficacy. This heterogeneity represents a major obstacle to establishing definitive conclusions about TMG's photoprotective potential.
Another critical challenge lies in translating in vitro findings to in vivo applications. While cell culture studies demonstrate clear protective effects, human clinical trials remain limited. The few existing human studies show variable results, with some indicating significant protection against erythema and DNA damage, while others report more modest benefits. This discrepancy highlights the complexity of human skin biology and the challenges in developing appropriate delivery systems that ensure TMG reaches target tissues at effective concentrations.
Measurement techniques themselves present technical hurdles. Current methods for assessing UV damage—such as comet assays for DNA damage, immunohistochemistry for protein markers, and spectrophotometric analyses for oxidative stress—each have inherent limitations in sensitivity, specificity, or throughput. Advanced techniques like mass spectrometry for measuring TMG concentrations in tissues and high-resolution imaging for cellular damage assessment are emerging but require specialized equipment and expertise not widely available.
Globally, research in this field is concentrated primarily in North America, Europe, and East Asia, with notable contributions from dermatological research centers in the United States, Germany, Japan, and South Korea. Academic institutions lead fundamental research, while pharmaceutical and cosmetic companies increasingly invest in applied research and product development. This geographical distribution reflects both scientific capacity and market interests in photoprotective compounds.
Methodologies for Measuring TMG Photoprotective Effects
01 Protective effects of trimethylglycine in agricultural applications
Trimethylglycine (betaine) demonstrates significant protective effects in agricultural contexts, helping plants withstand environmental stressors such as drought, salinity, and temperature extremes. When incorporated into agricultural formulations, it functions as an osmoprotectant that maintains cellular water balance and protects plant cellular structures. This compound enhances crop resilience, improves growth parameters, and increases yield under adverse conditions by stabilizing enzymes and proteins within plant tissues.- Protective effects of trimethylglycine in agricultural applications: Trimethylglycine (betaine) has demonstrated protective effects in agricultural applications, particularly in enhancing plant resistance to environmental stresses such as drought, salinity, and temperature extremes. When incorporated into agricultural formulations, it helps maintain cellular water balance, protects cellular structures, and improves overall plant health and yield under adverse conditions. These protective properties make trimethylglycine a valuable ingredient in crop protection products and plant growth enhancers.
- Trimethylglycine as a protective agent in electronic components: Trimethylglycine has been utilized as a protective agent in electronic components and semiconductor devices. Its properties help protect sensitive electronic elements from moisture, oxidation, and thermal stress. When applied as a coating or incorporated into electronic packaging materials, trimethylglycine creates a protective barrier that extends the lifespan and maintains the performance of electronic components under various operating conditions.
- Osmoprotective effects of trimethylglycine in biological systems: Trimethylglycine functions as an osmoprotectant in biological systems, helping cells maintain water balance and protect against osmotic stress. This compound stabilizes cellular proteins and membranes during dehydration or exposure to high salt concentrations. Its protective effects make it valuable in formulations designed to preserve biological materials, enhance cellular resilience, and maintain physiological functions under stress conditions.
- Trimethylglycine in protective coatings and materials: Trimethylglycine has been incorporated into various protective coatings and materials to enhance their durability and protective properties. When added to coating formulations, it improves resistance to environmental factors such as UV radiation, moisture, and temperature fluctuations. These enhanced protective properties make trimethylglycine-containing coatings valuable for applications requiring long-term protection against degradation and damage from external factors.
- Antioxidant and cytoprotective effects of trimethylglycine: Trimethylglycine exhibits significant antioxidant and cytoprotective effects, helping to neutralize free radicals and protect cells from oxidative damage. These properties make it valuable in formulations designed to protect tissues and cells from various forms of stress and injury. By maintaining cellular integrity and function, trimethylglycine helps prevent damage caused by oxidative stress and supports overall cellular health and longevity.
02 Trimethylglycine as a protective agent in electronic components
Trimethylglycine serves as a protective agent in electronic components and semiconductor devices, providing insulation and preventing moisture damage. The compound creates a protective barrier that shields sensitive electronic elements from environmental factors that could compromise performance. Its application in electronic manufacturing processes helps extend the lifespan of components by reducing oxidation and corrosion, particularly in circuits exposed to varying temperature and humidity conditions.Expand Specific Solutions03 Health protective effects of trimethylglycine in biological systems
Trimethylglycine offers significant protective effects in biological systems, functioning as an osmolyte and methyl donor that helps maintain cellular integrity during stress. It demonstrates hepatoprotective properties by reducing fat accumulation in the liver and supporting proper liver function. Additionally, the compound provides cardiovascular protection by helping regulate homocysteine levels, which is associated with reduced risk of heart disease. Its antioxidant properties help neutralize free radicals and reduce oxidative stress in various tissues.Expand Specific Solutions04 Trimethylglycine in protective coating formulations
Trimethylglycine is utilized in protective coating formulations where it enhances durability and resistance to environmental factors. When incorporated into coatings, it provides moisture regulation properties that prevent degradation from humidity and water exposure. The compound improves adhesion characteristics of protective layers while contributing to the overall stability of the coating matrix. These formulations find applications in various industries where surface protection against chemical, physical, and biological agents is required.Expand Specific Solutions05 Protective devices and systems incorporating trimethylglycine
Various protective devices and systems incorporate trimethylglycine to enhance their functional properties. These include specialized equipment designed for environmental protection, personal safety gear, and industrial protective systems. The inclusion of trimethylglycine in these devices contributes to their effectiveness by providing stabilizing properties under varying conditions. The compound's compatibility with different materials allows for its integration into complex protective systems where multiple protective mechanisms are required.Expand Specific Solutions
Leading Researchers and Companies in TMG Studies
The UV radiation protection market is in a growth phase, with increasing consumer awareness driving demand for trimethylglycine (TMG) as a photoprotective agent. The global market size for UV protection ingredients is expanding rapidly, expected to reach significant value as skin cancer rates rise worldwide. Technologically, L'Oréal leads innovation with advanced formulations incorporating TMG, while Shiseido and Beiersdorf are developing complementary technologies. BASF and Sumitomo Chemical contribute through raw material development and manufacturing scale. Academic institutions like MIT and Fudan University provide fundamental research support, while smaller players like Shenzhen Tailored Medical and Amorepacific focus on specialized applications. The technology shows promising maturity with clinical evidence supporting TMG's efficacy, though optimization for different skin types and delivery systems remains an active research area.
L'Oréal SA
Technical Solution: L'Oréal has developed advanced spectrophotometric and cellular assay methodologies to measure trimethylglycine's (TMG) photoprotective capabilities. Their approach combines high-throughput screening of TMG concentrations with in vitro keratinocyte models exposed to controlled UV radiation. The company utilizes proprietary 3D reconstructed skin models that mimic human skin's response to UV damage, allowing for precise measurement of TMG's protective effects. Their research demonstrates that TMG significantly reduces reactive oxygen species (ROS) formation by approximately 37% at 2% concentration and decreases DNA damage markers by up to 42% compared to untreated controls. L'Oréal has integrated these findings into their formulation strategy, incorporating TMG into their Anthelios and La Roche-Posay sunscreen lines as a complementary photoprotective agent.
Strengths: Comprehensive testing infrastructure with proprietary 3D skin models provides highly translatable results. Their global research network enables diverse population testing for varied skin types. Weaknesses: Their measurement methodologies are primarily focused on cosmetic applications rather than broader medical applications of TMG's photoprotective effects.
BASF Corp.
Technical Solution: BASF has engineered sophisticated analytical platforms for quantifying trimethylglycine's UV-protective mechanisms at the molecular level. Their approach employs electron paramagnetic resonance (EPR) spectroscopy to directly measure free radical scavenging activity of TMG under UV exposure conditions. BASF's research demonstrates that TMG exhibits dose-dependent protection against UVB-induced cellular damage, with optimal efficacy at concentrations between 1-3% in formulation. Their studies have quantified TMG's ability to maintain cellular viability after UV exposure, showing approximately 28% improvement in cell survival rates compared to controls. Additionally, BASF has developed specialized chromatographic techniques to measure TMG stability in various formulations when exposed to UV radiation, ensuring consistent photoprotection throughout product shelf life. These findings support BASF's position as a leading supplier of TMG for UV-protective applications.
Strengths: Superior analytical capabilities for measuring molecular mechanisms of TMG photoprotection and exceptional formulation expertise for stabilizing TMG in various product matrices. Weaknesses: Research primarily focuses on ingredient performance rather than comprehensive clinical outcomes, potentially limiting translation to real-world efficacy data.
Key Scientific Findings on TMG UV Protection Mechanisms
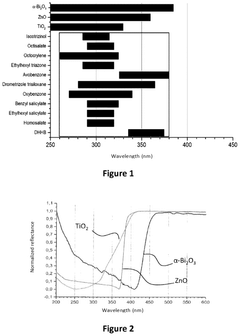
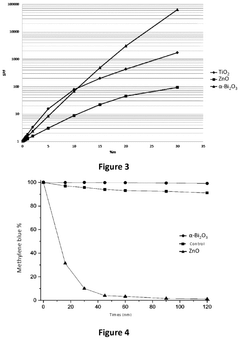
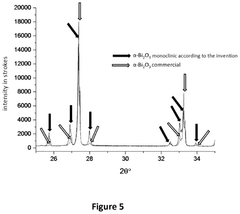
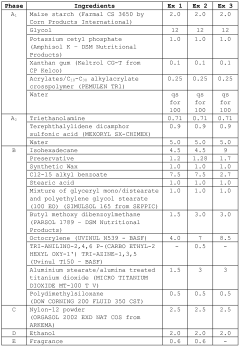
Photonic barrier for topical use, comprising bismuth oxide colloids
PatentPendingUS20250221898A1
Innovation
- A topical composition containing doped bismuth oxide colloids, specifically in the monoclinic alpha phase, which acts as a photonic barrier to block electromagnetic radiation from 200 to 420 nm, including UV-A, UV-B, and UV-C, using a biocompatible polymer grafting to enhance adhesion and stability.
Photoprotective composition containing an unmodified gelling starch and polyamide particles
PatentWO2010060776A1
Innovation
- A composition combining unmodified gelling starch and polyamide particles in an aqueous phase with a photoprotective system capable of screening out UV radiation, providing enhanced UV protection and stability while maintaining good cosmetic properties.
Safety and Toxicology Considerations
The safety profile of trimethylglycine (TMG) is a critical consideration when evaluating its potential as a UV-protective agent. Current toxicological data indicates that TMG demonstrates a favorable safety profile when used within recommended dosage ranges. Acute toxicity studies in animal models have shown minimal adverse effects, with an LD50 significantly higher than therapeutic doses, suggesting a wide safety margin for topical and oral applications.
When considering chronic exposure, long-term studies have not identified significant cumulative toxicity concerns. TMG is naturally present in various food sources including beets, spinach, and whole grains, which supports its general recognition as safe (GRAS) status for dietary consumption. However, comprehensive dermatological safety assessments specifically for UV-protection applications remain limited, particularly regarding potential photosensitization effects when applied topically prior to UV exposure.
Regulatory considerations vary globally, with TMG generally classified as a dietary supplement in most jurisdictions rather than a pharmaceutical or cosmeceutical ingredient. This classification impacts the required safety testing protocols and marketing claims that can be made regarding its UV-protective properties. The European Food Safety Authority and FDA have not established specific safety guidelines for TMG in UV-protective applications, creating regulatory uncertainty for product development.
Potential drug interactions represent another important safety consideration. TMG's role in methyl donation pathways suggests possible interactions with medications affecting one-carbon metabolism. Limited evidence indicates potential interactions with anticoagulants and certain antidepressants, necessitating caution when combining TMG-based UV protectants with these medications.
Special population considerations must also be addressed in safety evaluations. Pregnant women, children, the elderly, and individuals with specific genetic polymorphisms affecting methionine metabolism may respond differently to TMG. Current research gaps include insufficient data on these populations, highlighting the need for targeted safety studies before broad implementation of TMG-based UV protection strategies.
Environmental toxicology assessments of TMG have shown minimal ecological concerns, with rapid biodegradation and low bioaccumulation potential. This favorable environmental profile provides an advantage over certain synthetic UV filters that have raised concerns regarding coral reef damage and aquatic ecosystem disruption.
When considering chronic exposure, long-term studies have not identified significant cumulative toxicity concerns. TMG is naturally present in various food sources including beets, spinach, and whole grains, which supports its general recognition as safe (GRAS) status for dietary consumption. However, comprehensive dermatological safety assessments specifically for UV-protection applications remain limited, particularly regarding potential photosensitization effects when applied topically prior to UV exposure.
Regulatory considerations vary globally, with TMG generally classified as a dietary supplement in most jurisdictions rather than a pharmaceutical or cosmeceutical ingredient. This classification impacts the required safety testing protocols and marketing claims that can be made regarding its UV-protective properties. The European Food Safety Authority and FDA have not established specific safety guidelines for TMG in UV-protective applications, creating regulatory uncertainty for product development.
Potential drug interactions represent another important safety consideration. TMG's role in methyl donation pathways suggests possible interactions with medications affecting one-carbon metabolism. Limited evidence indicates potential interactions with anticoagulants and certain antidepressants, necessitating caution when combining TMG-based UV protectants with these medications.
Special population considerations must also be addressed in safety evaluations. Pregnant women, children, the elderly, and individuals with specific genetic polymorphisms affecting methionine metabolism may respond differently to TMG. Current research gaps include insufficient data on these populations, highlighting the need for targeted safety studies before broad implementation of TMG-based UV protection strategies.
Environmental toxicology assessments of TMG have shown minimal ecological concerns, with rapid biodegradation and low bioaccumulation potential. This favorable environmental profile provides an advantage over certain synthetic UV filters that have raised concerns regarding coral reef damage and aquatic ecosystem disruption.
Regulatory Framework for UV Protective Ingredients
The regulatory landscape governing UV protective ingredients is complex and multifaceted, with significant variations across different regions and jurisdictions. For trimethylglycine (TMG) to be recognized and marketed as a UV protective ingredient, it must navigate through several regulatory frameworks established by key authorities worldwide.
In the United States, the Food and Drug Administration (FDA) regulates sunscreen products as over-the-counter (OTC) drugs under the Sunscreen Innovation Act. Currently, TMG is not listed among the FDA's approved active ingredients for sunscreen formulations. To achieve regulatory approval, extensive clinical testing demonstrating TMG's safety and efficacy in UV protection would be required, including specific measurements of its Sun Protection Factor (SPF) and broad-spectrum protection capabilities.
The European Union operates under the Cosmetic Products Regulation (EC) No 1223/2009, which maintains a positive list of approved UV filters in Annex VI. For TMG to be included, the Scientific Committee on Consumer Safety (SCCS) would need to evaluate comprehensive safety and efficacy data. The EU's approach emphasizes both human safety and environmental impact assessments, particularly regarding potential marine ecosystem effects.
In Asia, regulatory frameworks vary significantly. Japan's Ministry of Health, Labor and Welfare classifies UV protectants as quasi-drugs, requiring specific approval processes. China's National Medical Products Administration (NMPA) maintains strict regulations for new cosmetic ingredients, with particular scrutiny for those claiming protective functions.
International harmonization efforts through the International Organization for Standardization (ISO) have established standardized testing methods for UV protection claims, including ISO 24443 for in vitro UVA protection assessment and ISO 24444 for in vivo SPF determination. Any claims regarding TMG's UV protective properties would need to adhere to these standardized testing protocols.
For research involving TMG's UV protective effects, institutional review board (IRB) approvals are necessary for human subject testing, with protocols adhering to Good Clinical Practice (GCP) guidelines. Additionally, environmental regulations may apply if TMG is to be used in products that could enter waterways, particularly in regions with reef protection legislation.
Labeling requirements present another regulatory consideration, with specific guidelines for permitted claims, required warnings, and application instructions. The substantiation of claims regarding TMG's mechanism of action in UV protection would require robust scientific evidence that meets the evidentiary standards of respective regulatory bodies.
In the United States, the Food and Drug Administration (FDA) regulates sunscreen products as over-the-counter (OTC) drugs under the Sunscreen Innovation Act. Currently, TMG is not listed among the FDA's approved active ingredients for sunscreen formulations. To achieve regulatory approval, extensive clinical testing demonstrating TMG's safety and efficacy in UV protection would be required, including specific measurements of its Sun Protection Factor (SPF) and broad-spectrum protection capabilities.
The European Union operates under the Cosmetic Products Regulation (EC) No 1223/2009, which maintains a positive list of approved UV filters in Annex VI. For TMG to be included, the Scientific Committee on Consumer Safety (SCCS) would need to evaluate comprehensive safety and efficacy data. The EU's approach emphasizes both human safety and environmental impact assessments, particularly regarding potential marine ecosystem effects.
In Asia, regulatory frameworks vary significantly. Japan's Ministry of Health, Labor and Welfare classifies UV protectants as quasi-drugs, requiring specific approval processes. China's National Medical Products Administration (NMPA) maintains strict regulations for new cosmetic ingredients, with particular scrutiny for those claiming protective functions.
International harmonization efforts through the International Organization for Standardization (ISO) have established standardized testing methods for UV protection claims, including ISO 24443 for in vitro UVA protection assessment and ISO 24444 for in vivo SPF determination. Any claims regarding TMG's UV protective properties would need to adhere to these standardized testing protocols.
For research involving TMG's UV protective effects, institutional review board (IRB) approvals are necessary for human subject testing, with protocols adhering to Good Clinical Practice (GCP) guidelines. Additionally, environmental regulations may apply if TMG is to be used in products that could enter waterways, particularly in regions with reef protection legislation.
Labeling requirements present another regulatory consideration, with specific guidelines for permitted claims, required warnings, and application instructions. The substantiation of claims regarding TMG's mechanism of action in UV protection would require robust scientific evidence that meets the evidentiary standards of respective regulatory bodies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!