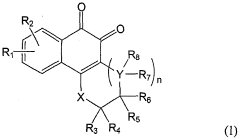
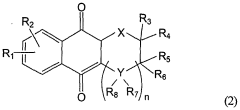
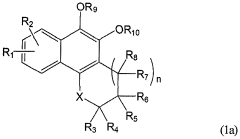
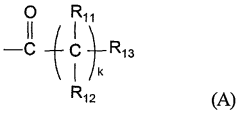
Impact of Geometric Isomers on Solubility Enhancing Techniques
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers and Solubility Enhancement Goals
Geometric isomers, a subset of stereoisomers, have long been recognized for their significant impact on various chemical and physical properties of compounds. In the context of solubility enhancement, understanding and leveraging the unique characteristics of geometric isomers have become increasingly important. This technological field has evolved from basic recognition of isomeric differences to sophisticated manipulation of molecular structures for optimized solubility profiles.
The primary goal of research in this area is to develop innovative techniques that exploit the structural nuances of geometric isomers to enhance the solubility of pharmaceuticals, agrochemicals, and other industrially relevant compounds. By focusing on the spatial arrangement of atoms within molecules, scientists aim to create more effective formulations and delivery systems for poorly soluble substances.
One key objective is to establish a comprehensive understanding of how geometric isomerization affects solubility across different chemical classes. This involves systematic studies of cis-trans isomers and their interactions with various solvents, as well as the development of predictive models to anticipate solubility changes based on isomeric configurations.
Another critical goal is the design of novel solubility-enhancing techniques that specifically target geometric isomers. These may include the development of smart excipients that can selectively interact with specific isomeric forms, or the creation of advanced formulation strategies that can dynamically respond to isomeric shifts in solution.
Researchers also aim to elucidate the mechanisms by which geometric isomers influence solubility at the molecular level. This includes investigating how isomeric changes affect crystal packing, hydration shells, and intermolecular forces, all of which play crucial roles in determining a compound's solubility profile.
The field is moving towards integrating computational approaches with experimental techniques to accelerate the discovery and optimization of solubility-enhancing strategies. Machine learning algorithms and molecular dynamics simulations are being employed to predict isomer-specific solubility behaviors and guide the rational design of more effective formulations.
Ultimately, the overarching goal is to translate these scientific insights into practical applications that can significantly improve drug bioavailability, reduce dosage requirements, and enhance the overall efficacy of therapeutic and industrial compounds. This requires close collaboration between chemists, pharmaceutical scientists, and formulation experts to bridge the gap between fundamental research and real-world implementation.
The primary goal of research in this area is to develop innovative techniques that exploit the structural nuances of geometric isomers to enhance the solubility of pharmaceuticals, agrochemicals, and other industrially relevant compounds. By focusing on the spatial arrangement of atoms within molecules, scientists aim to create more effective formulations and delivery systems for poorly soluble substances.
One key objective is to establish a comprehensive understanding of how geometric isomerization affects solubility across different chemical classes. This involves systematic studies of cis-trans isomers and their interactions with various solvents, as well as the development of predictive models to anticipate solubility changes based on isomeric configurations.
Another critical goal is the design of novel solubility-enhancing techniques that specifically target geometric isomers. These may include the development of smart excipients that can selectively interact with specific isomeric forms, or the creation of advanced formulation strategies that can dynamically respond to isomeric shifts in solution.
Researchers also aim to elucidate the mechanisms by which geometric isomers influence solubility at the molecular level. This includes investigating how isomeric changes affect crystal packing, hydration shells, and intermolecular forces, all of which play crucial roles in determining a compound's solubility profile.
The field is moving towards integrating computational approaches with experimental techniques to accelerate the discovery and optimization of solubility-enhancing strategies. Machine learning algorithms and molecular dynamics simulations are being employed to predict isomer-specific solubility behaviors and guide the rational design of more effective formulations.
Ultimately, the overarching goal is to translate these scientific insights into practical applications that can significantly improve drug bioavailability, reduce dosage requirements, and enhance the overall efficacy of therapeutic and industrial compounds. This requires close collaboration between chemists, pharmaceutical scientists, and formulation experts to bridge the gap between fundamental research and real-world implementation.
Market Demand Analysis for Improved Solubility
The market demand for improved solubility techniques, particularly those addressing geometric isomers, has been steadily increasing across various industries. Pharmaceutical companies are at the forefront of this demand, as they continually seek ways to enhance drug bioavailability and efficacy. The impact of geometric isomers on solubility is a critical factor in drug development, with many potential blockbuster drugs facing challenges due to poor solubility profiles.
In the pharmaceutical sector, there is a growing need for advanced solubility-enhancing techniques that can effectively handle geometric isomers. This demand is driven by the increasing complexity of drug molecules and the push towards personalized medicine. Improved solubility can lead to better drug absorption, reduced dosage requirements, and potentially fewer side effects, all of which are highly valued by both pharmaceutical companies and patients.
The agrochemical industry also shows significant interest in solubility-enhancing techniques for geometric isomers. Pesticides and herbicides often contain isomeric compounds, and their effectiveness can be greatly influenced by solubility. Improved solubility can lead to more efficient application methods, reduced environmental impact, and potentially lower costs for farmers.
In the food and beverage industry, there is a rising demand for natural flavors and colorants, many of which exist as geometric isomers. Enhancing the solubility of these compounds can lead to better incorporation into products, improved stability, and potentially enhanced sensory experiences for consumers.
The cosmetics and personal care industry is another sector showing increased interest in solubility-enhancing techniques for geometric isomers. Many active ingredients in skincare and haircare products are isomeric compounds, and their efficacy can be significantly improved through enhanced solubility.
Market analysts project that the global market for solubility-enhancing technologies, including those specifically addressing geometric isomers, will continue to grow at a substantial rate. This growth is expected to be driven by ongoing research and development efforts, increasing regulatory pressures for improved drug formulations, and the expanding applications of isomeric compounds across various industries.
As companies strive to gain a competitive edge, there is a clear trend towards investing in innovative solubility-enhancing techniques. This includes exploring novel formulation strategies, advanced particle engineering technologies, and cutting-edge analytical methods for characterizing and optimizing the solubility of geometric isomers.
In the pharmaceutical sector, there is a growing need for advanced solubility-enhancing techniques that can effectively handle geometric isomers. This demand is driven by the increasing complexity of drug molecules and the push towards personalized medicine. Improved solubility can lead to better drug absorption, reduced dosage requirements, and potentially fewer side effects, all of which are highly valued by both pharmaceutical companies and patients.
The agrochemical industry also shows significant interest in solubility-enhancing techniques for geometric isomers. Pesticides and herbicides often contain isomeric compounds, and their effectiveness can be greatly influenced by solubility. Improved solubility can lead to more efficient application methods, reduced environmental impact, and potentially lower costs for farmers.
In the food and beverage industry, there is a rising demand for natural flavors and colorants, many of which exist as geometric isomers. Enhancing the solubility of these compounds can lead to better incorporation into products, improved stability, and potentially enhanced sensory experiences for consumers.
The cosmetics and personal care industry is another sector showing increased interest in solubility-enhancing techniques for geometric isomers. Many active ingredients in skincare and haircare products are isomeric compounds, and their efficacy can be significantly improved through enhanced solubility.
Market analysts project that the global market for solubility-enhancing technologies, including those specifically addressing geometric isomers, will continue to grow at a substantial rate. This growth is expected to be driven by ongoing research and development efforts, increasing regulatory pressures for improved drug formulations, and the expanding applications of isomeric compounds across various industries.
As companies strive to gain a competitive edge, there is a clear trend towards investing in innovative solubility-enhancing techniques. This includes exploring novel formulation strategies, advanced particle engineering technologies, and cutting-edge analytical methods for characterizing and optimizing the solubility of geometric isomers.
Current Challenges in Isomer-Specific Solubilization
The field of isomer-specific solubilization faces several significant challenges that hinder the development of effective solubility-enhancing techniques for geometric isomers. One of the primary obstacles is the difficulty in selectively targeting and solubilizing specific isomers within a mixture. Geometric isomers, despite having the same molecular formula, possess different spatial arrangements of atoms, leading to distinct physicochemical properties. This structural diversity complicates the design of universal solubilization strategies.
Another major challenge lies in the limited understanding of the molecular interactions between geometric isomers and solubilizing agents. The subtle differences in molecular geometry can significantly impact how isomers interact with solvents, co-solvents, and other solubility-enhancing additives. This knowledge gap hampers the development of tailored approaches for isomer-specific solubilization.
The lack of robust analytical methods for accurately quantifying and characterizing geometric isomers in complex mixtures poses an additional hurdle. Current techniques often struggle to differentiate between closely related isomers, making it challenging to assess the effectiveness of solubilization strategies and optimize formulations.
Furthermore, the scalability of isomer-specific solubilization techniques presents a significant challenge. Many promising approaches demonstrated at the laboratory scale face difficulties when translated to industrial-scale processes. Issues such as cost-effectiveness, process efficiency, and maintaining isomer selectivity during scale-up need to be addressed.
The environmental impact and regulatory considerations surrounding solubility-enhancing techniques for geometric isomers also present challenges. There is a growing need for green and sustainable solubilization methods that minimize the use of harmful solvents and additives while maintaining efficacy.
Lastly, the dynamic nature of isomerization processes adds complexity to solubilization efforts. Some geometric isomers can interconvert under certain conditions, potentially altering the composition of the mixture during solubilization attempts. Developing strategies that account for these dynamic equilibria and maintain isomer-specific solubilization over time remains a significant challenge in the field.
Another major challenge lies in the limited understanding of the molecular interactions between geometric isomers and solubilizing agents. The subtle differences in molecular geometry can significantly impact how isomers interact with solvents, co-solvents, and other solubility-enhancing additives. This knowledge gap hampers the development of tailored approaches for isomer-specific solubilization.
The lack of robust analytical methods for accurately quantifying and characterizing geometric isomers in complex mixtures poses an additional hurdle. Current techniques often struggle to differentiate between closely related isomers, making it challenging to assess the effectiveness of solubilization strategies and optimize formulations.
Furthermore, the scalability of isomer-specific solubilization techniques presents a significant challenge. Many promising approaches demonstrated at the laboratory scale face difficulties when translated to industrial-scale processes. Issues such as cost-effectiveness, process efficiency, and maintaining isomer selectivity during scale-up need to be addressed.
The environmental impact and regulatory considerations surrounding solubility-enhancing techniques for geometric isomers also present challenges. There is a growing need for green and sustainable solubilization methods that minimize the use of harmful solvents and additives while maintaining efficacy.
Lastly, the dynamic nature of isomerization processes adds complexity to solubilization efforts. Some geometric isomers can interconvert under certain conditions, potentially altering the composition of the mixture during solubilization attempts. Developing strategies that account for these dynamic equilibria and maintain isomer-specific solubilization over time remains a significant challenge in the field.
Existing Solubility Enhancement Methods for Isomers
01 Separation techniques for geometric isomers
Various separation techniques can be employed to isolate geometric isomers based on their solubility differences. These methods may include crystallization, chromatography, or selective extraction, which exploit the distinct physical properties of different isomers to achieve separation.- Separation techniques for geometric isomers: Various separation techniques can be employed to isolate geometric isomers based on their solubility differences. These methods may include crystallization, chromatography, or selective extraction, which exploit the distinct physical properties of different isomers to achieve separation.
- Solvent selection for isomer solubility: The choice of solvent plays a crucial role in determining the solubility of geometric isomers. Different solvents can preferentially dissolve specific isomers, allowing for selective extraction or purification. Factors such as polarity, hydrogen bonding, and molecular size of the solvent influence isomer solubility.
- Temperature effects on isomer solubility: Temperature can significantly impact the solubility of geometric isomers. By manipulating temperature, it is possible to alter the relative solubilities of different isomers, facilitating their separation or purification. This principle can be applied in various processes, including crystallization and liquid-liquid extraction.
- pH-dependent solubility of isomers: The solubility of certain geometric isomers can be influenced by pH. By adjusting the pH of the solution, it is possible to alter the ionization state of the isomers, affecting their solubility and allowing for selective precipitation or extraction. This approach is particularly useful for isomers with ionizable functional groups.
- Complexation to modify isomer solubility: Complexation with specific agents can be used to modify the solubility of geometric isomers. By forming complexes with selective binding agents, the solubility properties of isomers can be altered, enabling their separation or purification. This technique can be particularly effective when traditional separation methods prove challenging.
02 Solvent selection for isomer solubility
The choice of solvent plays a crucial role in determining the solubility of geometric isomers. Different solvents can preferentially dissolve specific isomers, allowing for selective extraction or purification. Factors such as polarity, hydrogen bonding, and molecular size of the solvent influence isomer solubility.Expand Specific Solutions03 Temperature effects on isomer solubility
Temperature variations can significantly impact the solubility of geometric isomers. Some isomers may exhibit increased solubility at higher temperatures, while others may become less soluble. Understanding these temperature-dependent solubility differences can be utilized in separation and purification processes.Expand Specific Solutions04 pH-dependent solubility of geometric isomers
The solubility of certain geometric isomers can be influenced by pH changes in the solution. This property can be exploited for selective precipitation or extraction of specific isomers by adjusting the pH of the medium, allowing for efficient separation and purification.Expand Specific Solutions05 Computational modeling of isomer solubility
Advanced computational techniques can be employed to predict and model the solubility behavior of geometric isomers. These methods may involve molecular dynamics simulations, quantum mechanical calculations, or machine learning approaches to estimate solubility parameters and guide experimental design.Expand Specific Solutions
Key Players in Pharmaceutical Solubility Research
The competitive landscape for "Impact of Geometric Isomers on Solubility Enhancing Techniques" is in a developing stage, with growing market potential as pharmaceutical companies seek innovative solutions for drug delivery. The market size is expanding due to increased demand for improved solubility in drug formulations. Technologically, the field is advancing rapidly, with companies like BASF Corp., Eli Lilly & Co., and Novo Nordisk A/S leading research efforts. These firms are leveraging their expertise in chemical engineering and pharmaceutical development to explore novel approaches to solubility enhancement, focusing on the role of geometric isomers in drug formulations.
BASF Corp.
Technical Solution: BASF Corp. has developed a comprehensive approach to address the impact of geometric isomers on solubility enhancement techniques. Their strategy involves the use of advanced polymer excipients and solubilization technologies tailored to specific isomeric configurations. BASF's Soluplus®, a graft copolymer, has shown particular promise in enhancing the solubility of drugs with geometric isomerism[3]. The company has also explored the use of cyclodextrins and their derivatives to create inclusion complexes that can accommodate different geometric isomers, thereby improving their solubility profiles[4]. Additionally, BASF has invested in computational modeling techniques to predict the behavior of geometric isomers in various solubilization systems, allowing for more targeted formulation development[5].
Strengths: Broad portfolio of solubilization technologies; strong research capabilities in polymer science and computational modeling; global presence for widespread implementation. Weaknesses: Some solutions may be proprietary and costly; potential for drug-excipient interactions that could affect long-term stability.
Eli Lilly & Co.
Technical Solution: Eli Lilly & Co. has focused on developing integrated approaches to address the challenges posed by geometric isomers in solubility enhancement. Their strategy combines advanced formulation techniques with targeted drug design principles. Lilly has explored the use of co-crystal technology to improve the solubility of drugs with geometric isomerism, creating multi-component crystalline systems that can accommodate different isomeric forms[6]. The company has also invested in nanotechnology-based solutions, including lipid nanoparticles and polymeric micelles, which can encapsulate and solubilize geometric isomers more effectively[7]. Furthermore, Lilly has implemented high-throughput screening methods to rapidly assess the impact of various solubilization techniques on different geometric isomers, enabling more efficient formulation development[8].
Strengths: Comprehensive approach combining formulation and drug design; strong expertise in crystallization and nanotechnology; efficient screening capabilities. Weaknesses: Some approaches may be limited to specific drug classes; potential regulatory challenges with novel formulation technologies.
Innovative Approaches to Isomer-Specific Solubilization
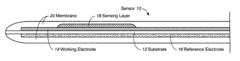
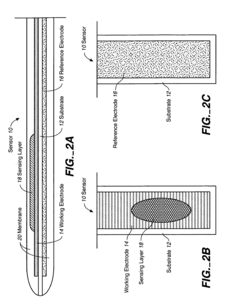
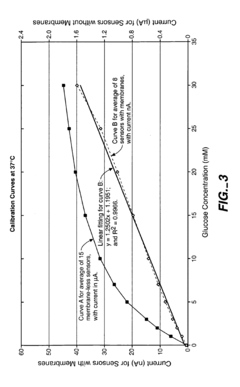
Biosensor Membranes Composed of Polymers Containing Heterocyclic Nitrogens
PatentInactiveUS20170027489A1
Innovation
- Development of crosslinked polymers containing heterocyclic nitrogen groups, such as polyvinylpyridine and polyvinylimidazole, which are formed in situ with a zwitterionic moiety and optional hydrophilic or hydrophobic modifiers, providing enhanced solubility, permeability, and biocompatibility, and are applied as a membrane to limit analyte flux and protect the sensing layer.
Pharmaceutical composition for treatment and prevention of restenosis
PatentWO2008066297A1
Innovation
- A pharmaceutical composition comprising therapeutically effective amounts of compounds represented by Formula 1 and Formula 2, or their pharmaceutically acceptable salts, prodrugs, solvates, or isomers, which activate NQOl to elevate NAD+ levels and subsequently AMPK activity, thereby inhibiting vascular smooth muscle cell proliferation.
Regulatory Considerations for Isomer-Specific Formulations
The regulatory landscape for isomer-specific formulations is complex and evolving, reflecting the growing understanding of the impact of geometric isomers on drug efficacy and safety. Regulatory bodies, such as the FDA and EMA, have established guidelines that address the development, manufacturing, and quality control of isomeric compounds in pharmaceutical products.
One key consideration is the requirement for comprehensive characterization of isomeric mixtures. Manufacturers must provide detailed information on the isomeric composition of their products, including the ratios of different isomers and their individual physicochemical properties. This information is crucial for assessing the potential impact on solubility and bioavailability, which are critical factors in drug formulation and delivery.
Regulatory agencies also emphasize the importance of consistent isomeric composition throughout the product lifecycle. This necessitates robust analytical methods for isomer separation and quantification, as well as stringent quality control measures to ensure batch-to-batch consistency. Manufacturers must demonstrate the ability to maintain a consistent isomeric profile during scale-up and commercial production.
Safety considerations play a significant role in the regulatory approach to isomer-specific formulations. Regulatory bodies require thorough toxicological evaluations of individual isomers and their mixtures, as different geometric isomers may exhibit varying pharmacological and toxicological profiles. This often involves conducting separate safety studies for each isomer and the racemic mixture.
The regulatory framework also addresses the potential for isomeric interconversion during storage or in vivo. Manufacturers must provide stability data demonstrating the maintenance of the intended isomeric composition under various storage conditions and throughout the product's shelf life. Additionally, they may need to conduct studies on potential in vivo isomerization and its impact on drug efficacy and safety.
Labeling requirements for isomer-specific formulations are another crucial regulatory aspect. Product labels must accurately reflect the isomeric composition and any specific handling or storage instructions related to maintaining isomeric integrity. In some cases, regulatory agencies may require separate marketing authorizations for different isomeric forms of the same active substance.
As the field of isomer-specific formulations continues to advance, regulatory bodies are likely to refine their guidelines further. This may include more specific requirements for solubility enhancement techniques that take into account the unique properties of geometric isomers. Manufacturers and researchers must stay abreast of these evolving regulations to ensure compliance and optimize their development strategies for isomer-specific drug products.
One key consideration is the requirement for comprehensive characterization of isomeric mixtures. Manufacturers must provide detailed information on the isomeric composition of their products, including the ratios of different isomers and their individual physicochemical properties. This information is crucial for assessing the potential impact on solubility and bioavailability, which are critical factors in drug formulation and delivery.
Regulatory agencies also emphasize the importance of consistent isomeric composition throughout the product lifecycle. This necessitates robust analytical methods for isomer separation and quantification, as well as stringent quality control measures to ensure batch-to-batch consistency. Manufacturers must demonstrate the ability to maintain a consistent isomeric profile during scale-up and commercial production.
Safety considerations play a significant role in the regulatory approach to isomer-specific formulations. Regulatory bodies require thorough toxicological evaluations of individual isomers and their mixtures, as different geometric isomers may exhibit varying pharmacological and toxicological profiles. This often involves conducting separate safety studies for each isomer and the racemic mixture.
The regulatory framework also addresses the potential for isomeric interconversion during storage or in vivo. Manufacturers must provide stability data demonstrating the maintenance of the intended isomeric composition under various storage conditions and throughout the product's shelf life. Additionally, they may need to conduct studies on potential in vivo isomerization and its impact on drug efficacy and safety.
Labeling requirements for isomer-specific formulations are another crucial regulatory aspect. Product labels must accurately reflect the isomeric composition and any specific handling or storage instructions related to maintaining isomeric integrity. In some cases, regulatory agencies may require separate marketing authorizations for different isomeric forms of the same active substance.
As the field of isomer-specific formulations continues to advance, regulatory bodies are likely to refine their guidelines further. This may include more specific requirements for solubility enhancement techniques that take into account the unique properties of geometric isomers. Manufacturers and researchers must stay abreast of these evolving regulations to ensure compliance and optimize their development strategies for isomer-specific drug products.
Environmental Impact of Solubility Enhancement Processes
The environmental impact of solubility enhancement processes for geometric isomers is a critical consideration in the development and implementation of these techniques. The use of various solvents, co-solvents, and surfactants in solubility enhancement can have significant implications for the environment, particularly in terms of waste generation, energy consumption, and potential ecological risks.
One of the primary environmental concerns associated with solubility enhancement processes is the generation of organic solvent waste. Many techniques rely on the use of organic solvents to improve the solubility of geometric isomers. These solvents, if not properly managed and disposed of, can lead to soil and water contamination. The release of volatile organic compounds (VOCs) from these solvents can also contribute to air pollution and the formation of ground-level ozone.
Energy consumption is another important factor to consider. Some solubility enhancement techniques, such as hot melt extrusion or spray drying, require significant energy inputs. This increased energy demand can result in higher greenhouse gas emissions if the energy source is not renewable. Additionally, the production of specialized excipients or surfactants used in solubility enhancement may have its own energy-intensive manufacturing processes, further contributing to the overall environmental footprint.
The impact on aquatic ecosystems is of particular concern when considering the environmental effects of solubility enhancement processes. Improved solubility of geometric isomers can lead to increased bioavailability of these compounds in water bodies. This may result in unintended exposure to aquatic organisms, potentially causing toxicological effects or disrupting ecosystem balance. The long-term consequences of such exposure are not always well understood and require careful monitoring and assessment.
Waste management is a crucial aspect of mitigating the environmental impact of solubility enhancement processes. Proper handling, treatment, and disposal of waste materials generated during these processes are essential to prevent environmental contamination. This includes not only the direct waste from the enhancement process but also secondary waste streams such as packaging materials and contaminated equipment.
To address these environmental concerns, there is a growing emphasis on developing more sustainable solubility enhancement techniques. Green chemistry principles are being applied to design processes that minimize the use of harmful solvents, reduce energy consumption, and decrease waste generation. For instance, the use of supercritical fluid technology or ionic liquids as alternatives to traditional organic solvents shows promise in reducing environmental impact while maintaining efficacy in solubility enhancement.
One of the primary environmental concerns associated with solubility enhancement processes is the generation of organic solvent waste. Many techniques rely on the use of organic solvents to improve the solubility of geometric isomers. These solvents, if not properly managed and disposed of, can lead to soil and water contamination. The release of volatile organic compounds (VOCs) from these solvents can also contribute to air pollution and the formation of ground-level ozone.
Energy consumption is another important factor to consider. Some solubility enhancement techniques, such as hot melt extrusion or spray drying, require significant energy inputs. This increased energy demand can result in higher greenhouse gas emissions if the energy source is not renewable. Additionally, the production of specialized excipients or surfactants used in solubility enhancement may have its own energy-intensive manufacturing processes, further contributing to the overall environmental footprint.
The impact on aquatic ecosystems is of particular concern when considering the environmental effects of solubility enhancement processes. Improved solubility of geometric isomers can lead to increased bioavailability of these compounds in water bodies. This may result in unintended exposure to aquatic organisms, potentially causing toxicological effects or disrupting ecosystem balance. The long-term consequences of such exposure are not always well understood and require careful monitoring and assessment.
Waste management is a crucial aspect of mitigating the environmental impact of solubility enhancement processes. Proper handling, treatment, and disposal of waste materials generated during these processes are essential to prevent environmental contamination. This includes not only the direct waste from the enhancement process but also secondary waste streams such as packaging materials and contaminated equipment.
To address these environmental concerns, there is a growing emphasis on developing more sustainable solubility enhancement techniques. Green chemistry principles are being applied to design processes that minimize the use of harmful solvents, reduce energy consumption, and decrease waste generation. For instance, the use of supercritical fluid technology or ionic liquids as alternatives to traditional organic solvents shows promise in reducing environmental impact while maintaining efficacy in solubility enhancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!