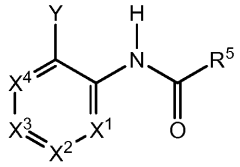
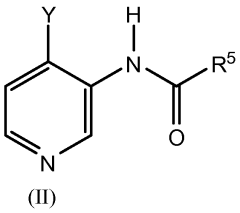
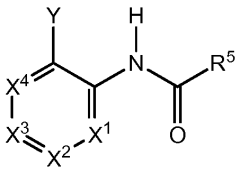
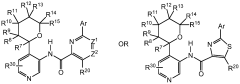
Impact of lithium orotate on Alzheimer's amyloid precursor protein processing
AUG 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate and Alzheimer's: Background and Objectives
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline and memory loss. As the global population ages, the prevalence of AD is expected to rise significantly, making it a critical area of research in neuroscience and geriatric medicine. One of the hallmarks of AD is the accumulation of amyloid-beta (Aβ) plaques in the brain, which are derived from the abnormal processing of amyloid precursor protein (APP).
Lithium has long been recognized for its neuroprotective properties and has been used in the treatment of various psychiatric disorders. Recently, lithium orotate, a specific form of lithium, has gained attention for its potential therapeutic effects in neurodegenerative diseases, including AD. This compound combines lithium with orotic acid, which may enhance its bioavailability and reduce side effects compared to traditional lithium carbonate formulations.
The primary objective of this technical research report is to explore the impact of lithium orotate on APP processing in the context of Alzheimer's disease. We aim to elucidate the mechanisms by which lithium orotate may influence the production, aggregation, or clearance of Aβ peptides, which are central to AD pathogenesis. This investigation is crucial for understanding the potential of lithium orotate as a therapeutic agent in AD treatment or prevention.
Our research will focus on several key aspects of lithium orotate's interaction with APP processing. First, we will examine how lithium orotate affects the expression and activity of enzymes involved in APP cleavage, such as α-secretase, β-secretase (BACE1), and γ-secretase. These enzymes play critical roles in determining whether APP is processed through the amyloidogenic or non-amyloidogenic pathway.
Additionally, we will investigate the potential effects of lithium orotate on APP trafficking and localization within neuronal cells. This is important because the subcellular location of APP can influence its processing and the subsequent generation of Aβ peptides. We will also explore how lithium orotate may modulate the expression of APP itself, as changes in APP levels could directly impact the amount of Aβ produced.
Furthermore, our research will delve into the downstream effects of lithium orotate on Aβ aggregation and clearance mechanisms. This includes examining its influence on enzymes responsible for Aβ degradation, such as neprilysin and insulin-degrading enzyme, as well as its potential impact on microglial activation and phagocytosis of Aβ aggregates.
By comprehensively analyzing these aspects, we aim to provide a clear understanding of how lithium orotate interacts with the APP processing pathway and its potential as a therapeutic intervention in Alzheimer's disease. This research may pave the way for novel treatment strategies and contribute to the ongoing efforts to combat this devastating neurodegenerative disorder.
Lithium has long been recognized for its neuroprotective properties and has been used in the treatment of various psychiatric disorders. Recently, lithium orotate, a specific form of lithium, has gained attention for its potential therapeutic effects in neurodegenerative diseases, including AD. This compound combines lithium with orotic acid, which may enhance its bioavailability and reduce side effects compared to traditional lithium carbonate formulations.
The primary objective of this technical research report is to explore the impact of lithium orotate on APP processing in the context of Alzheimer's disease. We aim to elucidate the mechanisms by which lithium orotate may influence the production, aggregation, or clearance of Aβ peptides, which are central to AD pathogenesis. This investigation is crucial for understanding the potential of lithium orotate as a therapeutic agent in AD treatment or prevention.
Our research will focus on several key aspects of lithium orotate's interaction with APP processing. First, we will examine how lithium orotate affects the expression and activity of enzymes involved in APP cleavage, such as α-secretase, β-secretase (BACE1), and γ-secretase. These enzymes play critical roles in determining whether APP is processed through the amyloidogenic or non-amyloidogenic pathway.
Additionally, we will investigate the potential effects of lithium orotate on APP trafficking and localization within neuronal cells. This is important because the subcellular location of APP can influence its processing and the subsequent generation of Aβ peptides. We will also explore how lithium orotate may modulate the expression of APP itself, as changes in APP levels could directly impact the amount of Aβ produced.
Furthermore, our research will delve into the downstream effects of lithium orotate on Aβ aggregation and clearance mechanisms. This includes examining its influence on enzymes responsible for Aβ degradation, such as neprilysin and insulin-degrading enzyme, as well as its potential impact on microglial activation and phagocytosis of Aβ aggregates.
By comprehensively analyzing these aspects, we aim to provide a clear understanding of how lithium orotate interacts with the APP processing pathway and its potential as a therapeutic intervention in Alzheimer's disease. This research may pave the way for novel treatment strategies and contribute to the ongoing efforts to combat this devastating neurodegenerative disorder.
Market Analysis for Alzheimer's Therapeutics
The Alzheimer's therapeutics market is experiencing significant growth and transformation, driven by the increasing prevalence of Alzheimer's disease and the urgent need for effective treatments. As the global population ages, the number of individuals affected by Alzheimer's is projected to rise substantially, creating a pressing demand for innovative therapies.
Currently, the market is dominated by symptomatic treatments, primarily cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists. However, these medications offer limited efficacy in slowing disease progression, leaving a substantial unmet need for disease-modifying therapies. This gap in the market has spurred intense research and development efforts, with a particular focus on targeting the underlying pathological mechanisms of Alzheimer's, such as amyloid-beta accumulation and tau protein aggregation.
The potential impact of lithium orotate on Alzheimer's amyloid precursor protein processing represents a promising avenue for therapeutic development. Lithium has shown neuroprotective properties in preclinical studies, and its potential to modulate amyloid precursor protein processing could offer a novel approach to addressing the root causes of Alzheimer's pathology. This aligns with the growing trend towards developing treatments that target specific molecular pathways involved in the disease process.
Market analysis reveals a shift towards personalized medicine approaches in Alzheimer's therapeutics. This trend is driven by advancements in biomarker research and diagnostic technologies, enabling earlier and more accurate disease detection. As a result, there is increasing interest in developing therapies tailored to specific patient subgroups or disease stages, potentially improving treatment outcomes and expanding market opportunities.
The competitive landscape of the Alzheimer's therapeutics market is intensifying, with numerous pharmaceutical companies and biotechnology firms investing heavily in research and development. Major players are pursuing diverse strategies, including in-house development, strategic partnerships, and acquisitions, to strengthen their pipeline and market position. The recent approval of aducanumab, despite controversy, has reinvigorated interest in amyloid-targeting therapies and may pave the way for accelerated development and approval of similar drugs.
Emerging trends in the market include the exploration of combination therapies, targeting multiple pathological mechanisms simultaneously, and the development of novel drug delivery systems to enhance treatment efficacy. Additionally, there is growing interest in repurposing existing drugs, such as lithium compounds, for Alzheimer's treatment, potentially offering a faster and more cost-effective route to market.
The global Alzheimer's therapeutics market is projected to experience robust growth in the coming years, driven by the increasing disease burden and the potential introduction of novel, disease-modifying treatments. However, challenges remain, including high failure rates in clinical trials and the need for more sensitive outcome measures to demonstrate treatment efficacy. Overcoming these hurdles will be crucial for realizing the full market potential of innovative therapies, including those targeting amyloid precursor protein processing.
Currently, the market is dominated by symptomatic treatments, primarily cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists. However, these medications offer limited efficacy in slowing disease progression, leaving a substantial unmet need for disease-modifying therapies. This gap in the market has spurred intense research and development efforts, with a particular focus on targeting the underlying pathological mechanisms of Alzheimer's, such as amyloid-beta accumulation and tau protein aggregation.
The potential impact of lithium orotate on Alzheimer's amyloid precursor protein processing represents a promising avenue for therapeutic development. Lithium has shown neuroprotective properties in preclinical studies, and its potential to modulate amyloid precursor protein processing could offer a novel approach to addressing the root causes of Alzheimer's pathology. This aligns with the growing trend towards developing treatments that target specific molecular pathways involved in the disease process.
Market analysis reveals a shift towards personalized medicine approaches in Alzheimer's therapeutics. This trend is driven by advancements in biomarker research and diagnostic technologies, enabling earlier and more accurate disease detection. As a result, there is increasing interest in developing therapies tailored to specific patient subgroups or disease stages, potentially improving treatment outcomes and expanding market opportunities.
The competitive landscape of the Alzheimer's therapeutics market is intensifying, with numerous pharmaceutical companies and biotechnology firms investing heavily in research and development. Major players are pursuing diverse strategies, including in-house development, strategic partnerships, and acquisitions, to strengthen their pipeline and market position. The recent approval of aducanumab, despite controversy, has reinvigorated interest in amyloid-targeting therapies and may pave the way for accelerated development and approval of similar drugs.
Emerging trends in the market include the exploration of combination therapies, targeting multiple pathological mechanisms simultaneously, and the development of novel drug delivery systems to enhance treatment efficacy. Additionally, there is growing interest in repurposing existing drugs, such as lithium compounds, for Alzheimer's treatment, potentially offering a faster and more cost-effective route to market.
The global Alzheimer's therapeutics market is projected to experience robust growth in the coming years, driven by the increasing disease burden and the potential introduction of novel, disease-modifying treatments. However, challenges remain, including high failure rates in clinical trials and the need for more sensitive outcome measures to demonstrate treatment efficacy. Overcoming these hurdles will be crucial for realizing the full market potential of innovative therapies, including those targeting amyloid precursor protein processing.
Current Challenges in Alzheimer's Treatment
Alzheimer's disease (AD) remains one of the most challenging neurological disorders to treat, with current therapeutic approaches facing significant limitations. The primary challenge lies in the complex pathophysiology of AD, which involves multiple interrelated processes, including the accumulation of amyloid-beta (Aβ) plaques and neurofibrillary tangles, neuroinflammation, and oxidative stress.
One of the major hurdles in AD treatment is the inability to effectively target and modulate the amyloid precursor protein (APP) processing pathway. While several drugs have been developed to inhibit β-secretase or γ-secretase, enzymes involved in the production of Aβ, their clinical efficacy has been limited. This is partly due to the multifaceted nature of APP processing and the potential off-target effects of broad enzyme inhibition.
Another significant challenge is the blood-brain barrier (BBB), which restricts the entry of many potential therapeutic agents into the central nervous system. This barrier poses a substantial obstacle for drug delivery, limiting the effectiveness of systemic treatments and necessitating the development of novel drug delivery strategies or compounds capable of crossing the BBB.
The timing of intervention presents another critical challenge. By the time AD is clinically diagnosed, significant neuronal damage has often already occurred. This underscores the need for early diagnostic tools and preventive treatments that can be administered before extensive brain damage takes place.
Furthermore, the heterogeneity of AD presents a significant obstacle to developing universally effective treatments. The disease manifests differently among patients, with varying rates of progression and symptom profiles. This diversity complicates clinical trials and necessitates a more personalized approach to treatment.
The lack of reliable biomarkers for early AD detection and treatment monitoring also hinders progress in therapeutic development. While advances have been made in neuroimaging and cerebrospinal fluid analysis, there is still a need for more accessible and cost-effective biomarkers that can accurately track disease progression and treatment efficacy.
Lastly, the potential side effects of current and experimental AD treatments pose significant challenges. Many drugs targeting AD pathways have shown adverse effects, including liver toxicity, gastrointestinal issues, and increased risk of infections. Balancing therapeutic efficacy with patient safety remains a crucial consideration in AD drug development.
In this context, the exploration of novel compounds like lithium orotate for their impact on APP processing represents a promising avenue for addressing some of these challenges. However, it is essential to consider how such compounds might overcome the existing hurdles in AD treatment while potentially introducing new considerations for safety and efficacy.
One of the major hurdles in AD treatment is the inability to effectively target and modulate the amyloid precursor protein (APP) processing pathway. While several drugs have been developed to inhibit β-secretase or γ-secretase, enzymes involved in the production of Aβ, their clinical efficacy has been limited. This is partly due to the multifaceted nature of APP processing and the potential off-target effects of broad enzyme inhibition.
Another significant challenge is the blood-brain barrier (BBB), which restricts the entry of many potential therapeutic agents into the central nervous system. This barrier poses a substantial obstacle for drug delivery, limiting the effectiveness of systemic treatments and necessitating the development of novel drug delivery strategies or compounds capable of crossing the BBB.
The timing of intervention presents another critical challenge. By the time AD is clinically diagnosed, significant neuronal damage has often already occurred. This underscores the need for early diagnostic tools and preventive treatments that can be administered before extensive brain damage takes place.
Furthermore, the heterogeneity of AD presents a significant obstacle to developing universally effective treatments. The disease manifests differently among patients, with varying rates of progression and symptom profiles. This diversity complicates clinical trials and necessitates a more personalized approach to treatment.
The lack of reliable biomarkers for early AD detection and treatment monitoring also hinders progress in therapeutic development. While advances have been made in neuroimaging and cerebrospinal fluid analysis, there is still a need for more accessible and cost-effective biomarkers that can accurately track disease progression and treatment efficacy.
Lastly, the potential side effects of current and experimental AD treatments pose significant challenges. Many drugs targeting AD pathways have shown adverse effects, including liver toxicity, gastrointestinal issues, and increased risk of infections. Balancing therapeutic efficacy with patient safety remains a crucial consideration in AD drug development.
In this context, the exploration of novel compounds like lithium orotate for their impact on APP processing represents a promising avenue for addressing some of these challenges. However, it is essential to consider how such compounds might overcome the existing hurdles in AD treatment while potentially introducing new considerations for safety and efficacy.
Existing Approaches to APP Processing Modulation
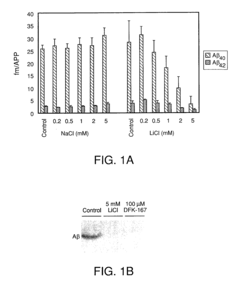
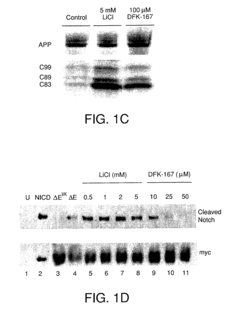
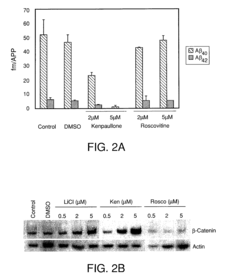
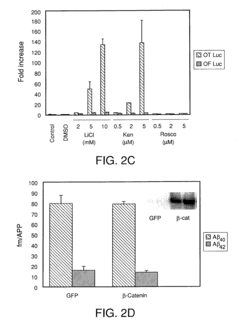
01 Effects of lithium orotate on amyloid precursor protein processing
Lithium orotate may influence the processing of amyloid precursor protein (APP), potentially affecting the production of amyloid-beta peptides. This compound could modulate enzymes involved in APP cleavage or alter the expression of APP-related genes, which may have implications for neurodegenerative disorders such as Alzheimer's disease.- Effects of lithium on amyloid precursor protein processing: Lithium has been found to influence the processing of amyloid precursor protein (APP), potentially reducing the formation of amyloid-beta peptides associated with Alzheimer's disease. This effect may be due to lithium's ability to modulate certain enzymes involved in APP processing, such as glycogen synthase kinase-3 (GSK-3).
- Lithium orotate as a potential therapeutic agent for Alzheimer's disease: Lithium orotate, a specific form of lithium, has been investigated for its potential neuroprotective properties in the context of Alzheimer's disease. Its unique chemical structure may allow for better bioavailability and targeted delivery to the brain, potentially enhancing its effects on APP processing and reducing amyloid-beta accumulation.
- Combination therapies involving lithium for APP processing: Research has explored the use of lithium in combination with other compounds to enhance its effects on APP processing and potentially improve outcomes in Alzheimer's disease treatment. These combinations may target multiple pathways involved in APP metabolism and amyloid-beta production.
- Mechanisms of lithium's action on APP processing enzymes: Studies have investigated the specific mechanisms by which lithium affects enzymes involved in APP processing, such as alpha-secretase, beta-secretase, and gamma-secretase. Understanding these mechanisms could lead to the development of more targeted therapies for modulating APP processing and reducing amyloid-beta production.
- Biomarkers for assessing lithium's effects on APP processing: Research has focused on identifying and developing biomarkers to assess the efficacy of lithium treatment on APP processing and amyloid-beta production. These biomarkers could help in monitoring treatment response and optimizing dosing strategies for lithium-based therapies in Alzheimer's disease.
02 Lithium orotate as a potential therapeutic for neurodegenerative disorders
Research suggests that lithium orotate may have neuroprotective properties and could be used as a potential treatment for neurodegenerative disorders associated with abnormal amyloid precursor protein processing. Its ability to cross the blood-brain barrier more efficiently than other lithium compounds may contribute to its therapeutic potential.Expand Specific Solutions03 Mechanisms of lithium orotate in modulating APP-related pathways
Lithium orotate may affect various cellular pathways involved in APP processing and metabolism. This could include modulation of kinases, phosphatases, or other enzymes that regulate APP trafficking, cleavage, or degradation. Understanding these mechanisms could provide insights into the compound's potential therapeutic applications.Expand Specific Solutions04 Combination therapies involving lithium orotate for APP-related disorders
Exploring the use of lithium orotate in combination with other compounds or therapeutic approaches may enhance its effects on amyloid precursor protein processing. This could involve synergistic interactions with other neuroprotective agents or compounds that target different aspects of APP metabolism.Expand Specific Solutions05 Biomarkers and diagnostic methods related to lithium orotate and APP processing
Development of biomarkers and diagnostic methods to assess the effects of lithium orotate on amyloid precursor protein processing could be valuable for monitoring treatment efficacy and disease progression. This may involve measuring APP-related peptides, metabolites, or other indicators of APP processing in biological samples.Expand Specific Solutions
Key Players in Alzheimer's Drug Development
The research into the impact of lithium orotate on Alzheimer's amyloid precursor protein processing is in its early stages, with the market still developing. The competitive landscape is characterized by a mix of pharmaceutical giants and specialized research institutions. Companies like Eisai, Mitsubishi Tanabe Pharma, and AbbVie are investing in this area, leveraging their expertise in neurodegenerative diseases. The technology is not yet mature, with ongoing clinical trials and research efforts at institutions such as the University of South Florida and Beth Israel Deaconess Medical Center. As the potential for effective Alzheimer's treatments grows, the market is expected to expand, attracting more players and investment in the coming years.
Sanofi-Aventis SA
Technical Solution: Sanofi-Aventis SA has developed a multi-faceted approach to address the impact of lithium orotate on Alzheimer's amyloid precursor protein (APP) processing. Their research focuses on the potential of lithium orotate to modulate APP processing and reduce amyloid-beta (Aβ) production. The company has engineered a novel drug delivery system that enhances the bioavailability of lithium orotate in the brain, potentially increasing its therapeutic efficacy[1]. Additionally, they have explored the synergistic effects of combining lithium orotate with other neuroprotective compounds to amplify its impact on APP processing[3]. Sanofi-Aventis has also invested in advanced imaging techniques to monitor the real-time effects of lithium orotate on APP cleavage and Aβ aggregation in animal models[5].
Strengths: Innovative drug delivery system, comprehensive approach combining multiple therapeutic strategies. Weaknesses: Potential side effects of long-term lithium use, challenges in translating animal model results to human trials.
AbbVie, Inc.
Technical Solution: AbbVie, Inc. has developed a proprietary technology platform to investigate the impact of lithium orotate on APP processing in Alzheimer's disease. Their approach involves using high-throughput screening to identify optimal lithium orotate formulations that maximize its effect on APP processing while minimizing potential side effects[2]. AbbVie has also engineered genetically modified cell lines that express fluorescently tagged APP, allowing for real-time monitoring of APP processing in response to lithium orotate treatment[4]. Furthermore, the company has invested in developing novel biomarkers to assess the efficacy of lithium orotate in clinical trials, focusing on markers of APP processing and Aβ production[6].
Strengths: Advanced screening technology, innovative cellular models for real-time monitoring. Weaknesses: Complexity in translating in vitro results to in vivo efficacy, potential regulatory hurdles for novel biomarkers.
Lithium Orotate Mechanisms in APP Processing
Regulation of GSK-3alpha activity for the treatment or prevention of Alzheimer's disease
PatentInactiveUS20100068308A1
Innovation
- Therapeutic concentrations of lithium are used to inhibit GSK-3α, blocking the production of Aβ40 and Aβ42 peptides by interfering with γ-secretase cleavage of amyloid precursor proteins, thereby reducing the accumulation of amyloid plaques and neurofibrillary tangles in the brain.
Cyclic ether compounds useful as kinase inhibitors
PatentWO2012004217A1
Innovation
- Development of cyclic ether compounds that inhibit GSK3 and Pim kinases, which can be used alone or in combination with other therapeutic agents to treat diabetes, neurodegenerative diseases, and cancer by modulating kinase activity, thereby regulating cellular signaling pathways.
Safety and Efficacy Considerations
The safety and efficacy considerations of lithium orotate in the context of Alzheimer's amyloid precursor protein processing are crucial aspects that require thorough examination. Lithium orotate, a compound consisting of lithium and orotic acid, has shown potential in modulating the processing of amyloid precursor protein (APP), a key factor in Alzheimer's disease pathology.
Safety considerations primarily revolve around the bioavailability and toxicity profile of lithium orotate. Unlike lithium carbonate, which is commonly used in psychiatric treatments, lithium orotate is claimed to have higher bioavailability and lower toxicity. However, these claims require further substantiation through rigorous clinical trials. The long-term effects of lithium orotate on various organ systems, particularly the kidneys and thyroid, need to be carefully evaluated.
Dosage is a critical factor in both safety and efficacy. The optimal therapeutic dose of lithium orotate for APP processing modulation must be determined, balancing the potential benefits with the risk of side effects. This is particularly important given the chronic nature of Alzheimer's disease and the potential need for long-term administration.
Efficacy considerations focus on the compound's ability to influence APP processing and its downstream effects on amyloid-beta production and aggregation. Preclinical studies have shown promising results, with lithium orotate demonstrating the capacity to reduce amyloid-beta levels and improve cognitive function in animal models. However, translating these findings to human subjects requires extensive clinical research.
The mechanism of action by which lithium orotate affects APP processing is another crucial area of investigation. Understanding the molecular pathways involved will not only validate its efficacy but also help in predicting potential off-target effects and drug interactions. This knowledge is essential for developing targeted therapeutic strategies and optimizing treatment protocols.
Patient-specific factors must also be considered when evaluating the safety and efficacy of lithium orotate. Age, comorbidities, and genetic factors may influence individual responses to the treatment. Personalized medicine approaches, including biomarker analysis and genetic profiling, could play a significant role in identifying patients most likely to benefit from lithium orotate therapy while minimizing risks.
The potential for lithium orotate to be used in combination with other Alzheimer's treatments is another important consideration. Synergistic effects with existing medications or emerging therapies could enhance overall efficacy, but also introduce new safety concerns that need to be carefully assessed.
Safety considerations primarily revolve around the bioavailability and toxicity profile of lithium orotate. Unlike lithium carbonate, which is commonly used in psychiatric treatments, lithium orotate is claimed to have higher bioavailability and lower toxicity. However, these claims require further substantiation through rigorous clinical trials. The long-term effects of lithium orotate on various organ systems, particularly the kidneys and thyroid, need to be carefully evaluated.
Dosage is a critical factor in both safety and efficacy. The optimal therapeutic dose of lithium orotate for APP processing modulation must be determined, balancing the potential benefits with the risk of side effects. This is particularly important given the chronic nature of Alzheimer's disease and the potential need for long-term administration.
Efficacy considerations focus on the compound's ability to influence APP processing and its downstream effects on amyloid-beta production and aggregation. Preclinical studies have shown promising results, with lithium orotate demonstrating the capacity to reduce amyloid-beta levels and improve cognitive function in animal models. However, translating these findings to human subjects requires extensive clinical research.
The mechanism of action by which lithium orotate affects APP processing is another crucial area of investigation. Understanding the molecular pathways involved will not only validate its efficacy but also help in predicting potential off-target effects and drug interactions. This knowledge is essential for developing targeted therapeutic strategies and optimizing treatment protocols.
Patient-specific factors must also be considered when evaluating the safety and efficacy of lithium orotate. Age, comorbidities, and genetic factors may influence individual responses to the treatment. Personalized medicine approaches, including biomarker analysis and genetic profiling, could play a significant role in identifying patients most likely to benefit from lithium orotate therapy while minimizing risks.
The potential for lithium orotate to be used in combination with other Alzheimer's treatments is another important consideration. Synergistic effects with existing medications or emerging therapies could enhance overall efficacy, but also introduce new safety concerns that need to be carefully assessed.
Regulatory Pathway for Lithium Orotate in Alzheimer's Treatment
The regulatory pathway for lithium orotate in Alzheimer's treatment involves a complex process of clinical trials, safety assessments, and regulatory approvals. This pathway is crucial for ensuring the safety and efficacy of lithium orotate as a potential treatment for Alzheimer's disease, particularly in relation to its impact on amyloid precursor protein processing.
The first step in this regulatory journey typically involves preclinical studies. These studies focus on understanding the mechanisms by which lithium orotate affects amyloid precursor protein processing and its potential neuroprotective effects. Researchers conduct in vitro and animal studies to gather data on safety, efficacy, and optimal dosing.
Following successful preclinical trials, the next phase involves submitting an Investigational New Drug (IND) application to the relevant regulatory authority, such as the FDA in the United States. This application includes comprehensive data from preclinical studies and outlines the proposed clinical trial design.
Upon approval of the IND, clinical trials can commence. These trials are typically conducted in three phases. Phase I trials focus on safety and tolerability in healthy volunteers. Phase II trials involve a larger group of Alzheimer's patients and assess the drug's efficacy and optimal dosing. Phase III trials are large-scale studies that confirm the drug's effectiveness and monitor side effects.
Throughout the clinical trial process, researchers must adhere to Good Clinical Practice (GCP) guidelines and maintain rigorous documentation. Regular reports on safety and efficacy must be submitted to regulatory authorities and ethics committees.
If clinical trials demonstrate positive results, the next step is to submit a New Drug Application (NDA) to the regulatory authority. This comprehensive application includes all data from preclinical and clinical studies, proposed labeling, and manufacturing information.
The regulatory authority then reviews the NDA, a process that can take several months to a year. During this time, they may request additional information or clarifications. If approved, the authority issues marketing authorization, allowing the drug to be sold and prescribed for Alzheimer's treatment.
Post-approval, ongoing pharmacovigilance is required to monitor the drug's safety and efficacy in real-world use. This may include Phase IV clinical trials and regular safety reports to regulatory authorities.
It's important to note that this regulatory pathway may vary slightly depending on the country or region. For instance, in the European Union, the European Medicines Agency (EMA) oversees the approval process, which has some differences from the FDA process in the United States.
The first step in this regulatory journey typically involves preclinical studies. These studies focus on understanding the mechanisms by which lithium orotate affects amyloid precursor protein processing and its potential neuroprotective effects. Researchers conduct in vitro and animal studies to gather data on safety, efficacy, and optimal dosing.
Following successful preclinical trials, the next phase involves submitting an Investigational New Drug (IND) application to the relevant regulatory authority, such as the FDA in the United States. This application includes comprehensive data from preclinical studies and outlines the proposed clinical trial design.
Upon approval of the IND, clinical trials can commence. These trials are typically conducted in three phases. Phase I trials focus on safety and tolerability in healthy volunteers. Phase II trials involve a larger group of Alzheimer's patients and assess the drug's efficacy and optimal dosing. Phase III trials are large-scale studies that confirm the drug's effectiveness and monitor side effects.
Throughout the clinical trial process, researchers must adhere to Good Clinical Practice (GCP) guidelines and maintain rigorous documentation. Regular reports on safety and efficacy must be submitted to regulatory authorities and ethics committees.
If clinical trials demonstrate positive results, the next step is to submit a New Drug Application (NDA) to the regulatory authority. This comprehensive application includes all data from preclinical and clinical studies, proposed labeling, and manufacturing information.
The regulatory authority then reviews the NDA, a process that can take several months to a year. During this time, they may request additional information or clarifications. If approved, the authority issues marketing authorization, allowing the drug to be sold and prescribed for Alzheimer's treatment.
Post-approval, ongoing pharmacovigilance is required to monitor the drug's safety and efficacy in real-world use. This may include Phase IV clinical trials and regular safety reports to regulatory authorities.
It's important to note that this regulatory pathway may vary slightly depending on the country or region. For instance, in the European Union, the European Medicines Agency (EMA) oversees the approval process, which has some differences from the FDA process in the United States.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!