Influence of Geometric Isomers on the Shelf-Life of Pharmaceuticals
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers in Pharmaceuticals: Background and Objectives
Geometric isomers, a subset of stereoisomers, have been a subject of significant interest in pharmaceutical research and development for decades. These compounds possess identical molecular formulas and bonding sequences but differ in the spatial arrangement of their atoms. The impact of geometric isomerism on drug efficacy, safety, and stability has become increasingly apparent, leading to a growing focus on understanding and controlling these molecular configurations in pharmaceutical products.
The evolution of geometric isomer research in pharmaceuticals can be traced back to the mid-20th century when scientists began to recognize the importance of molecular structure in drug action. As analytical techniques advanced, researchers gained the ability to identify and characterize geometric isomers with greater precision. This progress has led to a deeper understanding of how subtle differences in molecular geometry can profoundly affect a drug's pharmacological properties and shelf-life.
In recent years, the pharmaceutical industry has witnessed a surge in the development of drugs containing geometric isomers. This trend is driven by the potential for improved therapeutic outcomes and the ability to patent new molecular entities based on isomeric differences. However, the presence of geometric isomers in drug formulations introduces complexities in manufacturing, quality control, and stability assessment that must be carefully addressed.
The primary objective of studying geometric isomers in pharmaceuticals is to optimize drug performance and ensure product safety throughout its shelf-life. This involves investigating how different isomeric forms influence a drug's solubility, bioavailability, metabolism, and chemical stability. By understanding these factors, researchers aim to develop formulations that maintain their therapeutic efficacy over time and under various storage conditions.
Another critical goal is to establish robust analytical methods for detecting and quantifying geometric isomers in pharmaceutical products. This is essential for quality control and regulatory compliance, as regulatory agencies increasingly require detailed information on the isomeric composition of drug substances and products. The development of sensitive and specific analytical techniques is crucial for monitoring isomeric purity during manufacturing and throughout the product's shelf-life.
Furthermore, researchers are exploring strategies to control and manipulate geometric isomerism in drug molecules. This includes investigating methods to selectively synthesize desired isomers, prevent isomerization during storage, and stabilize preferred isomeric forms. Such efforts are aimed at enhancing drug stability, reducing variability in therapeutic outcomes, and potentially extending product shelf-life.
As the field progresses, there is a growing emphasis on predicting the behavior of geometric isomers in pharmaceutical formulations. This involves developing computational models and structure-activity relationship studies to forecast how isomeric differences might impact a drug's long-term stability and efficacy. These predictive tools are becoming increasingly valuable in early-stage drug development, helping to guide formulation strategies and stability testing protocols.
The evolution of geometric isomer research in pharmaceuticals can be traced back to the mid-20th century when scientists began to recognize the importance of molecular structure in drug action. As analytical techniques advanced, researchers gained the ability to identify and characterize geometric isomers with greater precision. This progress has led to a deeper understanding of how subtle differences in molecular geometry can profoundly affect a drug's pharmacological properties and shelf-life.
In recent years, the pharmaceutical industry has witnessed a surge in the development of drugs containing geometric isomers. This trend is driven by the potential for improved therapeutic outcomes and the ability to patent new molecular entities based on isomeric differences. However, the presence of geometric isomers in drug formulations introduces complexities in manufacturing, quality control, and stability assessment that must be carefully addressed.
The primary objective of studying geometric isomers in pharmaceuticals is to optimize drug performance and ensure product safety throughout its shelf-life. This involves investigating how different isomeric forms influence a drug's solubility, bioavailability, metabolism, and chemical stability. By understanding these factors, researchers aim to develop formulations that maintain their therapeutic efficacy over time and under various storage conditions.
Another critical goal is to establish robust analytical methods for detecting and quantifying geometric isomers in pharmaceutical products. This is essential for quality control and regulatory compliance, as regulatory agencies increasingly require detailed information on the isomeric composition of drug substances and products. The development of sensitive and specific analytical techniques is crucial for monitoring isomeric purity during manufacturing and throughout the product's shelf-life.
Furthermore, researchers are exploring strategies to control and manipulate geometric isomerism in drug molecules. This includes investigating methods to selectively synthesize desired isomers, prevent isomerization during storage, and stabilize preferred isomeric forms. Such efforts are aimed at enhancing drug stability, reducing variability in therapeutic outcomes, and potentially extending product shelf-life.
As the field progresses, there is a growing emphasis on predicting the behavior of geometric isomers in pharmaceutical formulations. This involves developing computational models and structure-activity relationship studies to forecast how isomeric differences might impact a drug's long-term stability and efficacy. These predictive tools are becoming increasingly valuable in early-stage drug development, helping to guide formulation strategies and stability testing protocols.
Market Analysis of Isomer-Sensitive Drug Products
The market for isomer-sensitive drug products has experienced significant growth in recent years, driven by the increasing awareness of the impact of geometric isomers on pharmaceutical efficacy and stability. This segment of the pharmaceutical industry is characterized by high-value, specialized products that require advanced manufacturing processes and stringent quality control measures.
The global market for isomer-sensitive drugs is estimated to be substantial, with a particularly strong presence in developed economies such as North America, Europe, and Japan. These regions have well-established regulatory frameworks that recognize the importance of isomeric purity in drug formulations, thereby driving demand for isomer-specific products.
Key therapeutic areas where isomer-sensitive drugs play a crucial role include cardiovascular diseases, central nervous system disorders, and oncology. In these fields, the specific geometric configuration of drug molecules can significantly influence their pharmacological properties, making isomer control a critical factor in drug development and production.
The market is characterized by a high degree of competition among pharmaceutical companies, with a focus on developing novel isomer-specific formulations and improving manufacturing processes to enhance isomeric purity. This has led to increased investment in research and development, as well as in advanced analytical technologies for isomer detection and separation.
A notable trend in the market is the growing emphasis on chiral switching, where pharmaceutical companies develop single-isomer versions of existing racemic drugs. This strategy aims to improve drug efficacy, reduce side effects, and extend patent protection, thereby creating new market opportunities for isomer-sensitive products.
The regulatory landscape plays a significant role in shaping the market for isomer-sensitive drug products. Regulatory agencies worldwide have implemented guidelines for the development and approval of chiral drugs, emphasizing the need for comprehensive isomeric characterization and control throughout the product lifecycle.
Looking ahead, the market for isomer-sensitive drug products is expected to continue its growth trajectory. Factors driving this growth include advancements in stereochemistry, increasing prevalence of chronic diseases requiring targeted therapies, and the pharmaceutical industry's ongoing pursuit of improved drug safety and efficacy profiles.
However, challenges such as complex manufacturing processes, higher production costs, and the need for specialized analytical techniques may impact market dynamics. These factors could influence pricing strategies and market access for isomer-sensitive drug products, particularly in emerging markets where cost considerations are paramount.
The global market for isomer-sensitive drugs is estimated to be substantial, with a particularly strong presence in developed economies such as North America, Europe, and Japan. These regions have well-established regulatory frameworks that recognize the importance of isomeric purity in drug formulations, thereby driving demand for isomer-specific products.
Key therapeutic areas where isomer-sensitive drugs play a crucial role include cardiovascular diseases, central nervous system disorders, and oncology. In these fields, the specific geometric configuration of drug molecules can significantly influence their pharmacological properties, making isomer control a critical factor in drug development and production.
The market is characterized by a high degree of competition among pharmaceutical companies, with a focus on developing novel isomer-specific formulations and improving manufacturing processes to enhance isomeric purity. This has led to increased investment in research and development, as well as in advanced analytical technologies for isomer detection and separation.
A notable trend in the market is the growing emphasis on chiral switching, where pharmaceutical companies develop single-isomer versions of existing racemic drugs. This strategy aims to improve drug efficacy, reduce side effects, and extend patent protection, thereby creating new market opportunities for isomer-sensitive products.
The regulatory landscape plays a significant role in shaping the market for isomer-sensitive drug products. Regulatory agencies worldwide have implemented guidelines for the development and approval of chiral drugs, emphasizing the need for comprehensive isomeric characterization and control throughout the product lifecycle.
Looking ahead, the market for isomer-sensitive drug products is expected to continue its growth trajectory. Factors driving this growth include advancements in stereochemistry, increasing prevalence of chronic diseases requiring targeted therapies, and the pharmaceutical industry's ongoing pursuit of improved drug safety and efficacy profiles.
However, challenges such as complex manufacturing processes, higher production costs, and the need for specialized analytical techniques may impact market dynamics. These factors could influence pricing strategies and market access for isomer-sensitive drug products, particularly in emerging markets where cost considerations are paramount.
Current Challenges in Isomer Stability and Shelf-Life
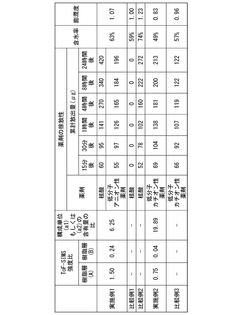
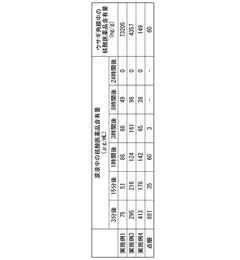
The stability and shelf-life of pharmaceutical products containing geometric isomers present significant challenges in drug development and manufacturing. One of the primary issues is the potential for interconversion between isomers during storage, which can lead to changes in the drug's efficacy and safety profile. This isomerization process is often influenced by environmental factors such as temperature, humidity, and light exposure, making it difficult to maintain consistent product quality over time.
Another major challenge is the differential stability of individual isomers within a mixture. In many cases, one isomer may be more prone to degradation than its counterpart, leading to changes in the isomeric ratio over time. This can be particularly problematic for drugs where the therapeutic effect is primarily attributed to one specific isomer, as the degradation of the active isomer can result in reduced potency and efficacy of the pharmaceutical product.
The presence of multiple isomers also complicates analytical procedures used for quality control and stability testing. Developing robust and sensitive methods to accurately quantify individual isomers and detect subtle changes in their ratios over time is crucial but often technically challenging. This is especially true for complex pharmaceutical formulations where matrix effects can interfere with isomer separation and quantification.
Furthermore, the impact of excipients and formulation components on isomer stability is a significant concern. Certain excipients may catalyze isomerization or preferentially stabilize one isomer over another, leading to unexpected changes in the product's isomeric composition during storage. Understanding and controlling these interactions is essential for developing stable formulations but requires extensive research and testing.
The regulatory landscape surrounding isomeric drugs adds another layer of complexity to shelf-life considerations. Regulatory agencies often require detailed stability data for individual isomers and their mixtures, as well as evidence of consistent isomeric ratios throughout the product's shelf-life. Meeting these requirements can be challenging, particularly for drugs with multiple chiral centers or those prone to racemization.
Packaging and storage conditions play a critical role in maintaining isomer stability, but finding optimal solutions can be difficult. Some isomers may require specialized packaging materials or storage conditions to prevent degradation or interconversion, which can increase manufacturing costs and limit distribution options. Balancing stability requirements with practical considerations for product handling and patient use remains an ongoing challenge in pharmaceutical development.
Another major challenge is the differential stability of individual isomers within a mixture. In many cases, one isomer may be more prone to degradation than its counterpart, leading to changes in the isomeric ratio over time. This can be particularly problematic for drugs where the therapeutic effect is primarily attributed to one specific isomer, as the degradation of the active isomer can result in reduced potency and efficacy of the pharmaceutical product.
The presence of multiple isomers also complicates analytical procedures used for quality control and stability testing. Developing robust and sensitive methods to accurately quantify individual isomers and detect subtle changes in their ratios over time is crucial but often technically challenging. This is especially true for complex pharmaceutical formulations where matrix effects can interfere with isomer separation and quantification.
Furthermore, the impact of excipients and formulation components on isomer stability is a significant concern. Certain excipients may catalyze isomerization or preferentially stabilize one isomer over another, leading to unexpected changes in the product's isomeric composition during storage. Understanding and controlling these interactions is essential for developing stable formulations but requires extensive research and testing.
The regulatory landscape surrounding isomeric drugs adds another layer of complexity to shelf-life considerations. Regulatory agencies often require detailed stability data for individual isomers and their mixtures, as well as evidence of consistent isomeric ratios throughout the product's shelf-life. Meeting these requirements can be challenging, particularly for drugs with multiple chiral centers or those prone to racemization.
Packaging and storage conditions play a critical role in maintaining isomer stability, but finding optimal solutions can be difficult. Some isomers may require specialized packaging materials or storage conditions to prevent degradation or interconversion, which can increase manufacturing costs and limit distribution options. Balancing stability requirements with practical considerations for product handling and patient use remains an ongoing challenge in pharmaceutical development.
Existing Strategies for Isomer Stability Enhancement
01 Stabilization techniques for geometric isomers
Various stabilization techniques can be employed to extend the shelf-life of geometric isomers. These may include the use of antioxidants, pH adjustments, or specific packaging materials that protect the isomers from light and oxygen exposure. Such techniques help maintain the structural integrity and efficacy of the geometric isomers over time.- Stabilization techniques for geometric isomers: Various stabilization techniques can be employed to extend the shelf-life of geometric isomers. These may include the use of antioxidants, protective coatings, or specific storage conditions to prevent isomerization or degradation. Such techniques help maintain the desired isomeric form and its properties over time.
- Packaging solutions for preserving geometric isomers: Specialized packaging solutions can significantly impact the shelf-life of geometric isomers. This may involve using light-resistant containers, oxygen-barrier materials, or modified atmosphere packaging to protect the isomers from environmental factors that could induce isomerization or degradation.
- Temperature control for geometric isomer stability: Maintaining appropriate temperature conditions during storage and transportation is crucial for preserving the shelf-life of geometric isomers. Specific temperature ranges may be required to prevent thermal-induced isomerization or decomposition, which can be achieved through controlled storage facilities or specialized shipping methods.
- Formulation strategies to enhance geometric isomer stability: Developing specific formulation strategies can help improve the shelf-life of geometric isomers. This may include the use of compatible excipients, pH adjustments, or the incorporation of stabilizing agents that interact favorably with the isomers to maintain their structural integrity over time.
- Analytical methods for monitoring geometric isomer stability: Implementing robust analytical methods is essential for monitoring the stability and shelf-life of geometric isomers. These may include chromatographic techniques, spectroscopic analyses, or other advanced methods that can accurately detect and quantify isomeric changes over time, allowing for better prediction and control of shelf-life.
02 Formulation strategies for improved shelf-life
Developing specific formulation strategies can significantly enhance the shelf-life of products containing geometric isomers. This may involve selecting compatible excipients, using microencapsulation techniques, or creating controlled-release systems that protect the isomers from degradation and maintain their stability over extended periods.Expand Specific Solutions03 Storage conditions and packaging innovations
Optimizing storage conditions and implementing innovative packaging solutions play a crucial role in extending the shelf-life of geometric isomers. This includes controlling temperature, humidity, and light exposure during storage and transportation. Advanced packaging materials and designs that minimize oxidation and degradation can significantly prolong the stability of these compounds.Expand Specific Solutions04 Analytical methods for shelf-life determination
Developing and utilizing advanced analytical methods is essential for accurately determining and predicting the shelf-life of geometric isomers. These methods may include chromatographic techniques, spectroscopic analyses, and stability-indicating assays that can detect and quantify any degradation products or changes in isomeric ratios over time.Expand Specific Solutions05 Regulatory considerations for shelf-life extension
Addressing regulatory requirements and guidelines is crucial when developing strategies to extend the shelf-life of products containing geometric isomers. This involves conducting thorough stability studies, establishing appropriate expiration dates, and ensuring compliance with good manufacturing practices (GMP) and quality control standards throughout the product lifecycle.Expand Specific Solutions
Key Pharmaceutical Companies and Research Institutions
The competitive landscape for research on geometric isomers' influence on pharmaceutical shelf-life is in a mature stage, with significant market potential due to the pharmaceutical industry's continuous growth. The technology's maturity is evident from the involvement of established players like Takeda Pharmaceutical, Merck Sharp & Dohme, and Bristol Myers Squibb. These companies, along with research institutions such as Vanderbilt University and École Polytechnique Fédérale de Lausanne, are driving innovation in this field. Smaller, specialized firms like Intra-Cellular Therapies and Verona Pharma are also contributing to advancements, indicating a diverse and competitive research landscape. The market size is substantial, given the critical importance of shelf-life in drug development and the global reach of major pharmaceutical companies involved.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme Corp. has developed a comprehensive approach to address the influence of geometric isomers on pharmaceutical shelf-life. Their strategy involves advanced stereochemical control during synthesis, utilizing chiral catalysts and asymmetric synthesis techniques to produce pure isomers[1]. They have implemented high-resolution analytical methods, including chiral HPLC and circular dichroism spectroscopy, to accurately quantify and monitor isomeric ratios throughout the product lifecycle[3]. Additionally, Merck has pioneered the use of computational modeling to predict isomerization rates and stability profiles under various storage conditions, enabling more accurate shelf-life predictions[5].
Strengths: Advanced stereochemical control, cutting-edge analytical techniques, and predictive modeling capabilities. Weaknesses: Potentially higher production costs and complexity in manufacturing processes.
Genentech, Inc.
Technical Solution: Genentech, Inc. has focused on a biotechnology-driven approach to address geometric isomer influences on pharmaceutical shelf-life. They have developed recombinant protein engineering techniques to produce biologics with enhanced conformational stability, reducing the risk of isomerization[7]. Genentech utilizes advanced biophysical characterization methods, including hydrogen-deuterium exchange mass spectrometry, to map isomer-prone regions in protein therapeutics[9]. The company has also pioneered the use of computational protein design algorithms to optimize amino acid sequences for improved isomeric stability[11]. Additionally, Genentech has implemented novel lyophilization processes tailored to preserve the desired isomeric state of complex biologics during long-term storage.
Strengths: Expertise in protein engineering, advanced biophysical characterization, and computational design. Weaknesses: Primarily focused on biologic drugs, potentially limited applicability to small molecule pharmaceuticals.
Breakthrough Technologies in Isomer Characterization
Process for isomerizing one of the geometric isomers of an " ," -unsaturated aldehyde to its corresponding other geometric isomer
PatentInactiveUS4145366A
Innovation
- Isomerization of one geometric isomer of α,β-unsaturated aldehyde to the corresponding other geometric isomer at a temperature of 30°C to 400°C in the presence of an acid with a pKa of 1 to 7, allowing for high selectivity and separation of isomers.
Ophthalmic medical instrument and manufacturing method therefor
PatentWO2024203883A1
Innovation
- An ophthalmic medical device comprising two resin layers (A and B) with specific structural units, where layer A has a cationic group and layer B contains a hydroxyl group, with controlled ratios of these units to enhance sustained drug release and shape stability, and a method involving photopolymerization to form these layers.
Regulatory Framework for Isomeric Pharmaceutical Products
The regulatory framework for isomeric pharmaceutical products is a complex and evolving landscape that significantly impacts the development, approval, and marketing of drugs containing geometric isomers. Regulatory agencies worldwide, including the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and Japan's Pharmaceuticals and Medical Devices Agency (PMDA), have established specific guidelines to address the unique challenges posed by isomeric compounds.
These regulatory bodies require pharmaceutical companies to thoroughly characterize and control the isomeric composition of their products throughout the drug development process. This includes providing detailed information on the stereochemistry of the active pharmaceutical ingredient (API), the rationale for selecting a specific isomer or mixture of isomers, and the potential impact of isomeric interconversion on drug safety and efficacy.
In the United States, the FDA's guidance on stereoisomeric drugs emphasizes the importance of developing single-isomer products when possible, as this can lead to improved therapeutic outcomes and reduced side effects. However, the agency also recognizes that in some cases, a racemic mixture may be more appropriate or necessary. Manufacturers must provide robust scientific justification for their chosen approach and demonstrate consistent control of the isomeric composition during production.
The EMA has similar requirements but places additional emphasis on the environmental impact of chiral drugs. Their guidelines mandate that pharmaceutical companies assess the potential ecological effects of individual isomers and their metabolites, reflecting a growing concern for environmental stewardship in drug development.
Japan's regulatory approach aligns closely with international standards but includes specific provisions for traditional Japanese medicines, which may contain complex mixtures of natural products with multiple chiral centers. The PMDA requires careful analysis and control of these compounds to ensure consistent quality and safety.
Globally, there is a trend towards harmonization of regulatory requirements for isomeric pharmaceuticals through initiatives like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). These efforts aim to streamline the drug development process and ensure consistent standards across different regions.
Regulatory frameworks also address the issue of patent protection for isomeric drugs. Many jurisdictions now allow separate patents for individual isomers, even when the racemic mixture is already known. This has significant implications for pharmaceutical companies' intellectual property strategies and the potential for developing improved versions of existing drugs.
As our understanding of the influence of geometric isomers on drug shelf-life continues to evolve, regulatory agencies are likely to update their guidelines to reflect new scientific insights. This may include more stringent requirements for stability testing of individual isomers and their potential interconversion products under various storage conditions.
These regulatory bodies require pharmaceutical companies to thoroughly characterize and control the isomeric composition of their products throughout the drug development process. This includes providing detailed information on the stereochemistry of the active pharmaceutical ingredient (API), the rationale for selecting a specific isomer or mixture of isomers, and the potential impact of isomeric interconversion on drug safety and efficacy.
In the United States, the FDA's guidance on stereoisomeric drugs emphasizes the importance of developing single-isomer products when possible, as this can lead to improved therapeutic outcomes and reduced side effects. However, the agency also recognizes that in some cases, a racemic mixture may be more appropriate or necessary. Manufacturers must provide robust scientific justification for their chosen approach and demonstrate consistent control of the isomeric composition during production.
The EMA has similar requirements but places additional emphasis on the environmental impact of chiral drugs. Their guidelines mandate that pharmaceutical companies assess the potential ecological effects of individual isomers and their metabolites, reflecting a growing concern for environmental stewardship in drug development.
Japan's regulatory approach aligns closely with international standards but includes specific provisions for traditional Japanese medicines, which may contain complex mixtures of natural products with multiple chiral centers. The PMDA requires careful analysis and control of these compounds to ensure consistent quality and safety.
Globally, there is a trend towards harmonization of regulatory requirements for isomeric pharmaceuticals through initiatives like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). These efforts aim to streamline the drug development process and ensure consistent standards across different regions.
Regulatory frameworks also address the issue of patent protection for isomeric drugs. Many jurisdictions now allow separate patents for individual isomers, even when the racemic mixture is already known. This has significant implications for pharmaceutical companies' intellectual property strategies and the potential for developing improved versions of existing drugs.
As our understanding of the influence of geometric isomers on drug shelf-life continues to evolve, regulatory agencies are likely to update their guidelines to reflect new scientific insights. This may include more stringent requirements for stability testing of individual isomers and their potential interconversion products under various storage conditions.
Economic Impact of Improved Drug Shelf-Life
The economic impact of improved drug shelf-life resulting from a better understanding of geometric isomers is significant and far-reaching. Extended shelf-life can lead to substantial cost savings for pharmaceutical companies, healthcare providers, and patients alike. By reducing the frequency of drug replacements due to expiration, companies can minimize production and distribution costs, potentially leading to lower drug prices for consumers.
Improved shelf-life also has implications for inventory management and supply chain efficiency. Pharmacies and hospitals can maintain larger stocks of medications without the risk of rapid expiration, reducing the likelihood of shortages and improving overall healthcare delivery. This is particularly crucial for rural or remote areas where frequent resupply may be challenging.
From a global perspective, extended shelf-life can have a profound impact on international aid and disaster relief efforts. Longer-lasting medications can be stockpiled more effectively and distributed to areas in need without the concern of imminent expiration. This can significantly enhance the efficacy of humanitarian aid programs and improve healthcare outcomes in developing regions.
The economic benefits extend to research and development as well. With a deeper understanding of how geometric isomers affect shelf-life, pharmaceutical companies can potentially streamline their drug development processes. This could lead to faster time-to-market for new drugs and reduced development costs, ultimately benefiting both the industry and patients.
Environmental considerations also play a role in the economic impact. Improved shelf-life means less pharmaceutical waste, reducing the environmental and financial costs associated with proper disposal of expired medications. This aligns with growing global initiatives for sustainability in healthcare and can contribute to a company's environmental, social, and governance (ESG) performance.
Lastly, the economic impact extends to regulatory compliance and quality control. With better shelf-life prediction and control, pharmaceutical companies can potentially reduce the frequency and cost of stability testing and regulatory submissions related to expiration date extensions. This can lead to significant savings in regulatory affairs and quality assurance departments, allowing resources to be redirected towards other critical areas of drug development and production.
Improved shelf-life also has implications for inventory management and supply chain efficiency. Pharmacies and hospitals can maintain larger stocks of medications without the risk of rapid expiration, reducing the likelihood of shortages and improving overall healthcare delivery. This is particularly crucial for rural or remote areas where frequent resupply may be challenging.
From a global perspective, extended shelf-life can have a profound impact on international aid and disaster relief efforts. Longer-lasting medications can be stockpiled more effectively and distributed to areas in need without the concern of imminent expiration. This can significantly enhance the efficacy of humanitarian aid programs and improve healthcare outcomes in developing regions.
The economic benefits extend to research and development as well. With a deeper understanding of how geometric isomers affect shelf-life, pharmaceutical companies can potentially streamline their drug development processes. This could lead to faster time-to-market for new drugs and reduced development costs, ultimately benefiting both the industry and patients.
Environmental considerations also play a role in the economic impact. Improved shelf-life means less pharmaceutical waste, reducing the environmental and financial costs associated with proper disposal of expired medications. This aligns with growing global initiatives for sustainability in healthcare and can contribute to a company's environmental, social, and governance (ESG) performance.
Lastly, the economic impact extends to regulatory compliance and quality control. With better shelf-life prediction and control, pharmaceutical companies can potentially reduce the frequency and cost of stability testing and regulatory submissions related to expiration date extensions. This can lead to significant savings in regulatory affairs and quality assurance departments, allowing resources to be redirected towards other critical areas of drug development and production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!