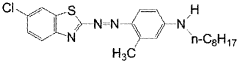
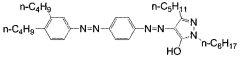
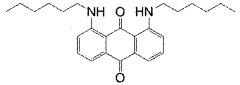
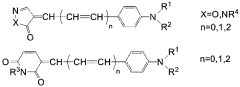
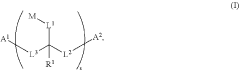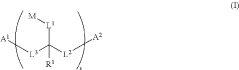Investigation into the Photochemistry of Geometric Isomers in Dyes
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photochemistry Background
Photochemistry, the study of chemical reactions induced by light, has been a cornerstone of scientific research for over a century. Its origins can be traced back to the early 19th century when scientists first observed the effects of light on chemical systems. The field gained significant momentum in the early 20th century with the development of quantum mechanics, which provided a theoretical framework for understanding light-matter interactions at the molecular level.
The photochemistry of geometric isomers in dyes represents a fascinating subset of this broader field. Geometric isomers are molecules with the same chemical formula but different spatial arrangements of atoms. In the context of dyes, these isomers can exhibit distinct optical and chemical properties, making them particularly interesting for both fundamental research and practical applications.
The study of photochemical reactions in dyes has been driven by their widespread use in various industries, including textiles, printing, and photography. The ability of certain dyes to undergo reversible photochemical transformations between different geometric isomers has led to the development of photochromic materials, which change color upon exposure to light.
One of the key principles underlying the photochemistry of geometric isomers is the concept of photoisomerization. This process involves the absorption of light energy by a molecule, leading to a change in its geometric configuration. In dyes, this often manifests as a cis-trans isomerization around a carbon-carbon double bond or a nitrogen-nitrogen double bond.
The mechanism of photoisomerization typically involves the excitation of electrons to higher energy states, followed by molecular rearrangement and relaxation back to the ground state. This process can occur on extremely fast timescales, often in the picosecond to nanosecond range, making it challenging to study experimentally.
Advances in spectroscopic techniques, particularly ultrafast laser spectroscopy, have revolutionized our ability to probe these rapid photochemical processes. Time-resolved absorption and emission spectroscopies have become invaluable tools for tracking the formation and decay of transient species during photoisomerization reactions.
The photochemistry of geometric isomers in dyes has found applications in various fields. In addition to photochromic materials, it has been exploited in the development of molecular switches and motors, data storage devices, and even in the design of smart materials that respond to light stimuli. Understanding and controlling these photochemical processes is crucial for optimizing the performance and stability of dye-based technologies.
The photochemistry of geometric isomers in dyes represents a fascinating subset of this broader field. Geometric isomers are molecules with the same chemical formula but different spatial arrangements of atoms. In the context of dyes, these isomers can exhibit distinct optical and chemical properties, making them particularly interesting for both fundamental research and practical applications.
The study of photochemical reactions in dyes has been driven by their widespread use in various industries, including textiles, printing, and photography. The ability of certain dyes to undergo reversible photochemical transformations between different geometric isomers has led to the development of photochromic materials, which change color upon exposure to light.
One of the key principles underlying the photochemistry of geometric isomers is the concept of photoisomerization. This process involves the absorption of light energy by a molecule, leading to a change in its geometric configuration. In dyes, this often manifests as a cis-trans isomerization around a carbon-carbon double bond or a nitrogen-nitrogen double bond.
The mechanism of photoisomerization typically involves the excitation of electrons to higher energy states, followed by molecular rearrangement and relaxation back to the ground state. This process can occur on extremely fast timescales, often in the picosecond to nanosecond range, making it challenging to study experimentally.
Advances in spectroscopic techniques, particularly ultrafast laser spectroscopy, have revolutionized our ability to probe these rapid photochemical processes. Time-resolved absorption and emission spectroscopies have become invaluable tools for tracking the formation and decay of transient species during photoisomerization reactions.
The photochemistry of geometric isomers in dyes has found applications in various fields. In addition to photochromic materials, it has been exploited in the development of molecular switches and motors, data storage devices, and even in the design of smart materials that respond to light stimuli. Understanding and controlling these photochemical processes is crucial for optimizing the performance and stability of dye-based technologies.
Market Analysis
The market for dyes and pigments has been experiencing steady growth, driven by increasing demand from various end-use industries such as textiles, paints and coatings, plastics, and printing inks. The global dye market size was valued at USD 33.2 billion in 2020 and is projected to reach USD 47.8 billion by 2028, growing at a CAGR of 4.9% during the forecast period.
The investigation into the photochemistry of geometric isomers in dyes has significant market implications, particularly in the development of advanced functional dyes and smart materials. This research area is gaining traction due to its potential applications in various high-value sectors, including photochromic materials, optical data storage, and photoswitchable molecular devices.
In the textile industry, which accounts for a substantial portion of the dye market, there is a growing interest in photochromic dyes that can change color in response to light exposure. These dyes offer opportunities for creating innovative fashion products and functional textiles with color-changing properties. The global smart textile market, which includes photochromic textiles, is expected to grow from USD 3.0 billion in 2020 to USD 5.9 billion by 2025, at a CAGR of 14.5%.
The printing and packaging industry is another significant market for photosensitive dyes. With the increasing demand for anti-counterfeiting measures and smart packaging solutions, photochromic inks derived from geometric isomers are finding new applications. The global anti-counterfeiting packaging market is projected to reach USD 189.9 billion by 2026, growing at a CAGR of 7.8% from 2021 to 2026.
In the field of optical data storage, the photochemistry of geometric isomers in dyes plays a crucial role in developing high-density storage media. As the demand for data storage continues to grow exponentially, there is a significant market opportunity for advanced optical storage technologies. The global optical data storage market is expected to reach USD 2.5 billion by 2025, growing at a CAGR of 6.7% from 2020 to 2025.
The research on geometric isomers in dyes also has potential applications in the development of photoswitchable molecular devices for use in sensors, displays, and molecular machines. This emerging field aligns with the growing market for nanotechnology and molecular electronics, which is projected to reach USD 9.9 billion by 2026, growing at a CAGR of 17.1% from 2021 to 2026.
As environmental concerns continue to shape consumer preferences and regulatory landscapes, there is an increasing demand for eco-friendly and sustainable dyes. Research into the photochemistry of geometric isomers could lead to the development of more environmentally benign dye formulations, addressing a growing market need. The global natural dyes market is expected to grow at a CAGR of 11.3% from 2021 to 2026, reaching USD 5.1 billion by the end of the forecast period.
The investigation into the photochemistry of geometric isomers in dyes has significant market implications, particularly in the development of advanced functional dyes and smart materials. This research area is gaining traction due to its potential applications in various high-value sectors, including photochromic materials, optical data storage, and photoswitchable molecular devices.
In the textile industry, which accounts for a substantial portion of the dye market, there is a growing interest in photochromic dyes that can change color in response to light exposure. These dyes offer opportunities for creating innovative fashion products and functional textiles with color-changing properties. The global smart textile market, which includes photochromic textiles, is expected to grow from USD 3.0 billion in 2020 to USD 5.9 billion by 2025, at a CAGR of 14.5%.
The printing and packaging industry is another significant market for photosensitive dyes. With the increasing demand for anti-counterfeiting measures and smart packaging solutions, photochromic inks derived from geometric isomers are finding new applications. The global anti-counterfeiting packaging market is projected to reach USD 189.9 billion by 2026, growing at a CAGR of 7.8% from 2021 to 2026.
In the field of optical data storage, the photochemistry of geometric isomers in dyes plays a crucial role in developing high-density storage media. As the demand for data storage continues to grow exponentially, there is a significant market opportunity for advanced optical storage technologies. The global optical data storage market is expected to reach USD 2.5 billion by 2025, growing at a CAGR of 6.7% from 2020 to 2025.
The research on geometric isomers in dyes also has potential applications in the development of photoswitchable molecular devices for use in sensors, displays, and molecular machines. This emerging field aligns with the growing market for nanotechnology and molecular electronics, which is projected to reach USD 9.9 billion by 2026, growing at a CAGR of 17.1% from 2021 to 2026.
As environmental concerns continue to shape consumer preferences and regulatory landscapes, there is an increasing demand for eco-friendly and sustainable dyes. Research into the photochemistry of geometric isomers could lead to the development of more environmentally benign dye formulations, addressing a growing market need. The global natural dyes market is expected to grow at a CAGR of 11.3% from 2021 to 2026, reaching USD 5.1 billion by the end of the forecast period.
Current Challenges
The investigation into the photochemistry of geometric isomers in dyes faces several significant challenges that hinder progress in this field. One of the primary obstacles is the complexity of the photochemical reactions involved. Geometric isomers can undergo various transformations upon light exposure, including cis-trans isomerization, cyclization, and rearrangements. These processes often occur simultaneously, making it difficult to isolate and study individual reaction pathways.
Another major challenge is the sensitivity of these systems to environmental factors. The photochemical behavior of geometric isomers can be greatly influenced by solvent polarity, pH, temperature, and the presence of other molecules. This sensitivity complicates the reproducibility of experiments and the interpretation of results, as slight variations in conditions can lead to significant changes in observed phenomena.
The transient nature of many intermediates formed during photochemical reactions poses a substantial technical hurdle. These short-lived species are crucial for understanding reaction mechanisms but are often difficult to detect and characterize using conventional analytical techniques. Advanced spectroscopic methods with high temporal resolution are required, which can be both expensive and technically demanding.
Furthermore, the development of predictive models for the photochemical behavior of geometric isomers in dyes remains a significant challenge. The complexity of the electronic structures and the multitude of possible excited states make it difficult to accurately simulate these systems using current computational methods. This limitation hampers the ability to design new photochromic dyes with tailored properties.
The scalability of photochemical processes involving geometric isomers is another area of concern. While many reactions work well on a laboratory scale, translating these to industrial applications can be problematic due to issues such as light penetration in larger reaction volumes and the potential for unwanted side reactions.
Lastly, the long-term stability of photochromic dyes based on geometric isomers is a persistent challenge. Many systems suffer from fatigue over repeated photochemical cycles, limiting their practical applications in areas such as smart materials and optical data storage. Developing more robust molecular designs that can withstand numerous isomerization cycles without degradation remains an active area of research.
Addressing these challenges requires a multidisciplinary approach, combining advanced spectroscopic techniques, computational modeling, and innovative molecular design. Progress in overcoming these obstacles will not only advance our fundamental understanding of photochemistry but also pave the way for new applications in fields ranging from materials science to biotechnology.
Another major challenge is the sensitivity of these systems to environmental factors. The photochemical behavior of geometric isomers can be greatly influenced by solvent polarity, pH, temperature, and the presence of other molecules. This sensitivity complicates the reproducibility of experiments and the interpretation of results, as slight variations in conditions can lead to significant changes in observed phenomena.
The transient nature of many intermediates formed during photochemical reactions poses a substantial technical hurdle. These short-lived species are crucial for understanding reaction mechanisms but are often difficult to detect and characterize using conventional analytical techniques. Advanced spectroscopic methods with high temporal resolution are required, which can be both expensive and technically demanding.
Furthermore, the development of predictive models for the photochemical behavior of geometric isomers in dyes remains a significant challenge. The complexity of the electronic structures and the multitude of possible excited states make it difficult to accurately simulate these systems using current computational methods. This limitation hampers the ability to design new photochromic dyes with tailored properties.
The scalability of photochemical processes involving geometric isomers is another area of concern. While many reactions work well on a laboratory scale, translating these to industrial applications can be problematic due to issues such as light penetration in larger reaction volumes and the potential for unwanted side reactions.
Lastly, the long-term stability of photochromic dyes based on geometric isomers is a persistent challenge. Many systems suffer from fatigue over repeated photochemical cycles, limiting their practical applications in areas such as smart materials and optical data storage. Developing more robust molecular designs that can withstand numerous isomerization cycles without degradation remains an active area of research.
Addressing these challenges requires a multidisciplinary approach, combining advanced spectroscopic techniques, computational modeling, and innovative molecular design. Progress in overcoming these obstacles will not only advance our fundamental understanding of photochemistry but also pave the way for new applications in fields ranging from materials science to biotechnology.
Existing Methodologies
01 Photochemical isomerization of dyes
Geometric isomers of dyes can undergo photochemical isomerization when exposed to light. This process involves the conversion between cis and trans isomers, which can affect the color and properties of the dye. Understanding and controlling this isomerization is crucial in developing photosensitive dyes and materials.- Photochemical isomerization of dyes: Geometric isomers of dyes can undergo photochemical isomerization when exposed to light. This process involves the transformation of one isomeric form to another, which can affect the color and properties of the dye. Understanding and controlling this isomerization is crucial in developing stable and efficient dye molecules for various applications.
- Synthesis of geometric isomers in dye production: The synthesis of specific geometric isomers is an important aspect of dye production. Different synthetic routes and reaction conditions can lead to the formation of various isomeric forms. Controlling the synthesis to favor desired geometric isomers can improve the efficiency and quality of dye products.
- Characterization and analysis of dye isomers: Advanced analytical techniques are employed to characterize and distinguish between geometric isomers of dyes. These methods may include spectroscopic techniques, chromatography, and computational modeling. Accurate characterization is essential for quality control and understanding the behavior of dye molecules in various applications.
- Application of geometric isomers in color technology: Different geometric isomers of dyes can exhibit varying color properties and stabilities. This characteristic is exploited in color technology to create a wide range of hues and effects. The selective use of specific isomers can lead to improved color fastness, brightness, and other desirable properties in textiles, paints, and other colored materials.
- Photostability and degradation of dye isomers: The photostability of geometric isomers in dyes is a critical factor in their long-term performance. Some isomers may be more susceptible to photodegradation than others, leading to color fading or changes over time. Research in this area focuses on understanding degradation mechanisms and developing strategies to enhance the photostability of dye molecules.
02 Synthesis of geometric isomers in dye production
The synthesis of specific geometric isomers is important in dye production. Different synthetic routes and reaction conditions can lead to the formation of different isomers, which may have distinct properties and applications. Controlling the isomer ratio during synthesis can be crucial for achieving desired dye characteristics.Expand Specific Solutions03 Separation and purification of geometric isomers
Separating and purifying geometric isomers of dyes is essential for obtaining pure compounds with specific properties. Various techniques such as chromatography, crystallization, and selective precipitation can be employed to isolate individual isomers from mixtures. This is particularly important when different isomers exhibit distinct photochemical behaviors.Expand Specific Solutions04 Application of geometric isomers in photosensitive materials
Geometric isomers of dyes find applications in various photosensitive materials, including photochromic compounds, optical data storage, and photodynamic therapy. The ability of these isomers to undergo reversible photochemical transformations makes them valuable in developing smart materials and advanced optical systems.Expand Specific Solutions05 Characterization and analysis of geometric isomers
Advanced analytical techniques are employed to characterize and analyze geometric isomers in dyes. These methods include spectroscopic techniques such as NMR, UV-Vis, and IR spectroscopy, as well as X-ray crystallography. Understanding the structural differences between isomers is crucial for predicting and optimizing their photochemical properties.Expand Specific Solutions
Key Industry Players
The investigation into the photochemistry of geometric isomers in dyes is in a developing stage, with a growing market driven by applications in various industries. The technology's maturity is advancing, as evidenced by the involvement of major players like Sony Group Corp., BASF Corp., and 3M Innovative Properties Co. These companies are leveraging their expertise in materials science and chemical engineering to explore novel applications. The competitive landscape is diverse, including established chemical firms, research institutions, and specialized dye manufacturers like DyStar Textilfarben GmbH & Co. As the field progresses, collaborations between academia and industry are likely to accelerate innovation and commercialization of new photochromic dye technologies.
BASF Corp.
Technical Solution: BASF Corp. has developed advanced photochromic dyes that undergo reversible structural changes upon exposure to light, enabling geometric isomerization. Their technology utilizes azobenzene derivatives with tailored substituents to fine-tune the photoisomerization process[1]. BASF's approach involves incorporating these photochromic dyes into various polymer matrices, allowing for applications in smart windows, optical data storage, and responsive coatings[2]. The company has also explored the use of metal-organic frameworks (MOFs) as hosts for photochromic dyes, enhancing the stability and efficiency of the isomerization process[3].
Strengths: Extensive expertise in chemical synthesis and formulation; large-scale production capabilities. Weaknesses: May face challenges in achieving rapid isomerization kinetics in certain polymer matrices.
3M Innovative Properties Co.
Technical Solution: 3M has developed a novel approach to investigating geometric isomers in dyes using advanced fluorescence spectroscopy techniques. Their method employs time-resolved fluorescence anisotropy measurements to study the rotational dynamics of dye molecules during photoisomerization[4]. By utilizing specially designed fluorescent probes, 3M researchers can monitor the real-time conformational changes of dye molecules with high sensitivity. This technology has been applied to optimize the performance of photochromic materials in applications such as adaptive optics and color-changing films[5]. Additionally, 3M has explored the use of plasmonic nanostructures to enhance the photoisomerization efficiency of azobenzene-based dyes[6].
Strengths: Strong expertise in optical materials and measurement techniques; diverse application portfolio. Weaknesses: May face challenges in scaling up production of specialized fluorescent probes.
Innovative Research
Ultra bright dimeric or polymeric dyes and methods for preparation of the same
PatentActiveUS12018159B2
Innovation
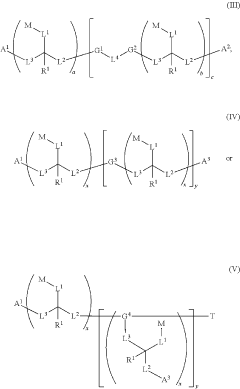
- The development of water-soluble dimeric and polymeric dyes through specific reaction methods involving compounds of structures (I), (II), (III), (IV), and (V), which allow for the formation of intensely colored or fluorescent compounds that can be used for visual detection of analyte molecules by reacting functional groups to create covalent bonds, thereby enhancing molar brightness.
Colored composition and image display structure
PatentWO2013100170A2
Innovation
- A colored composition comprising a mixture of stereoisomers of methine, anthraquinone, and azomethine dyes with optically active sites, specifically designed to have high solubility in non-polar solvents, particularly hydrocarbon solvents, is used in conjunction with a hydrophobic polymer layer and a hydrophilic liquid layer to enhance image display performance.
Environmental Impact
The environmental impact of photochemical reactions involving geometric isomers in dyes is a critical aspect that warrants thorough investigation. These reactions can have far-reaching consequences on ecosystems and human health, necessitating a comprehensive understanding of their effects.
One of the primary environmental concerns is the potential release of harmful byproducts during the photochemical isomerization process. When dyes undergo geometric changes upon exposure to light, they may form intermediate compounds or breakdown products that could be toxic to aquatic life or persist in the environment. This is particularly relevant in textile and printing industries, where large quantities of dyes are used and subsequently released into water systems.
The persistence of photochemically altered dyes in the environment is another significant issue. Some geometric isomers may be more resistant to biodegradation than their original counterparts, leading to prolonged environmental contamination. This can result in long-term effects on water quality, soil composition, and the overall health of ecosystems.
Light-induced changes in dye molecules can also alter their bioaccumulation potential. Certain geometric isomers may have increased lipophilicity, making them more likely to accumulate in the tissues of aquatic organisms. This bioaccumulation can lead to biomagnification up the food chain, potentially affecting higher-order consumers, including humans.
The photochemistry of geometric isomers in dyes may also impact the effectiveness of wastewater treatment processes. Traditional treatment methods may not be adequately equipped to handle the altered chemical structures, potentially allowing these compounds to pass through and enter natural water bodies.
Furthermore, the environmental impact extends to energy consumption and carbon footprint considerations. The deliberate use of photochemical reactions in industrial processes, while potentially beneficial for certain applications, may require significant energy input. This energy demand could contribute to increased greenhouse gas emissions if not managed sustainably.
On a positive note, understanding the photochemistry of geometric isomers in dyes could lead to the development of more environmentally friendly products. For instance, dyes that undergo beneficial isomerization when exposed to sunlight could be designed to break down more easily in the environment, reducing their long-term impact.
In conclusion, the environmental implications of geometric isomer photochemistry in dyes are multifaceted and significant. They encompass issues of toxicity, persistence, bioaccumulation, water treatment challenges, and energy consumption. As research in this field progresses, it is crucial to consider these environmental factors in the development and application of dye technologies.
One of the primary environmental concerns is the potential release of harmful byproducts during the photochemical isomerization process. When dyes undergo geometric changes upon exposure to light, they may form intermediate compounds or breakdown products that could be toxic to aquatic life or persist in the environment. This is particularly relevant in textile and printing industries, where large quantities of dyes are used and subsequently released into water systems.
The persistence of photochemically altered dyes in the environment is another significant issue. Some geometric isomers may be more resistant to biodegradation than their original counterparts, leading to prolonged environmental contamination. This can result in long-term effects on water quality, soil composition, and the overall health of ecosystems.
Light-induced changes in dye molecules can also alter their bioaccumulation potential. Certain geometric isomers may have increased lipophilicity, making them more likely to accumulate in the tissues of aquatic organisms. This bioaccumulation can lead to biomagnification up the food chain, potentially affecting higher-order consumers, including humans.
The photochemistry of geometric isomers in dyes may also impact the effectiveness of wastewater treatment processes. Traditional treatment methods may not be adequately equipped to handle the altered chemical structures, potentially allowing these compounds to pass through and enter natural water bodies.
Furthermore, the environmental impact extends to energy consumption and carbon footprint considerations. The deliberate use of photochemical reactions in industrial processes, while potentially beneficial for certain applications, may require significant energy input. This energy demand could contribute to increased greenhouse gas emissions if not managed sustainably.
On a positive note, understanding the photochemistry of geometric isomers in dyes could lead to the development of more environmentally friendly products. For instance, dyes that undergo beneficial isomerization when exposed to sunlight could be designed to break down more easily in the environment, reducing their long-term impact.
In conclusion, the environmental implications of geometric isomer photochemistry in dyes are multifaceted and significant. They encompass issues of toxicity, persistence, bioaccumulation, water treatment challenges, and energy consumption. As research in this field progresses, it is crucial to consider these environmental factors in the development and application of dye technologies.
Spectroscopic Techniques
Spectroscopic techniques play a crucial role in investigating the photochemistry of geometric isomers in dyes. These methods provide valuable insights into the structural changes and electronic transitions that occur during photochemical reactions. One of the most widely used techniques is UV-visible spectroscopy, which allows researchers to monitor the absorption spectra of dyes and their isomers. This technique is particularly useful for tracking the conversion between geometric isomers, as they often exhibit distinct absorption bands due to differences in their molecular configurations.
Fluorescence spectroscopy is another powerful tool in this field, enabling scientists to study the excited-state properties of dyes and their isomers. By analyzing the emission spectra and fluorescence lifetimes, researchers can gain information about the energy transfer processes and the formation of intermediate species during photochemical reactions. Time-resolved fluorescence spectroscopy, in particular, offers the ability to observe rapid changes in molecular structure on timescales as short as picoseconds.
Infrared (IR) spectroscopy provides complementary information about the vibrational modes of dye molecules, which can be sensitive to changes in geometric configuration. This technique is especially useful for identifying specific functional groups and monitoring their transformations during photochemical reactions. Fourier-transform infrared (FTIR) spectroscopy, with its high sensitivity and resolution, has become a standard method for characterizing dye isomers and their photoproducts.
Nuclear magnetic resonance (NMR) spectroscopy is indispensable for elucidating the molecular structure of dyes and their geometric isomers. Both 1H and 13C NMR can provide detailed information about the chemical environment of individual atoms within the molecule, allowing researchers to distinguish between different isomeric forms. Advanced NMR techniques, such as 2D correlation spectroscopy (COSY) and nuclear Overhauser effect spectroscopy (NOESY), offer additional insights into the spatial relationships between atoms in complex dye molecules.
Raman spectroscopy complements IR spectroscopy by providing information about vibrational modes that may not be IR-active. This technique is particularly useful for studying conjugated systems, which are common in many dyes. Surface-enhanced Raman spectroscopy (SERS) can dramatically increase the sensitivity of Raman measurements, allowing for the detection of trace amounts of dye isomers and reaction intermediates.
Circular dichroism (CD) spectroscopy is a valuable technique for investigating chiral dye molecules and their geometric isomers. CD can provide information about the absolute configuration of molecules and is sensitive to changes in molecular geometry, making it an excellent tool for monitoring photochemical isomerization reactions. Time-resolved CD spectroscopy can offer insights into the dynamics of these processes on short timescales.
Fluorescence spectroscopy is another powerful tool in this field, enabling scientists to study the excited-state properties of dyes and their isomers. By analyzing the emission spectra and fluorescence lifetimes, researchers can gain information about the energy transfer processes and the formation of intermediate species during photochemical reactions. Time-resolved fluorescence spectroscopy, in particular, offers the ability to observe rapid changes in molecular structure on timescales as short as picoseconds.
Infrared (IR) spectroscopy provides complementary information about the vibrational modes of dye molecules, which can be sensitive to changes in geometric configuration. This technique is especially useful for identifying specific functional groups and monitoring their transformations during photochemical reactions. Fourier-transform infrared (FTIR) spectroscopy, with its high sensitivity and resolution, has become a standard method for characterizing dye isomers and their photoproducts.
Nuclear magnetic resonance (NMR) spectroscopy is indispensable for elucidating the molecular structure of dyes and their geometric isomers. Both 1H and 13C NMR can provide detailed information about the chemical environment of individual atoms within the molecule, allowing researchers to distinguish between different isomeric forms. Advanced NMR techniques, such as 2D correlation spectroscopy (COSY) and nuclear Overhauser effect spectroscopy (NOESY), offer additional insights into the spatial relationships between atoms in complex dye molecules.
Raman spectroscopy complements IR spectroscopy by providing information about vibrational modes that may not be IR-active. This technique is particularly useful for studying conjugated systems, which are common in many dyes. Surface-enhanced Raman spectroscopy (SERS) can dramatically increase the sensitivity of Raman measurements, allowing for the detection of trace amounts of dye isomers and reaction intermediates.
Circular dichroism (CD) spectroscopy is a valuable technique for investigating chiral dye molecules and their geometric isomers. CD can provide information about the absolute configuration of molecules and is sensitive to changes in molecular geometry, making it an excellent tool for monitoring photochemical isomerization reactions. Time-resolved CD spectroscopy can offer insights into the dynamics of these processes on short timescales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!