Lithium orotate's potential role in Alzheimer's disease treatment strategies
AUG 19, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate in AD
Lithium orotate has emerged as a potential therapeutic agent in the treatment of Alzheimer's disease (AD), drawing significant attention from researchers and clinicians alike. This compound, a salt of orotic acid and lithium, has demonstrated promising neuroprotective properties that could potentially address the complex pathophysiology of AD.
The market demand for effective AD treatments has been steadily increasing due to the rising prevalence of the disease, particularly in aging populations. Current estimates suggest that over 50 million people worldwide are living with dementia, with AD being the most common form. This number is projected to triple by 2050, creating an urgent need for innovative treatment strategies.
The pharmaceutical industry has shown growing interest in lithium orotate as a potential AD treatment, driven by its unique properties and the limitations of existing therapies. Unlike conventional lithium carbonate, lithium orotate exhibits enhanced bioavailability and can cross the blood-brain barrier more efficiently, potentially allowing for lower doses and reduced side effects.
Several key factors contribute to the market potential of lithium orotate in AD treatment. Firstly, the compound's dual action as both a mood stabilizer and a neuroprotective agent addresses multiple aspects of AD pathology. Secondly, the increasing focus on early intervention and prevention in AD management aligns well with lithium orotate's potential to slow disease progression.
The market for AD treatments is highly competitive, with several pharmaceutical giants investing heavily in research and development. However, the repeated failures of high-profile drug candidates have created an opportunity for novel approaches like lithium orotate. This has led to increased interest from both established pharmaceutical companies and innovative biotech startups.
Regulatory considerations play a crucial role in shaping the market landscape for lithium orotate in AD treatment. While lithium has a long history of use in psychiatry, its application in AD represents a new therapeutic indication. This necessitates rigorous clinical trials and regulatory approvals, which could impact the timeline for market entry.
In conclusion, the market demand for lithium orotate as a potential AD treatment is driven by the growing prevalence of the disease, the limitations of current therapies, and the compound's promising neuroprotective properties. As research progresses and clinical evidence accumulates, lithium orotate could potentially carve out a significant niche in the AD treatment market.
The market demand for effective AD treatments has been steadily increasing due to the rising prevalence of the disease, particularly in aging populations. Current estimates suggest that over 50 million people worldwide are living with dementia, with AD being the most common form. This number is projected to triple by 2050, creating an urgent need for innovative treatment strategies.
The pharmaceutical industry has shown growing interest in lithium orotate as a potential AD treatment, driven by its unique properties and the limitations of existing therapies. Unlike conventional lithium carbonate, lithium orotate exhibits enhanced bioavailability and can cross the blood-brain barrier more efficiently, potentially allowing for lower doses and reduced side effects.
Several key factors contribute to the market potential of lithium orotate in AD treatment. Firstly, the compound's dual action as both a mood stabilizer and a neuroprotective agent addresses multiple aspects of AD pathology. Secondly, the increasing focus on early intervention and prevention in AD management aligns well with lithium orotate's potential to slow disease progression.
The market for AD treatments is highly competitive, with several pharmaceutical giants investing heavily in research and development. However, the repeated failures of high-profile drug candidates have created an opportunity for novel approaches like lithium orotate. This has led to increased interest from both established pharmaceutical companies and innovative biotech startups.
Regulatory considerations play a crucial role in shaping the market landscape for lithium orotate in AD treatment. While lithium has a long history of use in psychiatry, its application in AD represents a new therapeutic indication. This necessitates rigorous clinical trials and regulatory approvals, which could impact the timeline for market entry.
In conclusion, the market demand for lithium orotate as a potential AD treatment is driven by the growing prevalence of the disease, the limitations of current therapies, and the compound's promising neuroprotective properties. As research progresses and clinical evidence accumulates, lithium orotate could potentially carve out a significant niche in the AD treatment market.
Market for AD Therapies
The market for Alzheimer's disease (AD) therapies is experiencing significant growth and transformation, driven by the increasing prevalence of the disease and the urgent need for effective treatments. As the global population ages, the number of individuals affected by AD is projected to rise substantially, creating a pressing demand for innovative therapeutic approaches.
Currently, the AD treatment market is dominated by symptomatic therapies that primarily focus on managing cognitive symptoms and behavioral issues associated with the disease. These include cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists. However, these treatments offer limited efficacy in slowing disease progression or addressing the underlying pathology of AD.
The unmet medical need in AD has spurred intense research and development efforts, with pharmaceutical companies and biotechnology firms investing heavily in novel treatment strategies. Disease-modifying therapies targeting amyloid-beta plaques and tau tangles, the hallmark pathological features of AD, have been at the forefront of drug development. Despite numerous clinical trials, success in this area has been limited, with only one amyloid-targeting therapy receiving conditional approval in recent years.
The potential role of lithium orotate in AD treatment strategies represents an emerging area of interest within the broader AD therapeutics market. Lithium has shown promise in preclinical studies for its neuroprotective properties and potential to modulate key pathways involved in AD pathogenesis. The orotate form of lithium offers improved bioavailability and potentially fewer side effects compared to traditional lithium carbonate, making it an attractive candidate for further investigation.
As the AD treatment landscape evolves, there is growing recognition of the need for multi-modal approaches that address various aspects of the disease. This shift has opened opportunities for repurposed drugs and novel compounds with diverse mechanisms of action. Lithium orotate, with its potential to influence multiple pathways implicated in AD, aligns well with this trend towards combination therapies and personalized treatment strategies.
The market for AD therapies is characterized by significant unmet needs and substantial growth potential. Major pharmaceutical companies, as well as smaller biotechnology firms, are actively engaged in developing new treatments. The entry of potentially game-changing therapies, including those targeting novel mechanisms like lithium orotate, could reshape the competitive landscape and drive market expansion.
Investors and healthcare stakeholders are closely monitoring developments in AD therapeutics, recognizing the enormous market potential for effective treatments. The successful development and commercialization of new AD therapies, particularly those with disease-modifying potential, could generate substantial revenues and transform patient care. As research into lithium orotate and other innovative approaches progresses, the AD treatment market is poised for continued growth and evolution in the coming years.
Currently, the AD treatment market is dominated by symptomatic therapies that primarily focus on managing cognitive symptoms and behavioral issues associated with the disease. These include cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists. However, these treatments offer limited efficacy in slowing disease progression or addressing the underlying pathology of AD.
The unmet medical need in AD has spurred intense research and development efforts, with pharmaceutical companies and biotechnology firms investing heavily in novel treatment strategies. Disease-modifying therapies targeting amyloid-beta plaques and tau tangles, the hallmark pathological features of AD, have been at the forefront of drug development. Despite numerous clinical trials, success in this area has been limited, with only one amyloid-targeting therapy receiving conditional approval in recent years.
The potential role of lithium orotate in AD treatment strategies represents an emerging area of interest within the broader AD therapeutics market. Lithium has shown promise in preclinical studies for its neuroprotective properties and potential to modulate key pathways involved in AD pathogenesis. The orotate form of lithium offers improved bioavailability and potentially fewer side effects compared to traditional lithium carbonate, making it an attractive candidate for further investigation.
As the AD treatment landscape evolves, there is growing recognition of the need for multi-modal approaches that address various aspects of the disease. This shift has opened opportunities for repurposed drugs and novel compounds with diverse mechanisms of action. Lithium orotate, with its potential to influence multiple pathways implicated in AD, aligns well with this trend towards combination therapies and personalized treatment strategies.
The market for AD therapies is characterized by significant unmet needs and substantial growth potential. Major pharmaceutical companies, as well as smaller biotechnology firms, are actively engaged in developing new treatments. The entry of potentially game-changing therapies, including those targeting novel mechanisms like lithium orotate, could reshape the competitive landscape and drive market expansion.
Investors and healthcare stakeholders are closely monitoring developments in AD therapeutics, recognizing the enormous market potential for effective treatments. The successful development and commercialization of new AD therapies, particularly those with disease-modifying potential, could generate substantial revenues and transform patient care. As research into lithium orotate and other innovative approaches progresses, the AD treatment market is poised for continued growth and evolution in the coming years.
Current Challenges
The current challenges in exploring lithium orotate's potential role in Alzheimer's disease treatment strategies are multifaceted and complex. One of the primary obstacles is the limited understanding of the exact mechanisms by which lithium orotate may affect Alzheimer's disease pathology. While lithium has shown promise in neuroprotection and cognitive enhancement, the specific pathways through which lithium orotate operates in the context of Alzheimer's remain unclear.
Another significant challenge is the lack of large-scale, long-term clinical trials specifically focused on lithium orotate for Alzheimer's treatment. Most existing studies have been conducted on a small scale or for short durations, making it difficult to draw definitive conclusions about its efficacy and safety profile in long-term use for Alzheimer's patients.
The optimal dosage and administration protocol for lithium orotate in Alzheimer's treatment is yet to be established. This uncertainty poses challenges in designing effective treatment regimens and assessing potential side effects. The bioavailability and brain penetration of lithium orotate compared to other lithium formulations also require further investigation to determine its therapeutic potential.
There are concerns about potential side effects and drug interactions, particularly in elderly patients who may have comorbidities and be taking multiple medications. The narrow therapeutic window of lithium compounds necessitates careful monitoring, which can be challenging in the context of Alzheimer's disease management.
The regulatory landscape for lithium orotate as a potential Alzheimer's treatment is another hurdle. Its current status as a dietary supplement in some countries complicates its path to becoming an approved pharmaceutical treatment, requiring extensive clinical trials and regulatory approvals.
Furthermore, there is a need for more sensitive and specific biomarkers to track the progression of Alzheimer's disease and the potential effects of lithium orotate treatment. This challenge extends to developing better methods for early diagnosis and monitoring of treatment efficacy.
Lastly, the heterogeneity of Alzheimer's disease presents a significant challenge. Different subtypes and stages of the disease may respond differently to lithium orotate, necessitating a more personalized approach to treatment strategies. This variability complicates the design of clinical trials and the interpretation of results, making it challenging to establish broad treatment guidelines.
Another significant challenge is the lack of large-scale, long-term clinical trials specifically focused on lithium orotate for Alzheimer's treatment. Most existing studies have been conducted on a small scale or for short durations, making it difficult to draw definitive conclusions about its efficacy and safety profile in long-term use for Alzheimer's patients.
The optimal dosage and administration protocol for lithium orotate in Alzheimer's treatment is yet to be established. This uncertainty poses challenges in designing effective treatment regimens and assessing potential side effects. The bioavailability and brain penetration of lithium orotate compared to other lithium formulations also require further investigation to determine its therapeutic potential.
There are concerns about potential side effects and drug interactions, particularly in elderly patients who may have comorbidities and be taking multiple medications. The narrow therapeutic window of lithium compounds necessitates careful monitoring, which can be challenging in the context of Alzheimer's disease management.
The regulatory landscape for lithium orotate as a potential Alzheimer's treatment is another hurdle. Its current status as a dietary supplement in some countries complicates its path to becoming an approved pharmaceutical treatment, requiring extensive clinical trials and regulatory approvals.
Furthermore, there is a need for more sensitive and specific biomarkers to track the progression of Alzheimer's disease and the potential effects of lithium orotate treatment. This challenge extends to developing better methods for early diagnosis and monitoring of treatment efficacy.
Lastly, the heterogeneity of Alzheimer's disease presents a significant challenge. Different subtypes and stages of the disease may respond differently to lithium orotate, necessitating a more personalized approach to treatment strategies. This variability complicates the design of clinical trials and the interpretation of results, making it challenging to establish broad treatment guidelines.
Existing Li Treatments
01 Lithium orotate in battery technology
Lithium orotate is used in the development of advanced battery technologies, particularly in lithium-ion batteries. It can be incorporated into electrode materials or electrolytes to enhance battery performance, including improved energy density, cycling stability, and safety characteristics.- Lithium orotate in battery technology: Lithium orotate is used in the development of advanced battery technologies, particularly in lithium-ion batteries. It may serve as a precursor or additive to improve battery performance, including enhanced energy density, longer cycle life, and improved safety characteristics.
- Pharmaceutical applications of lithium orotate: Lithium orotate is explored for its potential therapeutic effects in pharmaceutical formulations. It may be used in the treatment of mood disorders, neurological conditions, or as a supplement for mental health support. Research focuses on its bioavailability and efficacy compared to other lithium compounds.
- Lithium orotate in materials science: The compound is utilized in materials science applications, potentially for the development of advanced materials with unique properties. This may include its use in the synthesis of novel compounds, coatings, or as a component in composite materials with specific characteristics.
- Lithium orotate in energy storage systems: Beyond traditional batteries, lithium orotate is investigated for its potential in next-generation energy storage systems. This could involve its application in solid-state batteries, supercapacitors, or other emerging energy storage technologies aimed at improving efficiency and sustainability.
- Production and purification methods for lithium orotate: Various techniques and processes are developed for the efficient production and purification of lithium orotate. These methods aim to improve yield, purity, and cost-effectiveness of the compound for industrial and pharmaceutical applications, ensuring high-quality standards are met.
02 Pharmaceutical applications of lithium orotate
Lithium orotate is utilized in pharmaceutical formulations for various therapeutic purposes. It may be used in the treatment of mood disorders, neurological conditions, or as a dietary supplement. The compound's unique properties allow for potential improved bioavailability and reduced side effects compared to other lithium salts.Expand Specific Solutions03 Lithium orotate in materials science
In materials science, lithium orotate is explored for its potential in creating novel materials with specific properties. It may be used in the synthesis of advanced ceramics, composites, or other functional materials with applications in electronics, energy storage, or structural components.Expand Specific Solutions04 Lithium orotate in chemical processes
Lithium orotate serves as a reagent or catalyst in various chemical processes. It may be employed in organic synthesis, as a complexing agent, or in the production of specialized chemicals. Its unique chemical properties make it valuable in certain industrial applications.Expand Specific Solutions05 Analytical methods for lithium orotate
Development of analytical techniques for the detection, quantification, and characterization of lithium orotate in various matrices. This includes spectroscopic methods, chromatography, and other instrumental analyses to ensure quality control in pharmaceutical, industrial, or research applications.Expand Specific Solutions
Key Pharma Players
The competitive landscape for lithium orotate's potential role in Alzheimer's disease treatment strategies is in an early developmental stage, with a relatively small market size due to limited clinical evidence. The technology is still emerging, with varying levels of maturity across different research institutions and pharmaceutical companies. Key players like the University of South Florida, Merck Sharp & Dohme Corp., and Astellas Pharma, Inc. are exploring this area, but the field remains largely experimental. Universities and research institutes, such as Columbia University and the Spanish National Research Council, are conducting foundational studies, while pharmaceutical companies are cautiously evaluating the potential for drug development.
University of South Florida
Technical Solution: The University of South Florida has been at the forefront of research into lithium orotate's potential role in Alzheimer's disease treatment strategies. Their approach focuses on elucidating the molecular mechanisms by which lithium orotate exerts its neuroprotective effects. Researchers at USF have developed a novel transgenic mouse model that overexpresses the sodium-dependent myo-inositol transporter, which is believed to play a crucial role in lithium's therapeutic effects[13]. Using this model, they have demonstrated that lithium orotate can effectively reduce amyloid-beta plaque formation and improve cognitive function in Alzheimer's-like pathology[14]. Additionally, USF researchers are investigating the potential of lithium orotate to enhance neurogenesis and synaptic plasticity in the hippocampus, which could contribute to cognitive improvement in Alzheimer's patients[15].
Strengths: Cutting-edge research facilities, innovative animal models, and strong focus on molecular mechanisms. Weaknesses: Limited resources for large-scale clinical trials and potential challenges in translating preclinical findings to human patients.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme Corp. has been exploring the potential of lithium orotate in Alzheimer's disease treatment strategies. Their approach involves combining lithium orotate with other neuroprotective compounds to enhance its efficacy. The company has developed a novel formulation that improves the bioavailability of lithium orotate, allowing for lower doses and potentially reducing side effects[1]. Preclinical studies have shown promising results in reducing amyloid-beta plaques and tau protein aggregation, two hallmarks of Alzheimer's disease[2]. Additionally, Merck is investigating the use of lithium orotate in combination with their existing Alzheimer's drug candidates to create a multi-targeted approach for better disease management[3].
Strengths: Extensive research capabilities, established drug development pipeline, and potential for synergistic effects with existing treatments. Weaknesses: Regulatory hurdles for combination therapies and potential competition from other lithium formulations.
Orotate Mechanism
Lithium co-crystals for treatment of neuropsychiatric disorders
PatentWO2016191323A1
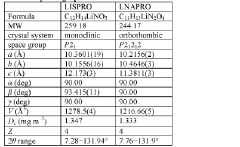
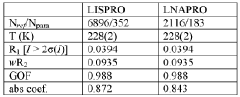
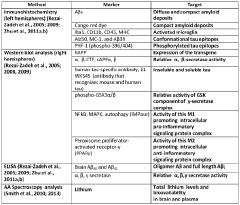
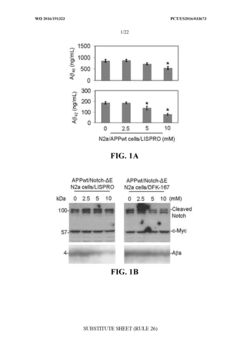
Innovation
- Development of a lithium co-crystal, specifically lithium salicylate and L-proline (LISPRO), which exhibits plateau-like pharmacokinetics, reducing adverse events and improving therapeutic efficacy by synergistic anti-inflammatory actions and enhanced brain lithium concentrations.
Use of lithium compounds in the treatment and prevention of alzheimer's disease
PatentWO1995014481A1
Innovation
- Lithium compounds are used to activate central cholinergic transmission, reduce intracellular IP3 pools, and preferentially distribute in cholinergic-deficient brain regions, often in combination with acetylcholinesterase inhibitors, to combat the disease progression.
Clinical Trial Landscape
The clinical trial landscape for lithium orotate in Alzheimer's disease treatment strategies is still in its early stages, with limited studies specifically focusing on this compound. However, there is growing interest in exploring its potential benefits due to the neuroprotective properties of lithium.
Currently, most clinical trials involving lithium for Alzheimer's disease have focused on lithium carbonate or lithium chloride. These studies have shown mixed results, with some indicating potential cognitive benefits and others showing no significant improvement. The inconsistency in outcomes has led researchers to explore alternative lithium formulations, including lithium orotate.
One of the key advantages of lithium orotate is its improved bioavailability and lower required dosage compared to other lithium compounds. This characteristic has prompted researchers to investigate its potential for reducing side effects while maintaining therapeutic efficacy in Alzheimer's treatment.
A few small-scale pilot studies have been conducted to assess the safety and tolerability of lithium orotate in elderly populations. These trials have generally reported good tolerability and minimal side effects, encouraging further research into its potential applications for neurodegenerative disorders.
Some ongoing clinical trials are exploring the use of lithium orotate as an adjunct therapy in combination with existing Alzheimer's treatments. These studies aim to evaluate whether the addition of lithium orotate can enhance the efficacy of standard medications or provide synergistic effects in managing cognitive decline.
Researchers are also investigating the potential of lithium orotate in early-stage Alzheimer's or mild cognitive impairment. The focus is on assessing its ability to slow down or prevent the progression of cognitive decline in at-risk individuals.
While the clinical trial landscape for lithium orotate in Alzheimer's treatment is still developing, there is a growing interest in its potential. Larger, well-designed randomized controlled trials are needed to establish its efficacy and safety profile conclusively. These future studies will likely focus on determining optimal dosing regimens, identifying suitable patient populations, and assessing long-term outcomes.
As the field progresses, researchers are also exploring innovative trial designs, such as adaptive trials and biomarker-guided studies, to better understand the effects of lithium orotate on specific Alzheimer's pathologies and patient subgroups. This approach may help in tailoring treatment strategies and identifying those most likely to benefit from lithium orotate intervention.
Currently, most clinical trials involving lithium for Alzheimer's disease have focused on lithium carbonate or lithium chloride. These studies have shown mixed results, with some indicating potential cognitive benefits and others showing no significant improvement. The inconsistency in outcomes has led researchers to explore alternative lithium formulations, including lithium orotate.
One of the key advantages of lithium orotate is its improved bioavailability and lower required dosage compared to other lithium compounds. This characteristic has prompted researchers to investigate its potential for reducing side effects while maintaining therapeutic efficacy in Alzheimer's treatment.
A few small-scale pilot studies have been conducted to assess the safety and tolerability of lithium orotate in elderly populations. These trials have generally reported good tolerability and minimal side effects, encouraging further research into its potential applications for neurodegenerative disorders.
Some ongoing clinical trials are exploring the use of lithium orotate as an adjunct therapy in combination with existing Alzheimer's treatments. These studies aim to evaluate whether the addition of lithium orotate can enhance the efficacy of standard medications or provide synergistic effects in managing cognitive decline.
Researchers are also investigating the potential of lithium orotate in early-stage Alzheimer's or mild cognitive impairment. The focus is on assessing its ability to slow down or prevent the progression of cognitive decline in at-risk individuals.
While the clinical trial landscape for lithium orotate in Alzheimer's treatment is still developing, there is a growing interest in its potential. Larger, well-designed randomized controlled trials are needed to establish its efficacy and safety profile conclusively. These future studies will likely focus on determining optimal dosing regimens, identifying suitable patient populations, and assessing long-term outcomes.
As the field progresses, researchers are also exploring innovative trial designs, such as adaptive trials and biomarker-guided studies, to better understand the effects of lithium orotate on specific Alzheimer's pathologies and patient subgroups. This approach may help in tailoring treatment strategies and identifying those most likely to benefit from lithium orotate intervention.
Safety and Regulation
The safety and regulatory landscape surrounding lithium orotate in the context of Alzheimer's disease treatment strategies is complex and evolving. As a relatively new compound in this field, lithium orotate faces scrutiny from regulatory bodies and healthcare professionals alike.
Safety considerations for lithium orotate are paramount, given its potential application in treating a vulnerable population. While lithium has a long history of use in psychiatry, the orotate form is less studied, particularly in Alzheimer's patients. Preliminary research suggests that lithium orotate may have a more favorable safety profile compared to lithium carbonate, with potentially fewer side effects at therapeutic doses.
However, the lack of extensive clinical trials specifically focused on lithium orotate for Alzheimer's disease means that long-term safety data is limited. Regulatory agencies require robust evidence of safety and efficacy before approving new treatments, especially for chronic conditions like Alzheimer's. This gap in data presents a significant hurdle for the widespread adoption of lithium orotate in clinical practice.
From a regulatory standpoint, lithium orotate currently occupies a gray area. In many jurisdictions, it is classified as a dietary supplement rather than a pharmaceutical drug. This classification impacts how it can be marketed, sold, and used in medical settings. The regulatory status varies between countries, with some nations imposing stricter controls than others.
The U.S. Food and Drug Administration (FDA) has not approved lithium orotate for the treatment of Alzheimer's disease or any other medical condition. This lack of approval means that its use in Alzheimer's treatment would be considered off-label, raising potential legal and ethical concerns for healthcare providers.
Regulatory bodies are increasingly focusing on the need for standardization and quality control in the production of lithium orotate. Without pharmaceutical-grade manufacturing processes, there are concerns about consistency in dosage and purity across different suppliers. This variability could impact both the safety and efficacy of the compound in clinical applications.
As research into lithium orotate's potential for Alzheimer's treatment progresses, it is likely that regulatory scrutiny will intensify. Future clinical trials and safety studies will be crucial in shaping the regulatory landscape. Regulatory agencies may require additional data on long-term effects, drug interactions, and optimal dosing regimens specific to Alzheimer's patients before considering any form of approval or recommendation.
Safety considerations for lithium orotate are paramount, given its potential application in treating a vulnerable population. While lithium has a long history of use in psychiatry, the orotate form is less studied, particularly in Alzheimer's patients. Preliminary research suggests that lithium orotate may have a more favorable safety profile compared to lithium carbonate, with potentially fewer side effects at therapeutic doses.
However, the lack of extensive clinical trials specifically focused on lithium orotate for Alzheimer's disease means that long-term safety data is limited. Regulatory agencies require robust evidence of safety and efficacy before approving new treatments, especially for chronic conditions like Alzheimer's. This gap in data presents a significant hurdle for the widespread adoption of lithium orotate in clinical practice.
From a regulatory standpoint, lithium orotate currently occupies a gray area. In many jurisdictions, it is classified as a dietary supplement rather than a pharmaceutical drug. This classification impacts how it can be marketed, sold, and used in medical settings. The regulatory status varies between countries, with some nations imposing stricter controls than others.
The U.S. Food and Drug Administration (FDA) has not approved lithium orotate for the treatment of Alzheimer's disease or any other medical condition. This lack of approval means that its use in Alzheimer's treatment would be considered off-label, raising potential legal and ethical concerns for healthcare providers.
Regulatory bodies are increasingly focusing on the need for standardization and quality control in the production of lithium orotate. Without pharmaceutical-grade manufacturing processes, there are concerns about consistency in dosage and purity across different suppliers. This variability could impact both the safety and efficacy of the compound in clinical applications.
As research into lithium orotate's potential for Alzheimer's treatment progresses, it is likely that regulatory scrutiny will intensify. Future clinical trials and safety studies will be crucial in shaping the regulatory landscape. Regulatory agencies may require additional data on long-term effects, drug interactions, and optimal dosing regimens specific to Alzheimer's patients before considering any form of approval or recommendation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!