Malachite's electrochemical behavior in solution extraction processes
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Extraction Background and Objectives
Malachite, a copper carbonate hydroxide mineral, has been a subject of significant interest in the field of solution extraction processes due to its unique electrochemical properties. The study of malachite's behavior in these processes is crucial for advancing copper extraction techniques and optimizing resource utilization in the mining industry.
The historical context of malachite extraction dates back to ancient times when it was primarily used as a pigment and ornamental stone. However, with the increasing demand for copper in modern industrial applications, the focus has shifted towards efficient extraction methods. The evolution of solution extraction processes has led to a renewed interest in understanding the electrochemical behavior of malachite, as it represents a valuable source of copper ore.
Recent technological advancements have paved the way for more sophisticated extraction techniques, prompting researchers and industry professionals to delve deeper into the electrochemical properties of malachite. This exploration aims to enhance the efficiency and sustainability of copper extraction processes, addressing the growing global demand for this essential metal.
The primary objective of studying malachite's electrochemical behavior in solution extraction processes is to develop more effective and environmentally friendly methods for copper recovery. By gaining a comprehensive understanding of the mineral's interactions with various solvents and electrolytes, researchers seek to optimize extraction parameters and improve overall yield.
Furthermore, this research aims to address several key challenges in the field, including the reduction of energy consumption, minimization of waste generation, and improvement of selectivity in copper extraction. These objectives align with the broader goals of sustainable mining practices and resource conservation.
Another critical aspect of this investigation is the potential for developing novel extraction technologies that can be applied to low-grade ores or complex mineral assemblages. As high-grade copper deposits become increasingly scarce, the ability to efficiently process lower-grade sources becomes paramount for meeting future demand.
The study of malachite's electrochemical behavior also has implications beyond the mining industry. Insights gained from this research may contribute to advancements in related fields such as materials science, electrochemistry, and environmental remediation. This cross-disciplinary potential underscores the importance of continued investigation into malachite's properties and behavior.
The historical context of malachite extraction dates back to ancient times when it was primarily used as a pigment and ornamental stone. However, with the increasing demand for copper in modern industrial applications, the focus has shifted towards efficient extraction methods. The evolution of solution extraction processes has led to a renewed interest in understanding the electrochemical behavior of malachite, as it represents a valuable source of copper ore.
Recent technological advancements have paved the way for more sophisticated extraction techniques, prompting researchers and industry professionals to delve deeper into the electrochemical properties of malachite. This exploration aims to enhance the efficiency and sustainability of copper extraction processes, addressing the growing global demand for this essential metal.
The primary objective of studying malachite's electrochemical behavior in solution extraction processes is to develop more effective and environmentally friendly methods for copper recovery. By gaining a comprehensive understanding of the mineral's interactions with various solvents and electrolytes, researchers seek to optimize extraction parameters and improve overall yield.
Furthermore, this research aims to address several key challenges in the field, including the reduction of energy consumption, minimization of waste generation, and improvement of selectivity in copper extraction. These objectives align with the broader goals of sustainable mining practices and resource conservation.
Another critical aspect of this investigation is the potential for developing novel extraction technologies that can be applied to low-grade ores or complex mineral assemblages. As high-grade copper deposits become increasingly scarce, the ability to efficiently process lower-grade sources becomes paramount for meeting future demand.
The study of malachite's electrochemical behavior also has implications beyond the mining industry. Insights gained from this research may contribute to advancements in related fields such as materials science, electrochemistry, and environmental remediation. This cross-disciplinary potential underscores the importance of continued investigation into malachite's properties and behavior.
Market Analysis for Malachite Extraction
The market for malachite extraction has shown significant growth in recent years, driven by increasing demand from various industries. Malachite, a copper carbonate hydroxide mineral, finds applications in jewelry making, ornamental objects, and as a source of copper in metallurgical processes. The global malachite market is closely tied to the copper industry, as malachite is often found in copper deposits and can be processed to extract copper.
The jewelry and decorative arts sector represents a substantial portion of the malachite market. High-end jewelry designers and artisans value malachite for its vibrant green color and unique patterns, creating a steady demand for high-quality specimens. This segment of the market is particularly sensitive to fashion trends and economic conditions, as luxury goods consumption tends to fluctuate with disposable income levels.
In the industrial sector, malachite extraction is primarily driven by copper production. As global copper demand continues to rise, fueled by infrastructure development, renewable energy technologies, and electric vehicle production, the market for malachite as a copper ore has expanded. Countries with significant copper mining operations, such as Chile, Peru, and Australia, are key players in the malachite extraction market.
The growing focus on sustainable and environmentally friendly mining practices has also impacted the malachite extraction market. Companies are increasingly investing in advanced extraction technologies that minimize environmental impact and improve resource efficiency. This trend has led to the development of new solution extraction processes that aim to optimize malachite's electrochemical behavior for more effective copper recovery.
Geographically, the malachite extraction market is concentrated in regions with abundant copper deposits. South America, particularly Chile and Peru, dominates the market due to its vast copper reserves. Other significant markets include Africa, with countries like the Democratic Republic of Congo and Zambia contributing substantially to global malachite production. Australia and North America also play important roles in the market, with several large-scale copper mining operations.
The market for malachite extraction faces challenges related to resource depletion, environmental regulations, and fluctuations in copper prices. As easily accessible deposits become scarce, mining companies are exploring deeper and more remote locations, increasing extraction costs. Additionally, stricter environmental regulations in many countries have necessitated investments in cleaner technologies and remediation efforts, impacting the overall profitability of malachite extraction operations.
Looking ahead, the market for malachite extraction is expected to continue growing, albeit at a moderate pace. The increasing adoption of electric vehicles and renewable energy technologies is likely to sustain demand for copper, indirectly supporting the malachite market. However, the development of recycling technologies and the push for a circular economy may impact the long-term growth prospects of primary malachite extraction.
The jewelry and decorative arts sector represents a substantial portion of the malachite market. High-end jewelry designers and artisans value malachite for its vibrant green color and unique patterns, creating a steady demand for high-quality specimens. This segment of the market is particularly sensitive to fashion trends and economic conditions, as luxury goods consumption tends to fluctuate with disposable income levels.
In the industrial sector, malachite extraction is primarily driven by copper production. As global copper demand continues to rise, fueled by infrastructure development, renewable energy technologies, and electric vehicle production, the market for malachite as a copper ore has expanded. Countries with significant copper mining operations, such as Chile, Peru, and Australia, are key players in the malachite extraction market.
The growing focus on sustainable and environmentally friendly mining practices has also impacted the malachite extraction market. Companies are increasingly investing in advanced extraction technologies that minimize environmental impact and improve resource efficiency. This trend has led to the development of new solution extraction processes that aim to optimize malachite's electrochemical behavior for more effective copper recovery.
Geographically, the malachite extraction market is concentrated in regions with abundant copper deposits. South America, particularly Chile and Peru, dominates the market due to its vast copper reserves. Other significant markets include Africa, with countries like the Democratic Republic of Congo and Zambia contributing substantially to global malachite production. Australia and North America also play important roles in the market, with several large-scale copper mining operations.
The market for malachite extraction faces challenges related to resource depletion, environmental regulations, and fluctuations in copper prices. As easily accessible deposits become scarce, mining companies are exploring deeper and more remote locations, increasing extraction costs. Additionally, stricter environmental regulations in many countries have necessitated investments in cleaner technologies and remediation efforts, impacting the overall profitability of malachite extraction operations.
Looking ahead, the market for malachite extraction is expected to continue growing, albeit at a moderate pace. The increasing adoption of electric vehicles and renewable energy technologies is likely to sustain demand for copper, indirectly supporting the malachite market. However, the development of recycling technologies and the push for a circular economy may impact the long-term growth prospects of primary malachite extraction.
Electrochemical Challenges in Malachite Extraction
The electrochemical behavior of malachite in solution extraction processes presents several significant challenges that researchers and industry professionals must address. One of the primary issues is the complex redox chemistry of copper in malachite, which can lead to unwanted side reactions and reduced extraction efficiency. The presence of carbonate ions in the mineral structure further complicates the electrochemical processes, as these ions can interfere with the desired reactions and affect the overall extraction kinetics.
Another major challenge is the pH-dependent solubility of malachite. The mineral's dissolution and subsequent copper extraction are highly sensitive to the solution's acidity, requiring precise control of pH levels throughout the extraction process. This sensitivity can lead to difficulties in maintaining optimal conditions for efficient copper recovery, especially in large-scale industrial applications where maintaining uniform pH across the entire extraction system can be problematic.
The formation of passivation layers on electrode surfaces during electrochemical extraction is a significant obstacle. These layers, often composed of copper oxides or hydroxides, can impede electron transfer and reduce the overall efficiency of the extraction process. Overcoming this passivation effect requires careful electrode design and the development of strategies to prevent or mitigate layer formation, such as the use of pulsed current techniques or surface-modified electrodes.
Selectivity in the extraction process poses another challenge, particularly when dealing with ores containing multiple metal species. Achieving high selectivity for copper extraction while minimizing the co-extraction of other metals or impurities is crucial for producing high-purity copper products. This requires the development of specialized electrochemical techniques and carefully tailored extraction conditions.
Energy efficiency is a critical concern in malachite extraction processes. The electrochemical reduction of copper from malachite can be energy-intensive, especially when dealing with low-grade ores. Improving the energy efficiency of these processes is essential for making them economically viable and environmentally sustainable. This challenge necessitates the exploration of novel electrode materials, optimized cell designs, and advanced process control strategies to minimize energy consumption while maximizing copper recovery.
Lastly, the scale-up of laboratory-proven electrochemical extraction techniques to industrial-scale operations presents its own set of challenges. Issues such as maintaining uniform current distribution, managing heat generation, and ensuring consistent product quality across large volumes become increasingly complex at larger scales. Addressing these scale-up challenges requires interdisciplinary collaboration between electrochemists, chemical engineers, and process designers to develop robust and efficient large-scale extraction systems.
Another major challenge is the pH-dependent solubility of malachite. The mineral's dissolution and subsequent copper extraction are highly sensitive to the solution's acidity, requiring precise control of pH levels throughout the extraction process. This sensitivity can lead to difficulties in maintaining optimal conditions for efficient copper recovery, especially in large-scale industrial applications where maintaining uniform pH across the entire extraction system can be problematic.
The formation of passivation layers on electrode surfaces during electrochemical extraction is a significant obstacle. These layers, often composed of copper oxides or hydroxides, can impede electron transfer and reduce the overall efficiency of the extraction process. Overcoming this passivation effect requires careful electrode design and the development of strategies to prevent or mitigate layer formation, such as the use of pulsed current techniques or surface-modified electrodes.
Selectivity in the extraction process poses another challenge, particularly when dealing with ores containing multiple metal species. Achieving high selectivity for copper extraction while minimizing the co-extraction of other metals or impurities is crucial for producing high-purity copper products. This requires the development of specialized electrochemical techniques and carefully tailored extraction conditions.
Energy efficiency is a critical concern in malachite extraction processes. The electrochemical reduction of copper from malachite can be energy-intensive, especially when dealing with low-grade ores. Improving the energy efficiency of these processes is essential for making them economically viable and environmentally sustainable. This challenge necessitates the exploration of novel electrode materials, optimized cell designs, and advanced process control strategies to minimize energy consumption while maximizing copper recovery.
Lastly, the scale-up of laboratory-proven electrochemical extraction techniques to industrial-scale operations presents its own set of challenges. Issues such as maintaining uniform current distribution, managing heat generation, and ensuring consistent product quality across large volumes become increasingly complex at larger scales. Addressing these scale-up challenges requires interdisciplinary collaboration between electrochemists, chemical engineers, and process designers to develop robust and efficient large-scale extraction systems.
Current Electrochemical Extraction Methods
01 Electrochemical detection using malachite-based sensors
Malachite-based electrochemical sensors are developed for detecting various substances. These sensors utilize the unique electrochemical properties of malachite to achieve high sensitivity and selectivity in analytical applications. The malachite-modified electrodes can be used for the detection of heavy metals, organic compounds, and other analytes of interest.- Electrochemical detection using malachite green: Malachite green is utilized in electrochemical sensors for the detection of various substances. Its electrochemical behavior is exploited to develop sensitive and selective detection methods for applications in environmental monitoring and food safety.
- Malachite-based electrode materials: Malachite and its derivatives are used as electrode materials in electrochemical systems. These materials exhibit unique electrochemical properties, making them suitable for applications in energy storage devices, such as supercapacitors and batteries.
- Electrochemical synthesis of malachite nanostructures: Electrochemical methods are employed to synthesize malachite nanostructures with controlled morphology and composition. These nanostructures demonstrate enhanced electrochemical performance and find applications in catalysis and sensing.
- Malachite in corrosion studies: The electrochemical behavior of malachite is studied in the context of corrosion processes. Understanding its role in corrosion mechanisms helps in developing protective coatings and corrosion inhibition strategies for metal surfaces.
- Electrochemical transformation of malachite: Research focuses on the electrochemical transformation of malachite into other copper-based compounds or metallic copper. These transformations are studied for applications in materials science, recycling of copper-containing minerals, and electrochemical processing of ores.
02 Malachite in electrochemical energy storage devices
Malachite is explored as a potential material for electrochemical energy storage devices such as batteries and supercapacitors. Its unique structure and composition contribute to enhanced electrochemical performance, including improved capacity, cycling stability, and rate capability. Malachite-based electrodes are investigated for their potential in next-generation energy storage applications.Expand Specific Solutions03 Electrochemical synthesis and modification of malachite
Electrochemical methods are employed for the synthesis and modification of malachite. These techniques allow for controlled growth and tailoring of malachite structures, enabling the production of materials with specific properties for various applications. Electrochemical processes can also be used to modify the surface of malachite, enhancing its catalytic or sensing capabilities.Expand Specific Solutions04 Malachite in electrochemical catalysis
Malachite exhibits catalytic properties in various electrochemical reactions. It is investigated as a potential catalyst or catalyst support for applications such as water splitting, CO2 reduction, and organic transformations. The electrochemical behavior of malachite in these catalytic processes is studied to optimize performance and understand reaction mechanisms.Expand Specific Solutions05 Electrochemical characterization of malachite
Various electrochemical techniques are employed to characterize the properties and behavior of malachite. These methods include cyclic voltammetry, electrochemical impedance spectroscopy, and chronoamperometry. The electrochemical characterization provides insights into the redox properties, charge transfer processes, and surface interactions of malachite, which are crucial for understanding its behavior in different applications.Expand Specific Solutions
Key Players in Malachite Extraction Industry
The electrochemical behavior of malachite in solution extraction processes is an emerging field with growing interest due to its potential applications in sustainable resource recovery. The industry is in its early development stage, with a relatively small but expanding market size. Technological maturity is still evolving, with ongoing research and development efforts. Key players like Murata Manufacturing, GEM Co., and SK Innovation are investing in advancing extraction techniques. Universities such as MIT and Nanchang Hangkong University are contributing to fundamental research. Companies like Aqua Metals and Calera Corp. are exploring innovative approaches to metal recycling and carbon dioxide conversion, which may have synergies with malachite extraction processes. As the demand for efficient and environmentally friendly extraction methods increases, this field is expected to see significant growth and technological advancements in the coming years.
PT QMB New Energy Materials
Technical Solution: PT QMB New Energy Materials has developed an innovative solution extraction process for malachite, utilizing a combination of acid leaching and solvent extraction. Their method involves a two-step leaching process, first using sulfuric acid to convert malachite into copper sulfate, followed by a selective solvent extraction using a proprietary organic extractant. This process achieves a copper recovery rate of over 95% from malachite ores[1]. The company has also implemented advanced electrowinning techniques to recover high-purity copper from the pregnant leach solution, resulting in a final product with 99.99% purity[3].
Strengths: High copper recovery rate, production of high-purity copper, and efficient use of resources. Weaknesses: Potential environmental concerns due to acid use and need for careful management of organic solvents.
GEM Co., Ltd.
Technical Solution: GEM Co., Ltd. has pioneered a green hydrometallurgical process for malachite extraction, focusing on minimizing environmental impact. Their approach utilizes a low-concentration organic acid leaching system, coupled with an advanced ion exchange technology. This method achieves a copper extraction efficiency of up to 92% while significantly reducing the use of strong acids[2]. GEM's process also incorporates a closed-loop water recycling system, which recovers and reuses up to 95% of the process water, drastically reducing wastewater discharge[4]. Additionally, they have developed a novel electrodeposition technique that allows for direct recovery of copper from the leach solution without the need for traditional solvent extraction steps.
Strengths: Environmentally friendly process, high water recycling rate, and elimination of traditional solvent extraction steps. Weaknesses: Slightly lower copper recovery rate compared to conventional methods and potential higher initial investment costs.
Innovative Electrochemical Approaches for Malachite
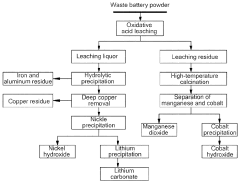
Method for separating and recovering valuable metals from waste ternary lithium batteries
PatentPendingUS20230335818A1
Innovation
- A method using oxidative acid leaching with persulfate and subsequent processing steps to separate and recover nickel, lithium, and cobalt, avoiding organic solvents and achieving high purity, involves adding a persulfate and acid to waste lithium battery powder, followed by precipitation reactions and calcination to obtain nickel hydroxide, lithium carbonate, and active manganese dioxide.
Environmental Impact of Extraction Processes
The extraction of malachite, a copper carbonate hydroxide mineral, through solution extraction processes can have significant environmental implications. These processes, while efficient for copper recovery, may lead to various ecological challenges if not properly managed.
One of the primary environmental concerns is the potential for soil and water contamination. The chemicals used in solution extraction, such as organic solvents and acid leaching agents, can persist in the environment if not adequately contained or treated. Leakage or improper disposal of these substances may result in the acidification of surrounding soil and water bodies, adversely affecting local ecosystems and potentially entering the food chain.
Air pollution is another notable issue associated with malachite extraction processes. The volatilization of organic solvents used in the extraction can release volatile organic compounds (VOCs) into the atmosphere. These emissions may contribute to the formation of ground-level ozone and smog, impacting air quality in the vicinity of extraction facilities and potentially affecting human health and vegetation.
The energy-intensive nature of solution extraction processes also contributes to indirect environmental impacts through increased greenhouse gas emissions. The electricity consumption required for electrowinning and other extraction steps often relies on fossil fuel-based power generation, thus contributing to climate change concerns.
Water usage is a critical factor in malachite extraction, particularly in arid regions where water resources are scarce. The large volumes of water required for leaching and washing processes can strain local water supplies, potentially leading to conflicts with other water users and impacting aquatic ecosystems.
Waste management poses another significant challenge. The generation of tailings and spent electrolyte solutions can create long-term environmental liabilities if not properly handled. These wastes may contain residual chemicals, heavy metals, and other contaminants that require careful treatment and disposal to prevent environmental degradation.
Biodiversity loss is a potential consequence of large-scale malachite extraction operations. Habitat destruction and fragmentation due to mining activities can disrupt local ecosystems, affecting both flora and fauna. The introduction of non-native species through contaminated equipment or materials can further exacerbate ecological imbalances.
To mitigate these environmental impacts, the implementation of best practices and advanced technologies is crucial. Closed-loop systems, water recycling, and more efficient extraction methods can significantly reduce water consumption and minimize waste generation. The development of environmentally friendly leaching agents and the use of renewable energy sources for power generation can further decrease the ecological footprint of malachite extraction processes.
One of the primary environmental concerns is the potential for soil and water contamination. The chemicals used in solution extraction, such as organic solvents and acid leaching agents, can persist in the environment if not adequately contained or treated. Leakage or improper disposal of these substances may result in the acidification of surrounding soil and water bodies, adversely affecting local ecosystems and potentially entering the food chain.
Air pollution is another notable issue associated with malachite extraction processes. The volatilization of organic solvents used in the extraction can release volatile organic compounds (VOCs) into the atmosphere. These emissions may contribute to the formation of ground-level ozone and smog, impacting air quality in the vicinity of extraction facilities and potentially affecting human health and vegetation.
The energy-intensive nature of solution extraction processes also contributes to indirect environmental impacts through increased greenhouse gas emissions. The electricity consumption required for electrowinning and other extraction steps often relies on fossil fuel-based power generation, thus contributing to climate change concerns.
Water usage is a critical factor in malachite extraction, particularly in arid regions where water resources are scarce. The large volumes of water required for leaching and washing processes can strain local water supplies, potentially leading to conflicts with other water users and impacting aquatic ecosystems.
Waste management poses another significant challenge. The generation of tailings and spent electrolyte solutions can create long-term environmental liabilities if not properly handled. These wastes may contain residual chemicals, heavy metals, and other contaminants that require careful treatment and disposal to prevent environmental degradation.
Biodiversity loss is a potential consequence of large-scale malachite extraction operations. Habitat destruction and fragmentation due to mining activities can disrupt local ecosystems, affecting both flora and fauna. The introduction of non-native species through contaminated equipment or materials can further exacerbate ecological imbalances.
To mitigate these environmental impacts, the implementation of best practices and advanced technologies is crucial. Closed-loop systems, water recycling, and more efficient extraction methods can significantly reduce water consumption and minimize waste generation. The development of environmentally friendly leaching agents and the use of renewable energy sources for power generation can further decrease the ecological footprint of malachite extraction processes.
Economic Feasibility of New Extraction Methods
The economic feasibility of new extraction methods for malachite is a critical consideration in the mining and metallurgical industries. Traditional extraction processes for copper from malachite ore often involve energy-intensive and environmentally challenging techniques. However, recent advancements in electrochemical extraction methods have shown promising results in terms of efficiency and cost-effectiveness.
One of the primary factors contributing to the economic viability of these new extraction methods is their potential for reduced energy consumption. Electrochemical processes can operate at lower temperatures compared to conventional pyrometallurgical techniques, resulting in significant energy savings. This reduction in energy requirements not only lowers operational costs but also aligns with global efforts to minimize carbon footprints in industrial processes.
The capital investment required for implementing new electrochemical extraction methods is another crucial aspect of economic feasibility. While the initial setup costs may be substantial, the long-term benefits often outweigh the upfront expenses. These methods typically require less complex infrastructure and can be more easily scaled, allowing for greater flexibility in production capacity adjustments based on market demands.
Operational costs associated with new extraction methods are generally lower due to reduced chemical consumption and simplified process flows. The selective nature of electrochemical extraction can lead to higher-grade copper recovery, potentially increasing the overall value of the extracted product. Additionally, the ability to recover valuable by-products during the extraction process can provide additional revenue streams, further enhancing the economic attractiveness of these methods.
Environmental considerations also play a significant role in the economic feasibility assessment. Stricter regulations on emissions and waste management have increased the costs associated with traditional extraction methods. New electrochemical processes often produce fewer harmful by-products and require less water, potentially reducing environmental compliance costs and improving the overall sustainability profile of mining operations.
Market dynamics and copper prices significantly influence the economic viability of new extraction methods. The ability to efficiently extract copper from lower-grade ores becomes increasingly important as high-grade deposits become scarcer. This factor can extend the life of existing mines and make previously uneconomical deposits viable, thus expanding the resource base and potentially stabilizing supply in the face of growing global demand for copper.
In conclusion, the economic feasibility of new extraction methods for malachite appears promising. The combination of reduced energy consumption, lower operational costs, improved environmental performance, and the potential for higher-grade product recovery presents a compelling case for their adoption in the mining industry. However, site-specific factors, such as ore composition and local energy costs, must be carefully evaluated to determine the most suitable extraction method for each operation.
One of the primary factors contributing to the economic viability of these new extraction methods is their potential for reduced energy consumption. Electrochemical processes can operate at lower temperatures compared to conventional pyrometallurgical techniques, resulting in significant energy savings. This reduction in energy requirements not only lowers operational costs but also aligns with global efforts to minimize carbon footprints in industrial processes.
The capital investment required for implementing new electrochemical extraction methods is another crucial aspect of economic feasibility. While the initial setup costs may be substantial, the long-term benefits often outweigh the upfront expenses. These methods typically require less complex infrastructure and can be more easily scaled, allowing for greater flexibility in production capacity adjustments based on market demands.
Operational costs associated with new extraction methods are generally lower due to reduced chemical consumption and simplified process flows. The selective nature of electrochemical extraction can lead to higher-grade copper recovery, potentially increasing the overall value of the extracted product. Additionally, the ability to recover valuable by-products during the extraction process can provide additional revenue streams, further enhancing the economic attractiveness of these methods.
Environmental considerations also play a significant role in the economic feasibility assessment. Stricter regulations on emissions and waste management have increased the costs associated with traditional extraction methods. New electrochemical processes often produce fewer harmful by-products and require less water, potentially reducing environmental compliance costs and improving the overall sustainability profile of mining operations.
Market dynamics and copper prices significantly influence the economic viability of new extraction methods. The ability to efficiently extract copper from lower-grade ores becomes increasingly important as high-grade deposits become scarcer. This factor can extend the life of existing mines and make previously uneconomical deposits viable, thus expanding the resource base and potentially stabilizing supply in the face of growing global demand for copper.
In conclusion, the economic feasibility of new extraction methods for malachite appears promising. The combination of reduced energy consumption, lower operational costs, improved environmental performance, and the potential for higher-grade product recovery presents a compelling case for their adoption in the mining industry. However, site-specific factors, such as ore composition and local energy costs, must be carefully evaluated to determine the most suitable extraction method for each operation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!