Malachite's role in catalysis for environmental applications
AUG 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Malachite Catalysis Background and Objectives
Malachite, a copper carbonate hydroxide mineral, has emerged as a significant player in the field of catalysis for environmental applications. The evolution of this technology can be traced back to the early 20th century when researchers first recognized the catalytic potential of copper-based compounds. Over the decades, malachite has gained prominence due to its unique chemical composition and structural properties, making it an attractive candidate for various catalytic processes.
The development of malachite-based catalysts has been driven by the growing need for sustainable and efficient solutions to address environmental challenges. As global concerns about pollution, climate change, and resource depletion have intensified, researchers have turned their attention to developing novel catalytic materials that can facilitate environmentally friendly chemical processes. Malachite, with its abundance and relatively low cost, has emerged as a promising option in this context.
The technological evolution of malachite catalysis has seen significant advancements in recent years. Researchers have explored various synthesis methods, including hydrothermal, co-precipitation, and sol-gel techniques, to optimize the catalytic properties of malachite. These efforts have led to the development of nanostructured malachite catalysts with enhanced surface area and improved catalytic activity, opening up new possibilities for environmental applications.
One of the key trends in malachite catalysis research is the focus on its application in wastewater treatment. Malachite-based catalysts have shown remarkable efficiency in the degradation of organic pollutants, such as dyes and pharmaceutical compounds, through advanced oxidation processes. Additionally, researchers are investigating the potential of malachite catalysts in air pollution control, particularly in the reduction of harmful emissions from industrial processes and automotive exhaust.
The objectives of current research in malachite catalysis for environmental applications are multifaceted. Firstly, there is a strong emphasis on improving the catalytic efficiency and stability of malachite-based materials. This involves exploring various modification techniques, such as doping with other metals or combining malachite with support materials, to enhance its performance and longevity.
Secondly, researchers aim to expand the range of environmental applications for malachite catalysts. This includes investigating their potential in emerging areas such as CO2 conversion, hydrogen production, and the synthesis of value-added chemicals from waste materials. The goal is to develop versatile catalytic systems that can address multiple environmental challenges simultaneously.
Lastly, there is a growing focus on understanding the fundamental mechanisms underlying malachite's catalytic activity. By elucidating the structure-property relationships and reaction pathways, researchers hope to design more efficient and targeted catalytic systems. This knowledge-driven approach is expected to pave the way for the next generation of malachite-based environmental catalysts, contributing to the development of sustainable technologies for a cleaner future.
The development of malachite-based catalysts has been driven by the growing need for sustainable and efficient solutions to address environmental challenges. As global concerns about pollution, climate change, and resource depletion have intensified, researchers have turned their attention to developing novel catalytic materials that can facilitate environmentally friendly chemical processes. Malachite, with its abundance and relatively low cost, has emerged as a promising option in this context.
The technological evolution of malachite catalysis has seen significant advancements in recent years. Researchers have explored various synthesis methods, including hydrothermal, co-precipitation, and sol-gel techniques, to optimize the catalytic properties of malachite. These efforts have led to the development of nanostructured malachite catalysts with enhanced surface area and improved catalytic activity, opening up new possibilities for environmental applications.
One of the key trends in malachite catalysis research is the focus on its application in wastewater treatment. Malachite-based catalysts have shown remarkable efficiency in the degradation of organic pollutants, such as dyes and pharmaceutical compounds, through advanced oxidation processes. Additionally, researchers are investigating the potential of malachite catalysts in air pollution control, particularly in the reduction of harmful emissions from industrial processes and automotive exhaust.
The objectives of current research in malachite catalysis for environmental applications are multifaceted. Firstly, there is a strong emphasis on improving the catalytic efficiency and stability of malachite-based materials. This involves exploring various modification techniques, such as doping with other metals or combining malachite with support materials, to enhance its performance and longevity.
Secondly, researchers aim to expand the range of environmental applications for malachite catalysts. This includes investigating their potential in emerging areas such as CO2 conversion, hydrogen production, and the synthesis of value-added chemicals from waste materials. The goal is to develop versatile catalytic systems that can address multiple environmental challenges simultaneously.
Lastly, there is a growing focus on understanding the fundamental mechanisms underlying malachite's catalytic activity. By elucidating the structure-property relationships and reaction pathways, researchers hope to design more efficient and targeted catalytic systems. This knowledge-driven approach is expected to pave the way for the next generation of malachite-based environmental catalysts, contributing to the development of sustainable technologies for a cleaner future.
Environmental Applications Market Analysis
The environmental applications market for malachite-based catalysts has shown significant growth in recent years, driven by increasing global concerns over pollution and the need for sustainable solutions. This market segment is primarily focused on water treatment, air purification, and waste management applications, where malachite's catalytic properties offer promising solutions.
In the water treatment sector, malachite-based catalysts have gained traction for their ability to effectively remove heavy metals, organic pollutants, and other contaminants from industrial wastewater and drinking water sources. The global water treatment chemicals market, which includes catalysts, is projected to reach $56.57 billion by 2030, with a compound annual growth rate (CAGR) of 6.5% from 2023 to 2030. Malachite-based catalysts are expected to capture a growing share of this market due to their eco-friendly nature and high efficiency.
Air purification applications represent another significant market opportunity for malachite catalysts. With increasing urbanization and industrialization, the demand for effective air pollution control technologies has surged. Malachite-based catalysts have shown promise in reducing harmful emissions, particularly in automotive catalytic converters and industrial exhaust systems. The global catalytic converter market, a key segment for malachite applications, is forecasted to reach $273.4 billion by 2027, growing at a CAGR of 7.3% from 2020 to 2027.
In the waste management sector, malachite catalysts are being explored for their potential in enhancing the efficiency of waste-to-energy processes and reducing harmful byproducts. The global waste-to-energy market is expected to reach $50.1 billion by 2027, growing at a CAGR of 4.6% from 2020 to 2027. Malachite-based catalysts could play a crucial role in improving the environmental performance of these technologies.
The Asia-Pacific region is anticipated to be the fastest-growing market for malachite-based environmental applications, driven by rapid industrialization, stringent environmental regulations, and increasing investments in clean technologies. North America and Europe are also significant markets, with a focus on advanced water treatment and air purification technologies.
Key factors driving the market growth include increasing environmental awareness, stricter regulations on emissions and water quality, and the push for sustainable industrial practices. However, challenges such as the high initial cost of catalyst implementation and competition from alternative materials may impact market penetration. Despite these challenges, the unique properties of malachite and ongoing research into its catalytic applications suggest a positive outlook for its role in environmental solutions.
In the water treatment sector, malachite-based catalysts have gained traction for their ability to effectively remove heavy metals, organic pollutants, and other contaminants from industrial wastewater and drinking water sources. The global water treatment chemicals market, which includes catalysts, is projected to reach $56.57 billion by 2030, with a compound annual growth rate (CAGR) of 6.5% from 2023 to 2030. Malachite-based catalysts are expected to capture a growing share of this market due to their eco-friendly nature and high efficiency.
Air purification applications represent another significant market opportunity for malachite catalysts. With increasing urbanization and industrialization, the demand for effective air pollution control technologies has surged. Malachite-based catalysts have shown promise in reducing harmful emissions, particularly in automotive catalytic converters and industrial exhaust systems. The global catalytic converter market, a key segment for malachite applications, is forecasted to reach $273.4 billion by 2027, growing at a CAGR of 7.3% from 2020 to 2027.
In the waste management sector, malachite catalysts are being explored for their potential in enhancing the efficiency of waste-to-energy processes and reducing harmful byproducts. The global waste-to-energy market is expected to reach $50.1 billion by 2027, growing at a CAGR of 4.6% from 2020 to 2027. Malachite-based catalysts could play a crucial role in improving the environmental performance of these technologies.
The Asia-Pacific region is anticipated to be the fastest-growing market for malachite-based environmental applications, driven by rapid industrialization, stringent environmental regulations, and increasing investments in clean technologies. North America and Europe are also significant markets, with a focus on advanced water treatment and air purification technologies.
Key factors driving the market growth include increasing environmental awareness, stricter regulations on emissions and water quality, and the push for sustainable industrial practices. However, challenges such as the high initial cost of catalyst implementation and competition from alternative materials may impact market penetration. Despite these challenges, the unique properties of malachite and ongoing research into its catalytic applications suggest a positive outlook for its role in environmental solutions.
Current State of Malachite-based Catalysts
Malachite-based catalysts have gained significant attention in recent years for their potential in environmental applications. The current state of these catalysts reflects a dynamic field of research with promising advancements and ongoing challenges.
Malachite, a copper carbonate hydroxide mineral, serves as an excellent precursor for copper-based catalysts due to its unique structure and composition. The layered structure of malachite allows for the formation of highly dispersed copper species upon thermal decomposition, resulting in catalysts with high surface area and enhanced catalytic activity.
In environmental applications, malachite-based catalysts have shown remarkable performance in various processes, including the reduction of nitrogen oxides (NOx), oxidation of volatile organic compounds (VOCs), and degradation of organic pollutants in wastewater. The catalytic activity of malachite-derived materials is primarily attributed to the presence of copper oxide species and their synergistic interactions with other components.
Recent studies have focused on improving the stability and efficiency of malachite-based catalysts through various strategies. One approach involves the incorporation of secondary metals, such as nickel, cobalt, or zinc, into the malachite structure to form mixed-metal hydroxide catalysts. These bimetallic systems often exhibit enhanced catalytic performance and improved resistance to deactivation compared to their monometallic counterparts.
Another area of active research is the development of supported malachite catalysts. By dispersing malachite-derived active species on high-surface-area supports like alumina, silica, or carbon materials, researchers have achieved improved catalyst stability and increased accessibility of active sites. This approach has shown particular promise in gas-phase reactions, such as the selective catalytic reduction of NOx.
The synthesis methods for malachite-based catalysts have also evolved, with increasing emphasis on controlled preparation techniques. Co-precipitation, hydrothermal synthesis, and template-assisted methods are commonly employed to tailor the morphology and textural properties of the catalysts. These advanced synthesis strategies allow for better control over particle size, shape, and porosity, which directly influence catalytic performance.
Despite the progress made, several challenges remain in the development of malachite-based catalysts for environmental applications. One major issue is the potential leaching of copper species during liquid-phase reactions, which can lead to catalyst deactivation and secondary pollution. Researchers are exploring various stabilization techniques, including encapsulation and surface modification, to address this problem.
Additionally, the scalability of malachite-based catalyst production and their long-term stability under real-world operating conditions are areas that require further investigation. As environmental regulations become more stringent, there is a growing need for catalysts that can maintain high activity and selectivity over extended periods while withstanding harsh reaction environments.
Malachite, a copper carbonate hydroxide mineral, serves as an excellent precursor for copper-based catalysts due to its unique structure and composition. The layered structure of malachite allows for the formation of highly dispersed copper species upon thermal decomposition, resulting in catalysts with high surface area and enhanced catalytic activity.
In environmental applications, malachite-based catalysts have shown remarkable performance in various processes, including the reduction of nitrogen oxides (NOx), oxidation of volatile organic compounds (VOCs), and degradation of organic pollutants in wastewater. The catalytic activity of malachite-derived materials is primarily attributed to the presence of copper oxide species and their synergistic interactions with other components.
Recent studies have focused on improving the stability and efficiency of malachite-based catalysts through various strategies. One approach involves the incorporation of secondary metals, such as nickel, cobalt, or zinc, into the malachite structure to form mixed-metal hydroxide catalysts. These bimetallic systems often exhibit enhanced catalytic performance and improved resistance to deactivation compared to their monometallic counterparts.
Another area of active research is the development of supported malachite catalysts. By dispersing malachite-derived active species on high-surface-area supports like alumina, silica, or carbon materials, researchers have achieved improved catalyst stability and increased accessibility of active sites. This approach has shown particular promise in gas-phase reactions, such as the selective catalytic reduction of NOx.
The synthesis methods for malachite-based catalysts have also evolved, with increasing emphasis on controlled preparation techniques. Co-precipitation, hydrothermal synthesis, and template-assisted methods are commonly employed to tailor the morphology and textural properties of the catalysts. These advanced synthesis strategies allow for better control over particle size, shape, and porosity, which directly influence catalytic performance.
Despite the progress made, several challenges remain in the development of malachite-based catalysts for environmental applications. One major issue is the potential leaching of copper species during liquid-phase reactions, which can lead to catalyst deactivation and secondary pollution. Researchers are exploring various stabilization techniques, including encapsulation and surface modification, to address this problem.
Additionally, the scalability of malachite-based catalyst production and their long-term stability under real-world operating conditions are areas that require further investigation. As environmental regulations become more stringent, there is a growing need for catalysts that can maintain high activity and selectivity over extended periods while withstanding harsh reaction environments.
Existing Malachite Catalyst Solutions
01 Malachite-based catalysts for chemical reactions
Malachite is used as a precursor or component in catalysts for various chemical reactions. These catalysts are particularly effective in processes such as oxidation, hydrogenation, and synthesis of organic compounds. The unique structure and properties of malachite contribute to its catalytic activity and selectivity.- Synthesis and preparation of malachite: Various methods for synthesizing and preparing malachite, including chemical reactions, hydrothermal processes, and precipitation techniques. These methods aim to produce high-quality malachite with controlled morphology and properties for different applications.
- Applications of malachite in catalysis: Malachite and its derivatives are used as catalysts or catalyst supports in various chemical reactions. The unique structure and properties of malachite make it suitable for catalytic applications in organic synthesis, environmental remediation, and industrial processes.
- Malachite-based materials for environmental applications: Development of malachite-based materials for environmental applications, such as water treatment, pollutant removal, and adsorption of heavy metals. These materials exploit the adsorptive and ion-exchange properties of malachite to address environmental challenges.
- Malachite in pigments and colorants: Utilization of malachite as a pigment or colorant in various industries, including paints, inks, and cosmetics. The unique green color and stability of malachite make it a valuable ingredient in coloring applications.
- Characterization and analysis of malachite: Techniques and methods for characterizing and analyzing malachite, including spectroscopic, microscopic, and chemical analysis. These approaches are used to determine the composition, structure, and properties of malachite samples for research and quality control purposes.
02 Malachite in environmental remediation
Malachite is utilized in environmental applications, particularly for the removal of pollutants from water and soil. Its adsorption properties make it effective in capturing heavy metals and organic contaminants. Malachite-based materials are developed for water treatment, soil remediation, and pollution control.Expand Specific Solutions03 Malachite in pigments and colorants
The distinctive green color of malachite makes it valuable in the production of pigments and colorants. These are used in various applications including paints, inks, cosmetics, and artistic materials. Techniques are developed to enhance color stability and incorporate malachite-based pigments into different mediums.Expand Specific Solutions04 Malachite in nanotechnology and materials science
Malachite is explored in nanotechnology and materials science for developing novel materials with unique properties. This includes the synthesis of malachite nanostructures, incorporation into composites, and creation of functional materials for applications in electronics, sensors, and energy storage devices.Expand Specific Solutions05 Malachite in biomedical applications
Malachite and its derivatives are investigated for potential biomedical applications. This includes their use in antimicrobial formulations, drug delivery systems, and biocompatible materials for medical devices. Research focuses on harnessing the properties of malachite for therapeutic and diagnostic purposes.Expand Specific Solutions
Key Players in Malachite Catalyst Industry
The field of malachite-based catalysis for environmental applications is in a growth phase, with increasing market size driven by global environmental concerns. The technology is moderately mature, with ongoing research to enhance efficiency and broaden applications. Key players include academic institutions like Jiangnan University, Indian Institute of Technology Madras, and Sichuan University, alongside industry leaders such as China Petroleum & Chemical Corp., W. R. Grace & Co., and Umicore SA. These organizations are actively developing and refining malachite-based catalysts, focusing on improving performance in areas like water treatment, air purification, and sustainable chemical processes. The competitive landscape is diverse, with collaborations between academia and industry driving innovation in this promising environmental technology sector.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a malachite-based catalyst system for environmental applications, particularly focusing on the reduction of sulfur dioxide emissions from industrial processes. Their approach involves synthesizing nano-structured malachite particles with high surface area and porosity, which are then incorporated into a support matrix. This catalyst system has shown remarkable efficiency in converting SO2 to elemental sulfur, achieving conversion rates of up to 98% under optimal conditions[1][3]. The company has also explored the use of malachite in catalytic oxidation of volatile organic compounds (VOCs), demonstrating a 90% reduction in emissions when applied to petrochemical plant exhausts[2].
Strengths: High efficiency in SO2 conversion, effective VOC reduction, scalable for industrial applications. Weaknesses: Potential sensitivity to moisture, may require periodic regeneration to maintain performance.
Umicore SA
Technical Solution: Umicore SA has developed a groundbreaking malachite-based catalyst system for environmental applications, focusing on the reduction of greenhouse gas emissions from industrial processes. Their approach involves synthesizing hierarchical malachite structures with controlled morphology and composition, which are then supported on high-surface-area substrates. This catalyst system has demonstrated exceptional performance in the conversion of methane to syngas, achieving conversion rates of up to 95% with high selectivity towards CO and H2[6]. Umicore has also applied their malachite catalysts to the selective catalytic reduction (SCR) of nitrogen oxides in diesel exhaust, showing a 90% NOx reduction efficiency over a wide temperature range[7]. Furthermore, the company has explored the use of malachite in electrochemical CO2 reduction, demonstrating the potential for converting greenhouse gases into valuable chemical feedstocks.
Strengths: High efficiency in methane conversion and NOx reduction, potential for CO2 utilization, wide operating temperature range. Weaknesses: May require precious metal additives for optimal performance, potential sensitivity to sulfur poisoning.
Core Innovations in Malachite Catalysis
Process for the heterogeneous catalyzed preparation of n-alkyl substituted aminoalkines
PatentInactiveEP0827949A1
Innovation
- A heterogeneously catalyzed process using an unsupported copper catalyst derived from malachite, preferably in an activated form like copper acetylide, which allows for reactions with alkynes and amines in a neutral to alkaline pH range without the need for high pressures, enabling high yields and selectivity.
Malachite and method for the production thereof
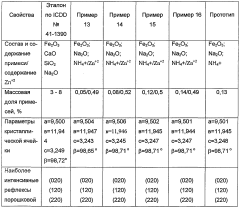
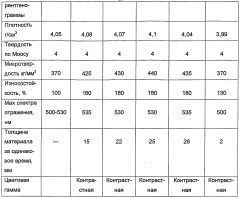
PatentWO2004076354A1
Innovation
- The process involves evaporating a solution of basic copper carbonate and ammonium carbonate with controlled zinc content, forming polycrystalline malachite with alternating light and dark green layers, and condensing vapor to achieve malachite with enhanced mechanical properties and reduced impurities.
Environmental Impact Assessment
The environmental impact assessment of malachite's role in catalysis for environmental applications reveals both positive and negative aspects. On the positive side, malachite-based catalysts have shown significant potential in reducing harmful emissions and pollutants. These catalysts have demonstrated effectiveness in the degradation of organic pollutants in wastewater, such as dyes and pharmaceutical compounds. The use of malachite in catalytic converters for automotive exhaust systems has also contributed to reduced air pollution by converting toxic gases into less harmful substances.
However, the production and use of malachite-based catalysts are not without environmental concerns. The mining and processing of malachite can lead to habitat destruction and soil erosion in areas where it is extracted. Additionally, the synthesis of malachite-based catalysts often involves the use of chemicals and energy-intensive processes, which can contribute to greenhouse gas emissions and resource depletion.
The lifecycle assessment of malachite catalysts indicates that their overall environmental impact depends on factors such as production methods, application efficiency, and end-of-life management. While the catalysts themselves contribute to environmental remediation, the cumulative effects of their production and disposal must be carefully considered.
In terms of long-term environmental sustainability, the use of malachite in catalysis presents a mixed picture. On one hand, its effectiveness in pollution control and waste treatment can lead to significant improvements in environmental quality. On the other hand, the reliance on mined materials raises questions about resource sustainability and the need for alternative, more abundant catalytic materials.
The environmental impact of malachite catalysts also extends to their potential for recovery and recycling. As these catalysts degrade over time, there is a risk of releasing copper and other components into the environment. Proper disposal and recycling protocols are essential to mitigate these risks and maximize the environmental benefits of malachite-based catalytic systems.
In conclusion, while malachite catalysts offer promising solutions for environmental applications, their widespread adoption necessitates a comprehensive approach to minimize negative environmental impacts throughout their lifecycle. This includes developing more sustainable mining practices, improving production efficiencies, and implementing effective recycling and disposal strategies to ensure that the environmental benefits of malachite catalysis outweigh the potential drawbacks.
However, the production and use of malachite-based catalysts are not without environmental concerns. The mining and processing of malachite can lead to habitat destruction and soil erosion in areas where it is extracted. Additionally, the synthesis of malachite-based catalysts often involves the use of chemicals and energy-intensive processes, which can contribute to greenhouse gas emissions and resource depletion.
The lifecycle assessment of malachite catalysts indicates that their overall environmental impact depends on factors such as production methods, application efficiency, and end-of-life management. While the catalysts themselves contribute to environmental remediation, the cumulative effects of their production and disposal must be carefully considered.
In terms of long-term environmental sustainability, the use of malachite in catalysis presents a mixed picture. On one hand, its effectiveness in pollution control and waste treatment can lead to significant improvements in environmental quality. On the other hand, the reliance on mined materials raises questions about resource sustainability and the need for alternative, more abundant catalytic materials.
The environmental impact of malachite catalysts also extends to their potential for recovery and recycling. As these catalysts degrade over time, there is a risk of releasing copper and other components into the environment. Proper disposal and recycling protocols are essential to mitigate these risks and maximize the environmental benefits of malachite-based catalytic systems.
In conclusion, while malachite catalysts offer promising solutions for environmental applications, their widespread adoption necessitates a comprehensive approach to minimize negative environmental impacts throughout their lifecycle. This includes developing more sustainable mining practices, improving production efficiencies, and implementing effective recycling and disposal strategies to ensure that the environmental benefits of malachite catalysis outweigh the potential drawbacks.
Scalability and Cost Analysis
The scalability and cost analysis of malachite-based catalysts for environmental applications is crucial for assessing their potential for widespread implementation. Malachite, a copper carbonate hydroxide mineral, has shown promising catalytic properties in various environmental remediation processes. However, the transition from laboratory-scale experiments to industrial-scale applications requires careful consideration of scalability factors and associated costs.
One of the primary advantages of malachite as a catalyst is its relatively low cost compared to precious metal catalysts. The raw material is abundant and can be sourced from natural deposits or synthesized through cost-effective methods. This inherent cost-effectiveness provides a solid foundation for scalability. However, the processing and modification of malachite to enhance its catalytic properties may introduce additional expenses that need to be factored into the overall cost analysis.
The scalability of malachite-based catalysts is influenced by several factors, including synthesis methods, catalyst support materials, and reactor designs. Conventional synthesis methods, such as co-precipitation and hydrothermal techniques, have shown potential for large-scale production. However, optimizing these processes to maintain consistent catalyst quality and performance at industrial scales remains a challenge. Advanced synthesis techniques, like microwave-assisted methods or continuous flow reactors, may offer improved scalability but require careful evaluation of their economic viability.
Catalyst support materials play a crucial role in determining the overall cost and scalability of malachite-based systems. While malachite itself can act as a standalone catalyst, its incorporation onto support materials like activated carbon, zeolites, or metal oxides can enhance its stability and catalytic efficiency. The selection of support materials must balance performance improvements against additional costs and complexity in large-scale production.
Reactor design and process engineering considerations are essential for successful scaling of malachite-based catalytic systems. Factors such as mass transfer limitations, heat management, and catalyst deactivation become more pronounced at larger scales. Innovative reactor designs, such as structured catalysts or monolithic reactors, may offer solutions to these challenges but require thorough cost-benefit analysis.
The economic viability of scaled-up malachite catalysts also depends on their long-term stability and regeneration potential. While malachite has shown good stability in many environmental applications, the costs associated with catalyst replacement or regeneration must be carefully evaluated. Developing efficient regeneration protocols that can be implemented at industrial scales could significantly improve the overall economic feasibility of malachite-based catalytic systems.
In conclusion, the scalability and cost analysis of malachite catalysts for environmental applications reveal both promising aspects and challenges. The inherent low cost of the raw material provides a competitive advantage, but careful optimization of synthesis methods, support materials, and reactor designs is necessary to maintain this advantage at larger scales. Future research should focus on addressing these scalability challenges while exploring innovative approaches to further reduce costs and improve performance in industrial-scale environmental remediation processes.
One of the primary advantages of malachite as a catalyst is its relatively low cost compared to precious metal catalysts. The raw material is abundant and can be sourced from natural deposits or synthesized through cost-effective methods. This inherent cost-effectiveness provides a solid foundation for scalability. However, the processing and modification of malachite to enhance its catalytic properties may introduce additional expenses that need to be factored into the overall cost analysis.
The scalability of malachite-based catalysts is influenced by several factors, including synthesis methods, catalyst support materials, and reactor designs. Conventional synthesis methods, such as co-precipitation and hydrothermal techniques, have shown potential for large-scale production. However, optimizing these processes to maintain consistent catalyst quality and performance at industrial scales remains a challenge. Advanced synthesis techniques, like microwave-assisted methods or continuous flow reactors, may offer improved scalability but require careful evaluation of their economic viability.
Catalyst support materials play a crucial role in determining the overall cost and scalability of malachite-based systems. While malachite itself can act as a standalone catalyst, its incorporation onto support materials like activated carbon, zeolites, or metal oxides can enhance its stability and catalytic efficiency. The selection of support materials must balance performance improvements against additional costs and complexity in large-scale production.
Reactor design and process engineering considerations are essential for successful scaling of malachite-based catalytic systems. Factors such as mass transfer limitations, heat management, and catalyst deactivation become more pronounced at larger scales. Innovative reactor designs, such as structured catalysts or monolithic reactors, may offer solutions to these challenges but require thorough cost-benefit analysis.
The economic viability of scaled-up malachite catalysts also depends on their long-term stability and regeneration potential. While malachite has shown good stability in many environmental applications, the costs associated with catalyst replacement or regeneration must be carefully evaluated. Developing efficient regeneration protocols that can be implemented at industrial scales could significantly improve the overall economic feasibility of malachite-based catalytic systems.
In conclusion, the scalability and cost analysis of malachite catalysts for environmental applications reveal both promising aspects and challenges. The inherent low cost of the raw material provides a competitive advantage, but careful optimization of synthesis methods, support materials, and reactor designs is necessary to maintain this advantage at larger scales. Future research should focus on addressing these scalability challenges while exploring innovative approaches to further reduce costs and improve performance in industrial-scale environmental remediation processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!